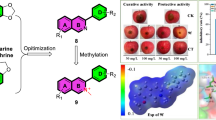
Abstract
A series of 2-phenyl-4-aminoquinolines were designed, synthesized and evaluated for their antifungal activities against three phytopathogenic fungi in vitro. All of the target compounds were fully elucidated by 1H NMR, 13C NMR and HRMS spectra. The results indicated that most of the target compounds demonstrated significant activities against the tested fungi. Among them, compound 6e exhibited more promising inhibitory activities against C. lunata (EC50 = 13.3 μg/mL), P. grisea (EC50 = 14.4 μg/mL) and A. alternate (EC50 = 15.6 μg/mL), superior to azoxystrobin, a commercial agricultural fungicide. The structure–activity relationship (SAR) revealed that the aniline moiety at position 4 of the quinoline scaffold played a key role in the potency of a compound. And the substitution positions of the aniline moiety significantly influenced the activities. These encouraging results yielded a variety of 2-phenylquinolines bearing an aniline moiety acting as promising antifungal agents.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytopathogenic fungi have long been known as a severe threat to plant species. They cause serious economic loss to global agricultural production and even lead to food safety problem due to the mycotoxins produced by some kinds of fungi [1, 2]. Although some agricultural antifungal agents are currently available on the market, there is still an extremely urgent demand for new fungicides on account of some inevitable defects of the traditional antifungal agents, including toxicity to non-target organisms, high residue, growing resistance and so on [3, 4].
Quinoline and its derivatives, a class of important bioactive natural products, usually serve as a core fragment in a variety of active molecules, which exhibit extensive biological activities [5,6,7,8,9,10,11,12], including antifungal property [13,14,15,16]. Over the past decades, some agricultural chemicals containing a quinoline moiety have been put on the market, such as the fungicides quinoxyfen and tebufloquin, the insecticide flometoquin and the herbicide quinclorac. Of note, a growing number of investigations have been directed toward the modification of 2-phenylquinoline and 4-aminoquinoline due to their versatile biological activities, such as antimicrobial [17,18,19], antiviral [20, 21], antimalarial [22, 23], antitumor [24, 25] and antifungal activities [26,27,28,29]. As shown in Fig. 1, some reported antifungal agents containing 2-phenylquinoline or 4-aminoquinoline were listed. In previous work, we also found that some 2-phenyl-4-thioquinolines exhibited moderate to good antifungal activities [30]. Nevertheless, to our best knowledge, the preparation and antifungal activity of 2-phenyl-4-aminoquinolines were less studied.
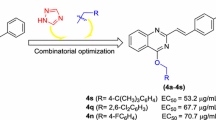
Inspired by the above considerations and as part of our continuing efforts to achieve high-efficacy and broad-spectrum fungicides, herein we have designed and synthesized a series of 2-phenyl-4-aminoquinolines as potential antifungal agents by incorporation of 2-phenylquinoline and 4-aminoquinoline in one molecule (Scheme 1). Quinolines 6a–6u contain various substituted aniline at position 4 of quinoline scaffold, while quinolines 7, 8 carry a dimethylamine or 1,2,4-1H-triazole at position 4, respectively. And all synthesized compounds were investigated for their antifungal activities against some common phytopathogenic fungi in vitro.
Results and discussion
Chemistry
As outlined in Scheme 1, o-aminoacetophenone 1 was coupled to benzoyl chloride 2 in the presence of base (TEA) to give 3 in quantitative yield. Aldol condensation of intermediate 3 afforded quinolinone 4 in 94% yield, which was then converted into 4-chloroquinoline 5 in 64% yield using phosphorus oxychloride in refluxing dioxane. Finally, the target compounds were smoothly generated in moderate to good yields (28–93%) by reaction of 4-chloroquinoline 5 with amine, amide or 1,2,4-triazolylsodium under suitable conditions [31, 32].
All target compounds were fully characterized by means of 1H NMR, 13C NMR and HRMS spectra. Taking compound 6o (R = 4′-CH3) as a representative example, two downfield proton signals were found at 14.35 and 11.18 ppm in DMSO-d6, which proved that imine was transformed into a form of hydrochloride. A singlet appeared at 6.91 ppm was attributed to H-3 resonance of quinoline skeleton. Additionally, CH3 protons occurred as a singlet at 2.41 ppm. The other signals (13H) appeared in the range of 8.95–7.39 were attributed to Ar–H. Due to the influence of symmetry, eighteen signals, including four overlapped peaks, were observed in the 13C NMR spectrum. Finally, high-resolution mass spectrum (HRMS) of 6o displayed a characteristic ion peak at m/z = 311.1526, which was attributed to the chemical species of [M-Cl]+.
Antifungal activity
All the target compounds (6a–6u, 7 and 8), as well as azoxystrobin (a commercial fungicide), were evaluated for their antifungal activities in vitro against three phytopathogenic fungi (C. lunata, P. grisea and A. alternate) at 100 and 50 μg/mL based on mycelium growth rate method. As described in Table 1, more than half of the target compounds exhibited comparable or better inhibition activities at 100 and 50 µg/mL, relative to the positive control. Especially, compounds 6a, 6b, 6e, 6i, 6j, 6o and 6r revealed better potency with inhibition rates over 65% at 50 μg/mL in most cases.
In order to more reliably explore the antifungal potential and structure–activity relationship (SAR), compounds with inhibition rates over 50% at 50 μg/mL were selected to obtain their EC50 (half maximal effective concentration) values (Table 2). As summarized in Table 2, most compounds displayed good antifungal activities against the tested fungi. Compared with the positive control azoxystrobin (EC50 = 72.5 μg/mL) against C. lunata, at least twelve compounds exhibited superior potency with EC50 values of 13.3–48.7 μg/mL. It was worth noting that the EC50 values of compounds 6e, 6i and 6k were lower than 16 μg/mL against C. lunata. For P. grisea, all thirteen tested compounds showed good activities with EC50 values of 14.4–44.2 μg/mL, and 6e exhibited the best activity with an EC50 value of 14.4 μg/mL, superior to azoxystrobin (EC50 = 34.5 μg/mL). Regarding A. alternate, the twelve tested compounds also displayed good activities with EC50 values of 15.6–49.8 μg/mL. Interestingly, compounds 6e (EC50 = 15.6 μg/mL) and 6j (EC50 = 16.9 μg/mL) showed comparable inhibitory potency compared with azoxystrobin (EC50 = 16.0 μg/mL).
In case of anilinoquinolines 6a–6u, it was clearly seen that different substituents on the aniline moiety had a remarkable effect on the inhibitory activity. First, it was apparent that the one bearing a para-substituent on the aniline moiety was more potent than ortho- or meta-substituted analogs (see compounds 6e, 6h, 6j, 6l, 6o and 6r). In addition, compounds bearing substituents at ortho position of the aniline ring were the least active compounds followed by meta-substituted counterparts in most cases, implying that steric bulk has in general a negative effect on potency. For example, Compound 6e (4′-Cl) displayed the most potent antifungal activities in the set against C. lunata (EC50 = 13.3 μg/mL), P. grisea (EC50 = 14.4 μg/mL) and A. alternate (EC50 = 15.6 μg/mL). This compound was also found to be most potent among all target compounds investigated in the current work. Meanwhile, the above mentioned pattern of a decreased activity from meta (EC50 of 6f: ≥ 50, 34.7, 40.5 μg/mL, respectively) to ortho substitution (6g: ≥ 50, ≥ 50, 41.4 μg/mL, respectively) applied. Similar cases could easily be found elsewhere, such as compounds 6b vs 6d, 6h vs 6i, 6j vs 6k and 6r vs 6s, 6t. Furthermore, the type of substituents on the aniline moiety to some extent affected the potency of the compounds as well. For para substitutions, as a representative, the exchange of Cl (6e) with F (6b) or CF3 (6l) resulted in an about twofold decrease in activities from 14.4 to 27.2 or 31.6 μg/mL (mean of three EC50, Table 2), respectively. In comparison with the precursor structure 6a (R=H), it was found that the introduction of 4-Cl, 4-OMe, 4-I, 4-Br or 4-Me (6e, 6r, 6j, 6h and 6o, respectively) to the aniline moiety yielded an increase in the activities in most cases. Indeed, the presence of 4-F (6b) or 4-CF3 (6l) only resulted in similar or slightly decreased activities. Furthermore, a disubstitution of 3′,5′-diOMe on the aniline moiety (6u) gave nearly equivalent inhibitory activities compared with the 3′-OMe monosubstituted derivative 6s. It was worth noting that the electronic effect (electron donating or electron withdrawing) of the substituents on the aniline moiety played a minor role.
To confirm the importance of the aniline moiety at position 4 of the quinoline scaffold, the aniline residue was substituted with a dimethylamine or a 1,2,4-1H-triazole moiety yielding compound 7 and 8, respectively. This modification dramatically decreased the inhibitory potency, whose EC50 values were more than 100 μg/mL in all cases. It was apparent that the aniline moiety at position 4 played a key role in the inhibitory potency. A graphical summary of the discussed SAR is given in Fig. 2.
Conclusion
In summary, a series of 2-phenyl-4-aminoquinolines were synthesized and evaluated for their antifungal activities in vitro. Some of them were more potent than the positive control azoxystrobin against part or all of the tested fungi. Among them, compound 6e showed remarkable antifungal abilities with EC50 values of 13.3–15.6 μg/mL, which was of great potential to be developed as new antifungal agent. SAR analysis showed that the aniline moiety at position 4 of the quinoline scaffold was crucial for the potency of a compound. The compounds bearing substituents at para position of the aniline ring yielded higher inhibitory activities than that of ortho- or meta-substituted analogs. Moreover, the electronic effect of the substituents on the aniline moiety played a minor role.
Experimental
All starting materials were obtained from commercial sources and used without further purification. Azoxystrobin was purchased from Jiangsu Frey Agrochemical Co. Ltd. (Jiangsu, China). Melting points were measured using X-4 melting point apparatus (Shanghai instrument physical optics instrument Co. Ltd., China) and were uncorrected. 1H and 13C NMR spectra were recorded on a Bruker Advance 400 or 500 instrument using CDCl3 or DMSO-d6 as deuterated solvent. HRMS-ESI spectra were recorded on a SCIEX X500R QTOF mass spectrometer.
The phytopathogenic fungi, Curvularia lunata (C. lunata), Pyricularia grisea (P. grisea) and Alternaria alternate (A. alternate), were provided by the Institute of Pesticides, Northwest A&F University, China. These fungi were cultured on potato dextrose agar (PDA) at 28 °C and maintained at 4 °C with periodic subculturing.
Synthesis of intermediate 3
To a mixture of o-aminoacetophenone 1 (0.1 mol), anhydrous CH2Cl2 (100 mL) and triethylamine (0.1 mol), a solution of benzoyl chloride 2 (0.1 mol) in CH2Cl2 (50 mL) was added in dropwise under ice bath. The reaction mixture was stirred for 3 h at room temperature. Then, the mixture was extracted with CH2Cl2 (3 × 80 mL), and the combined organic layers washed with water (3 × 100 mL) and then dried with Na2SO4. After removal of the solvent, the crude product was recrystallized from petroleum ether/ethyl acetate (10:1, v/v) to afford 3 as a white solid. Yield: 97%; m.p. 97.0–98.4 °C; 1H NMR (CDCl3, 500 MHz) δ: 8.98 (d, J = 6.6 Hz, 1H), 8.07 (d, J = 6.6 Hz, 2H), 7.96–7.93 (m, 1H), 7.63–7.60 (m, 1H), 7.57–7.51 (m, 3H), 7.17–7.13 (m, 1H), 2.71 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ: 203.8, 166.6, 142.0, 136.0, 135.3, 132.6, 132.4, 129.4 (2 × C), 128.0 (2 × C), 123.0, 122.5, 121.3, 29.2.
Synthesis of intermediate 4
NaOH (0.27 mol) was added to a solution of amide 3 (0.09 mol) in 1,4-dioxane (200 mL). The mixture was heated to 110 °C for 2 h under stirring. After the reaction mixture was cooled to room temperature; the solvent was removed under vacuum. The residue was dissolved in water and adjusted to pH = 5–6 by addition of diluted HCl. With acidification of the solution, copious precipitate appeared. The precipitate was collected and washed successively with water and a cold mixture of CH2Cl2 and EtOAc (1:1, v/v) to give the pure product as a brown solid. Yield: 94%; m.p. 247.7–249.5 °C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.75 (s, 1H), 8.10 (d, J = 8.1 Hz, 1H), 7.84 (d-like, J = 3.7 Hz, 2H), 7.78 (d, J = 8.3 Hz, 1H), 7.68 (t, J = 8.1 Hz, 1H), 7.59–7.58 (m, 3H), 7.34 (t, J = 7.5 Hz, 1H), 6.34 (s, 1H); 13C NMR (DMSO-d6, 125 MHz) δ: 177.4, 150.5, 141.0, 134.7, 132.3, 130.9, 129.5 (2 × C), 127.9 (2 × C), 125.3, 125.2, 123.8, 119.2, 107.8.
Synthesis of intermediate 5
To a solution of quinolinone 4 (0.08 mol) in 1,4-dioxane (50 mL), POCl3 (80 mL) was added. The resulting mixture was heated to reflux for 6 h. After cooling, the solvent was removed by evaporation and the residue was poured into cold water. Then, the mixture was neutralized with a cold saturated solution of NaOH and extracted with CH2Cl2 (3 × 80 mL). After extraction and evaporation of the solvent, the product was recrystallized from petroleum ether/ethyl acetate (20:1, v/v) to yield 5 as a white solid. Yield: 64%; m.p. 81.8–83.5 °C; 1H NMR (CDCl3, 400 MHz) δ: 8.13 (t, J = 9.4 Hz, 2H), 8.06 (d-like, J = 8.4 Hz, 2H), 7.89 (s, 1H), 7.69 (td, J = 7.1, 1.2 Hz, 1H), 7.54 (td, J = 7.6, 0.5 Hz, 1H), 7.47–7.38 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 157.3, 149.1, 143.1, 138.6, 130.6, 130.1, 129.8, 128.9 (2 × C), 127.5 (2 × C), 127.2, 125.3, 124.0, 119.1.
General procedure for the synthesis of 6a–6u
The 4-chloroquinoline 5 (3 mmol) and corresponding arylamine (5 mmol) were dissolved in dry 1,4-dioxane (8 mL), and the above mixture was stirred at 110 °C for several hours until the reaction was complete (as indicated by TLC analysis). After the reaction mixture was cooled to room temperature, the resultant precipitate was filtered, washed with petroleum ether/ethyl acetate (20:1, v/v) to yield the target compounds 6a–6u.
N ,2-Diphenylquinolin-4-amine hydrochloride (6a)
Yield: 70%; yellow solid; m.p. 252.4–253.6 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.53 (s, 1H), 11.36 (s, 1H), 9.03 (d, J = 8.3 Hz, 1H), 8.52 (d, J = 8.3 Hz, 1H), 8.04 (t, J = 7.4 Hz, 1H), 7.93 (d, J = 6.7 Hz, 2H), 7.79 (t, J = 7.4 Hz, 1H), 7.67–7.56 (m, 7H), 7.32 (br s, 1H), 6.95 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 155.1, 153.2, 139.7, 137.8, 134.4, 132.6, 132.3, 130.4 (2 × C), 129.7 (2 × C), 129.0 (2 × C), 127.8, 127.3, 125.8 (2 × C), 124.4, 121.2, 117.1, 99.2. HR-MS: 297.1369 ([M-Cl]+, C21H17N2+; calc. 297.1386).
N -(4-Fluorophenyl)-2-phenylquinolin-4-amine hydrochloride (6b)
Yield: 78%; yellow solid; m.p. 277.2–279.0 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.48 (s, 1H), 11.30 (s, 1H), 8.99 (d, J = 8.3 Hz, 1H), 8.48 (d, J = 8.3 Hz, 1H), 8.04 (t, J = 7.4 Hz, 1H), 7.94 (d, J = 6.8 Hz, 2H), 7.78 (t, J = 7.4 Hz, 1H), 7.68–7.59 (m, 5H), 7.41 (t, J = 8.8 Hz, 2H), 6.90 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 161.2 (d, J = 244.4 Hz), 155.3, 153.3, 139.7, 134.4, 134.1 (d, J = 2.8 Hz), 132.6, 132.3, 129.7 (2 × C), 129.1 (2 × C), 128.2 (d, J = 8.6 Hz), 127.3, 124.3, 121.2, 117.2 (d, J = 22.8 Hz), 117.0, 99.1. HR-MS: 315.1275 ([M-Cl]+, C21H16FN2+; calc. 315.1292).
N -(3-Fluorophenyl)-2-phenylquinolin-4-amine hydrochloride (6c)
Yield: 53%; yellow solid; m.p. 119.7–120.3 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.63 (s, 1H), 11.43 (s, 1H), 9.05 (d, J = 8.4 Hz, 1H), 8.53 (d, J = 8.4 Hz, 1H), 8.05 (t, J = 7.7 Hz, 1H), 7.99 (d, J = 6.8 Hz, 2H), 7.80 (t, J = 7.7 Hz, 1H), 7.70 ~ 7.51 (m, 6H), 7.25 (td, J = 12.7, 2.0 Hz, 1H), 7.10 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 163.0 (d, J = 244.8 Hz), 154.8, 153.5, 139.8, 139.7 (d, J = 7.0 Hz), 134.4, 132.5, 132.3, 131.9 (d, J = 9.3 Hz), 129.6 (2 × C), 129.1 (2 × C), 127.4, 124.4, 121.5 (d, J = 2.4 Hz), 121.2, 117.2, 114.3 (d, J = 20.9 Hz), 112.8 (d, J = 23.9 Hz), 99.8. HR-MS: 315.1277 ([M-Cl]+, C21H16FN2+; calc. 315.1292).
N -(2-Fluorophenyl)-2-phenylquinolin-4-amine hydrochloride (6d)
Yield: 87%; light yellow solid; m.p. 141.2–142.2 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.73 (s, 1H), 11.44 (s, 1H), 9.09 (d, J = 8.5 Hz, 1H), 8.58 (d, J = 8.5 Hz, 1H), 8.03 (t, J = 7.8 Hz, 1H), 7.91 (d, J = 7.7 Hz, 2H), 7.78 (t, J = 7.7 Hz, 1H), 7.71 (t, J = 7.8 Hz, 1H), 7.64 ~ 7.48 (m, 5H), 7.42 (t, J = 7.2 Hz, 1H), 6.59 (d, J = 2.1 Hz, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 157.2 (d, J = 249.2 Hz), 155.4, 153.4, 139.5, 134.4, 132.3, 132.2, 130.4 (d, J = 7.8 Hz), 129.6 (2 × C), 129.4, 129.0 (2 × C), 127.5, 126.2 (d, J = 3.1 Hz), 124.9 (d, J = 12.1 Hz), 124.4, 121.1, 117.5 (d, J = 19.4 Hz), 116.8, 99.5. HR-MS: 315.1277 ([M-Cl]+, C21H16FN2+; calc. 315.1292).
N -(4-Chlorophenyl)-2-phenylquinolin-4-amine hydrochloride (6e)
Yield: 64%; yellow solid; m.p. 272.4–274.0 °C; 1H NMR (DMSO-d6, 500 MHz) δ: 14.43 (s, 1H), 11.25 (s, 1H), 8.95 (d, J = 8.4 Hz, 1H), 8.44 (d, J = 8.4 Hz, 1H), 8.08 (t, J = 7.7 Hz, 1H), 7.98 (d, J = 7.3 Hz, 2H), 7.84 (t, J = 7.7 Hz, 1H), 7.72–7.64 (m, 7H), 7.05 (s, 1H). 13C NMR (DMSO-d6, 125 MHz) δ: 154.9, 153.5, 139.7, 136.8, 134.5, 132.6, 132.4, 131.7, 130.3 (2 × C), 129.7 (2 × C), 129.1 (2 × C), 127.5 (2 × C), 124.2, 121.2, 117.2, 99.5. HR-MS: 331.0976 ([M-Cl]+, C21H16ClN2+; calc. 331.0997).
N -(3-Chlorophenyl)-2-phenylquinolin-4-amine hydrochloride (6f)
Yield: 48%; light yellow solid; m.p. 141.2–142.4 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.64 (s, 1H), 11.48 (s, 1H), 9.06 (d, J = 8.4 Hz, 1H), 8.54 (d, J = 8.4 Hz, 1H), 8.04 (t, J = 7.6 Hz, 1H), 7.98 (d, J = 6.8 Hz, 2H), 7.78 (t, J = 7.6 Hz, 1H), 7.73 (t, J = 1.8 Hz, 1H), 7.69 ~ 7.57 (m, 5H), 7.46 (dd, J = 7.9, 0.7 Hz, 1H), 7.06 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 154.8, 153.4, 139.7, 139.5, 134.4, 132.5, 132.3, 131.8, 129.6 (2 × C), 129.1 (2 × C), 127.4, 125.5, 124.5, 124.1, 121.2, 117.2, 99.8. HR-MS: 331.0990 ([M-Cl]+, C21H16ClN2+; calc. 331.0997).
N -(2-Chlorophenyl)-2-phenylquinolin-4-amine hydrochloride (6g)
Yield: 55%; gray solid; m.p. 151.8–153.2 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.66 (s, 1H), 11.43 (s, 1H), 9.01 (d, J = 8.5 Hz, 1H), 8.55 (d, J = 8.5 Hz, 1H), 8.09 (t, J = 7.7 Hz, 1H), 7.89 (d, J = 7.0 Hz, 2H), 7.85 (t, J = 7.8 Hz, 1H), 7.78 (dd, J = 7.7, 1.5 Hz, 1H), 7.72 (dd, J = 7.6, 1.5 Hz, 1H), 7.68 ~ 7.55 (m, 5H), 6.42 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 155.5, 153.4, 139.5, 134.6, 132.4, 132.3, 131.4, 131.3, 130.6, 130.3, 129.7 (2 × C), 129.5, 129.0 (2 × C), 127.7, 124.2, 121.3, 116.6, 99.5. HR-MS: 331.0991 ([M-Cl]+, C21H16ClN2+; calc. 331.0997).
N -(4-Bromophenyl)-2-phenylquinolin-4-amine hydrochloride (6h)
Yield: 75%; light yellow solid; m.p. 187.3–189.2 °C; 1H NMR (DMSO-d6, 500 MHz) δ: 14.45 (s, 1H), 11.24 (s, 1H), 8.96 (d, J = 8.4 Hz, 1H), 8.44 (d, J = 8.4 Hz, 1H), 8.08 (t, J = 7.6 Hz, 1H), 7.98 (d, J = 7.3 Hz, 2H), 7.83 (t, J = 7.6 Hz, 1H), 7.77 (d, J = 8.3 Hz, 2H), 7.71–7.64 (m, 3H), 7.61 (d, J = 8.3 Hz, 2H), 7.07 (s, 1H). 13C NMR (DMSO-d6, 125 MHz) δ: 154.7, 153.5, 139.7, 137.3, 134.4, 133.3 (2 × C), 132.7, 132.3, 129.7 (2 × C), 129.1 (2 × C), 127.7 (2 × C), 127.5, 124.2, 121.3, 120.0, 117.2, 99.5. HR-MS: 375.0473 ([M-Cl]+, C21H16BrN2+; calc. 375.0491).
N -(3-Bromophenyl)-2-phenylquinolin-4-amine hydrochloride (6i)
Yield: 40%; yellow solid; m.p. 222.1–223.3 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.55 (s, 1H), 11.36 (s, 1H), 9.00 (d, J = 8.5 Hz, 1H), 8.48 (d, J = 8.5 Hz, 1H), 8.07 (t, J = 7.8 Hz, 1H), 7.97 (d, J = 7.5 Hz, 2H), 7.85 (s, 1H), 7.82 (t, J = 7.7 Hz, 1H), 7.70 ~ 7.61 (m, 5H), 7.54 (t, J = 8.0 Hz, 1H), 7.08 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 154.8, 153.5, 139.7, 139.6, 134.5, 132.6, 132.4, 132.1, 130.3, 129.7 (2 × C), 129.1 (2 × C), 128.4, 127.5, 124.5, 124.3, 122.7, 121.2, 117.2, 99.8. HR-MS: 375.0487 ([M-Cl]+, C21H16BrN2+; calc. 375.0491).
N -(4-Iodophenyl)-2-phenylquinolin-4-amine hydrochloride (6j)
Yield: 77%; gray solid; m.p. 271.1–273.0 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.31 (s, 1H), 11.10 (s, 1H), 8.87 (d, J = 8.5 Hz, 1H), 8.36 (d, J = 8.5 Hz, 1H), 8.06 (t, J = 7.7 Hz, 1H), 7.94 (d, J = 8.3 Hz, 2H), 7.91 (d, J = 8.5 Hz, 2H), 7.82 (t, J = 7.6 Hz, 1H), 7.70–7.59 (m, 3H), 7.42 (d, J = 8.5 Hz, 2H), 7.06 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 154.1, 152.8, 139.1, 138.5 (2 × C), 137.2, 133.8, 132.0, 131.8, 129.1 (2 × C), 128.6 (2 × C), 127.1 (2 × C), 126.9, 123.7, 120.6, 116.7, 99.0, 92.2. HR-MS: 423.0327 ([M-Cl]+, C21H16IN2+; calc. 423.0353).
N -(3-Iodophenyl)-2-phenylquinolin-4-amine hydrochloride (6k)
Yield: 41%; light yellow solid; m.p. 212.4–214.0 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.51 (s, 1H), 11.29 (s, 1H), 8.97 (d, J = 8.5 Hz, 1H), 8.47 (d, J = 8.5 Hz, 1H), 8.07 (t, J = 7.7 Hz, 1H), 7.98 (d, J = 6.1 Hz, 2H), 7.96 (s, 1H), 7.83 (d, J = 7.6 Hz, 1H), 7.79 (d, J = 8.6 Hz, 1H), 7.71 ~ 7.63 (m, 4H), 7.38 (t, J = 8.0 Hz, 1H), 7.05 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 154.8, 153.4, 139.7, 139.4, 136.2, 134.4, 134.0, 132.6, 132.4, 132.1, 129.7 (2 × C), 129.1 (2 × C), 127.5, 124.9, 124.2, 121.2, 117.2, 99.7, 95.8. HR-MS: 423.0341 ([M-Cl]+, C21H16IN2+; calc. 423.0353).
2-Phenyl- N -(4-(trifluoromethyl)phenyl)quinolin-4-amine hydrochloride (6l)
Yield: 75%; light yellow solid; m.p. 294.7–296.3 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.57 (s, 1H), 11.33 (s, 1H), 8.97 (d, J = 8.5 Hz, 1H), 8.44 (d, J = 8.5 Hz, 1H), 8.07 (t, J = 7.7 Hz, 1H), 8.00 (d, J = 6.8 Hz, 2H), 7.92–7.81 (m, 5H), 7.70–7.62 (m, 3H), 7.27 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 154.3, 153.8, 142.1, 140.0, 134.5, 132.7, 132.4, 129.7 (2 × C), 129.2 (2 × C), 127.6, 127.4 (d, J = 4.2 Hz), 125.3, 124.6 (d, J = 271.8 Hz, -CF3), 124.3, 121.5, 117.6, 100.4. HR-MS: 365.1240 ([M-Cl]+, C22H16F3N2+; calc. 365.1260).
2-Phenyl- N -(3-(trifluoromethyl)phenyl)quinolin-4-amine hydrochloride (6m)
Yield: 43%; light yellow solid; m.p. 177.3–178.1 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.70 (s, 1H), 11.60 (s, 1H), 9.09 (d, J = 8.5 Hz, 1H), 8.56 (d, J = 8.5 Hz, 1H), 8.08 ~ 7.98 (m, 5H), 7.82 ~ 7.74 (m, 3H), 7.69 ~ 7.60 (m, 3H), 7.12 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 154.8, 153.5, 139.8, 139.0, 134.4, 132.5, 132.4, 131.5, 130.9 (q, J = 32.2 Hz), 129.6 (2 × C), 129.2, 129.1 (2 × C), 127.5, 124.4, 124.3 (q, J = 272.5 Hz, –CF3), 123.8 (d, J = 3.2 Hz), 122.3 (d, J = 3.5 Hz), 121.2, 117.4, 99.8. HR-MS: 365.1248 ([M-Cl]+, C22H16F3N2+; calc. 365.1260).
2-Phenyl- N -(2-(trifluoromethyl)phenyl)quinolin-4-amine hydrochloride (6n)
Yield: 28%; white solid; m.p. 204.5–206.3 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.79 (s, 1H), 11.41 (s, 1H), 9.02 (d, J = 8.5 Hz, 1H), 8.63 (d, J = 8.5 Hz, 1H), 8.09 (t, J = 7.8 Hz, 1H), 8.03 (d, J = 7.8 Hz, 1H), 7.97 (t, J = 7.5 Hz, 1H), 7.87 ~ 7.78 (m, 5H), 7.67 ~ 7.56 (m, 3H), 6.33 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ: 156.9, 153.3, 139.5, 135.4, 134.5, 132.4, 132.2, 131.6, 130.2, 129.6 (2 × C), 129.1 (2 × C), 128.9, 128.3 (d, J = 4.8 Hz), 127.8 (q, J = 29.9 Hz), 127.7, 124.1, 123.7 (q, J = 274.0 Hz, -CF3), 121.3, 116.6, 99.5. HR-MS: 365.1248 ([M-Cl]+, C22H16F3N2+; calc. 365.1260).
2-Phenyl- N -( p -tolyl)quinolin-4-amine hydrochloride (6o)
Yield: 62%; yellow solid; m.p. 201.5–203.4 °C; 1H NMR (DMSO-d6, 500 MHz) δ: 14.35 (s, 1H), 11.18 (s, 1H), 8.94 (d, J = 8.4 Hz, 1H), 8.44 (d, J = 8.4 Hz, 1H), 8.06 (t, J = 7.7 Hz, 1H), 7.92 (d, J = 7.1 Hz, 2H), 7.81 (t, J = 7.7 Hz, 1H), 7.70–7.62 (m, 3H), 7.49 (d, J = 8.1 Hz, 2H), 7.40 (d, J = 8.1 Hz, 2H), 6.91 (s, 1H), 2.41 (s, 3H). 13C NMR (DMSO-d6, 125 MHz) δ: 155.2, 153.2, 139.7, 137.3, 135.0, 134.3, 132.8, 132.3, 130.9 (2 × C), 129.7 (2 × C), 128.9 (2 × C), 127.3, 125.7 (2 × C), 124.1, 121.2, 116.9, 99.0, 21.2. HR-MS: 311.1526 ([M-Cl]+, C22H19N2+; calc. 311.1543).
2-Phenyl- N -( m -tolyl)quinolin-4-amine hydrochloride (6p)
Yield: 93%; yellow solid; m.p. 146.0–147.2 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.53 (s, 1H), 11.35 (s, 1H), 9.05 (d, J = 8.5 Hz, 1H), 8.55 (d, J = 8.5 Hz, 1H), 8.01 (t, J = 7.7 Hz, 1H), 7.93 (d, J = 7.2 Hz, 2H), 7.75 (t, J = 7.7 Hz, 1H), 7.66 ~ 7.58 (m, 3H), 7.47 ~ 7.40 (m, 3H), 7.23 (d, J = 7.2 Hz, 1H), 6.91 (s, 1H), 2.39 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ: 155.1, 153.1, 140.0, 139.6, 137.6, 134.2, 132.5, 132.2, 130.1, 129.6 (2 × C), 129.0 (2 × C), 128.4, 127.2, 126.3, 124.4, 122.8, 121.1, 117.0, 99.1, 21.4. HR-MS: 311.1530 ([M-Cl]+, C22H19N2+; calc. 311.1543).
2-Phenyl- N -( o -tolyl)quinolin-4-amine hydrochloride (6q)
Yield: 56%; light yellow solid; m.p. 182.0–182.9 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.56 (s, 1H), 11.39 (s, 1H), 9.15 (d, J = 8.5 Hz, 1H), 8.59 (d, J = 8.5 Hz, 1H), 8.03 (t, J = 7.7 Hz, 1H), 7.85 (d, J = 7.0 Hz, 2H), 7.78 (t, J = 7.7 Hz, 1H), 7.63 ~ 7.54 (m, 3H), 7.50 ~ 7.40 (m, 4H), 6.33 (s, 1H), 2.30 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ: 156.0, 153.2, 139.6, 136.1, 135.8, 134.3, 132.5, 132.3, 132.1, 129.7 (2 × C), 129.0, 128.9 (2 × C), 128.1, 128.1, 127.3, 124.5, 121.2, 116.7, 98.6, 18.0. HR-MS: 311.1535 ([M-Cl]+, C22H19N2+; calc. 311.1543).
N -(4-Methoxyphenyl)-2-phenylquinolin-4-amine hydrochloride (6r)
Yield: 82%; yellow solid; m.p. 174.9–176.1 °C; 1H NMR (DMSO-d6, 500 MHz) δ: 14.39 (s, 1H), 11.22 (s, 1H), 8.98 (d, J = 8.5 Hz, 1H), 8.48 (d, J = 8.4 Hz, 1H), 8.04 (t, J = 7.7 Hz, 1H), 7.92 (d, J = 7.2 Hz, 2H), 7.78 (t, J = 7.7 Hz, 1H), 7.68–7.61 (m, 3H), 7.52 (d, J = 8.8 Hz, 2H), 7.14 (d, J = 8.8 Hz, 2H), 6.83 (s, 1H), 3.85 (s, 3H). 13C NMR (DMSO-d6, 125 MHz) δ: 158.7, 155.5, 153.1, 139.6, 134.3, 132.7, 132.2, 130.1, 129.7 (2 × C), 128.9 (2 × C), 127.5 (2 × C), 127.2, 124.1, 121.1, 116.8, 115.5 (2 × C), 98.8, 55.9. HR-MS: 327.1471 ([M-Cl]+, C22H19N2O+; calc. 327.1492).
N -(3-Methoxyphenyl)-2-phenylquinolin-4-amine hydrochloride (6s)
Yield: 51%; yellow solid; m.p. 200.9–202.1 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.63 (s, 1H), 11.40 (s, 1H), 9.07 (d, J = 8.5 Hz, 1H), 8.58 (d, J = 8.5 Hz, 1H), 8.00 (t, J = 7.8 Hz, 1H), 7.94 (d, J = 6.9 Hz, 2H), 7.73 (t, J = 7.7 Hz, 1H), 7.65 ~ 7.57 (m, 3H), 7.47 (t, J = 8.4 Hz, 1H), 7.21 ~ 7.20 (m, 2H), 7.00 ~ 6.97 (m, 2H), 3.82 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ: 160.8, 155.0, 153.1, 139.7, 139.0, 134.2, 132.5, 132.2, 131.1, 129.6 (2 × C), 129.0 (2 × C), 127.2, 124.4, 121.1, 117.6, 117.1, 113.3, 111.5, 99.5, 55.9. HR-MS: 327.1480 ([M-Cl]+, C22H19N2O+; calc. 327.1492).
N -(2-Methoxyphenyl)-2-phenylquinolin-4-amine hydrochloride (6t)
Yield: 54%; yellow solid; m.p. 116.8–117.5 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.56 (s, 1H), 11.14 (s, 1H), 9.01 (d, J = 8.4 Hz, 1H), 8.58 (d, J = 8.5 Hz, 1H), 8.03 (t, J = 7.7 Hz, 1H), 7.88 (d, J = 6.8 Hz, 2H), 7.78 (t, J = 7.7 Hz, 1H), 7.64 ~ 7.57 (m, 3H), 7.52 ~ 7.48 (m, 2H), 7.32 (d, J = 8.1 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 6.41 (s, 1H), 3.85 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ: 155.5, 154.7, 152.8, 139.5, 134.2, 132.5, 132.2, 130.0, 129.7 (2 × C), 128.9 (2 × C), 128.6, 127.2, 125.3, 124.2, 121.7, 121.1, 116.6, 113.4, 99.5, 56.3. HR-MS: 327.1482 ([M-Cl]+, C22H19N2O+; calc. 327.1492).
N -(3,5-Dimethoxyphenyl)-2-phenylquinolin-4-amine hydrochloride (6u)
Yield: 68%; yellow solid; m.p. 151.4–153.0 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.36 (s, 1H), 11.08 (s, 1H), 8.88 (d, J = 8.5 Hz, 1H), 8.41 (d, J = 8.5 Hz, 1H), 8.05 (t, J = 7.7 Hz, 1H), 7.91 (d, J = 8.5 Hz, 2H), 7.80 (t, J = 7.7 Hz, 1H), 7.68–7.63 (m, 3H), 7.05 (s, 1H), 6.77 (d, J = 2.1 Hz, 2H), 6.57 (t, J = 2.1 Hz, 1H), 3.80 (s, 6H). 13C NMR (DMSO-d6, 100 MHz) δ: 161.2 (2 × C), 154.5, 152.6, 139.1, 139.0, 133.8, 132.0, 131.7, 129.1 (2 × C), 128.5 (2 × C), 126.7, 123.8, 120.6, 116.5, 103.3 (2 × C), 99.3, 99.1, 55.5 (2 × C). HR-MS: 357.1575 ([M-Cl]+, C23H21N2O2+; calc. 357.1598).
General procedure for the synthesis of 7 and 8
The 4-chloroquinoline 5 (3 mmol) was dissolved in dry DMF (10 mL), and the mixture was maintained at 150 °C for several hours until the reaction was complete. For the synthesis of compound 8, additional 1,2,4-triazole sodium (3.6 mmol) was added to the above mixture. After the reaction was finished, distilled water was added until copious solid appeared. The solid was collected and washed with petroleum ether/ethyl acetate (20:1, v/v) to yield the target compounds.
N,N -Dimethyl-2-phenylquinolin-4-amine hydrochloride (7)
Yield: 64%; yellow solid; m.p. 243.7–245.5 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 14.10 (s, 1H), 8.42 (d, J = 8.6 Hz, 1H), 8.39 (d, J = 8.5 Hz, 1H), 8.13 (d, J = 7.7 Hz, 2H), 7.97 (t, J = 7.7 Hz, 1H), 7.73 ~ 7.65 (m, 4H), 7.15 (s, 1H), 3.56 (s, 6H). 13C NMR (DMSO-d6, 100 MHz) δ: 159.8, 151.2, 140.8, 133.5, 132.8, 132.2, 129.6 (2 × C), 129.2 (2 × C), 127.5, 125.7, 121.0, 117.5, 102.5, 44.9 (2 × C). HR-MS: 249.1377 ([M-Cl]+, C17H17N2+; calc. 249.1386).
2-Phenyl-4-(1 H -1,2,4-triazol-1-yl)quinoline (8)
Yield: 44%; white solid; m.p. 172.8–173.4 °C; 1H NMR (DMSO-d6, 400 MHz) δ: 9.39 (s, 1H), 8.53 (s, 1H), 8.42 (s, 1H), 8.39 (d, J = 6.9 Hz, 2H), 8.23 (d, J = 8.4 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.89 (td, J = 7.6, 0.9 Hz, 1H), 7.69 (t, J = 7.7 Hz, 1H), 7.61 ~ 7.53 (m, 3H). 13C NMR (DMSO-d6, 100 MHz) δ: 157.0, 153.6, 149.5, 146.7, 141.8, 138.2, 131.2, 130.6, 130.0, 129.3 (2 × C), 128.1, 127.9 (2 × C), 123.9, 121.2, 114.2. HR-MS: 273.1134 ([M + H]+, C17H13N4+; calc. 273.1140).
Antifungal assay
Antifungal activities of all the target compounds were evaluated using mycelium growth rate method against three phytopathogenic fungi (C. lunata, P. grisea and A. alternate) as previously reported.[30, 33] The concentration of each tested compound was 100 or 50 µg/mL in PDA medium. A 5-mm-diameter mycelium disk was inoculated to the center of the medium to incubate at 28 °C for 72 h. Each experiment was performed in triplicate. Meanwhile, 0.5% DMSO (v/v) in PDA medium and azoxystrobin (100 or 50 µg/mL) were utilized as negative control and positive control, respectively. After 72 h of treatment, the mycelium diameter (in mm) of each fungus on the medium was measured and the inhibition rate of the tested compounds was calculated based on the following formula and expressed as mean ± standard deviation.
In the formula, C represents the average diameter of mycelia in the negative control test, and T represents the average diameter of mycelia in the compound-treated test.
Serial dilution method was used for determination of EC50 values according to the same method described above, ranging from 100 to 6.25 μg/mL. And EC50 values with their confidence intervals at 95% probability (95% CI) were calculated by Graphpad Prism 7.0.4 (GraphPad Software Inc., San Diego, CA, USA).
References
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484(7393):186–194. https://doi.org/10.1038/nature10947
Bräse S, Encinas A, Keck J, Nising CF (2009) Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 109(9):3903–3990. https://doi.org/10.1021/cr050001f
Zhang J, Peng JF, Wang T, Kang Y, Jing S, Zhang ZT (2017) Synthesis and biological evaluation of arylpyrazoles as fungicides against phytopathogenic fungi. Mol Divers 21(2):317–323. https://doi.org/10.1007/s11030-017-9727-x
Wang X, Fu X, Yan J, Wang A, Wang M, Chen M, Yang C, Song Y (2019) Design and synthesis of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N’-phenylacethydrazide derivatives as potential fungicides. Mol Divers 23(3):573–583. https://doi.org/10.1007/s11030-018-9891-7
Karekal MR, Biradar V, Bennikallu HMM (2013) Synthesis, characterization, antimicrobial, DNA cleavage, and antioxidant studies of some metal complexes derived from schiff base containing indole and quinoline moieties. Bioinorg Chem Appl 2013:315972. https://doi.org/10.1155/2013/315972
Minocheherhomji FP, Vaidya KK (2016) Potential therapeutic values of quinoline derivatives based on their antibacterial activity. Int J Pharma Bio Sci 7(4):412–415. https://doi.org/10.22376/ijpbs.2016.7.4.b412-415
Emami S, Ghafouri E, Faramarzi MA, Samadi N, Irannejad H, Foroumadi A (2013) Mannich bases of 7-piperazinylquinolones and kojic acid derivatives: synthesis, in vitro antibacterial activity and in silico study. Eur J Med Chem 68:185–191. https://doi.org/10.1016/j.ejmech.2013.07.032
Arafa RK, Hegazy GH, Piazza GA, Abadi AH (2013) Synthesis and in vitro antiproliferative effect of novel quinoline-based potential anticancer agents. Eur J Med Chem 63:826–832. https://doi.org/10.1016/j.ejmech.2013.03.008
Köprülü TK, Ökten S, Tekin Ş, Çakmak O (2019) Biological evaluation of some quinoline derivatives with different functional groups as anticancer agents. J Biochem Mol Toxicol 33(3):e22260. https://doi.org/10.1002/jbt.22260
Vandekerckhove S, Desmet T, Tran HG, de Kock C, Smith PJ, Chibale K, Dhooghe M (2014) Synthesis of halogenated 4-quinolones and evaluation of their antiplasmodial activity. Bioorg Med Chem Lett 24(4):1214–1217. https://doi.org/10.1016/j.bmcl.2013.12.067
Plevová K, Briestenská K, Colobert F, Mistríková J, Milata V, Leroux FR (2015) Synthesis and biological evaluation of new nucleosides derived from trifluoromethoxy-4-quinolones. Tetrahedron Lett 56(36):5112–5115. https://doi.org/10.1016/j.tetlet.2015.07.031
Qu TF, Qu LL, Wang XG, Xu T, Xiao X, Ding M, Deng L, Guo Y (2017) Design, synthesis, and antibacterial activity of novel 8-methoxyquinoline-2-carboxamide compounds containing 1,3,4-thiadiazole moiety. Zeitschrift für Naturforschung C 73(3–4):117–122. https://doi.org/10.1515/znc-2017-0063
Musiol R, Serda M, Hensel-Bielowka S, Polanski J (2010) Quinoline-based antifungals. Curr Med Chem 17(18):1960–1973. https://doi.org/10.2174/092986710791163966
Kouznetsov VV, Meléndez Gómez CM, Derita MG, Svetaz L, del Olmo E, Zacchino SA (2012) Synthesis and antifungal activity of diverse C-2 pyridinyl and pyridinylvinyl substituted quinolines. Bioorg Med Chem 20(21):6506–6512. https://doi.org/10.1016/j.bmc.2012.08.036
Yaakov DB, Shadkchan Y, Albert N, Kontoyiannis DP, Osherov N (2017) The quinoline bromoquinol exhibits broad-spectrum antifungal activity and induces oxidative stress and apoptosis in Aspergillus fumigatus. J Antimicrob Chemother 72(8):2263–2272. https://doi.org/10.1093/jac/dkx117
Fang YM, Zhang RR, Shen ZH, Wu HK, Tan CX, Weng JQ, Xu TM, Liu XH (2018) Synthesis, antifungal activity, and SAR study of some new 6-perfluoropropanyl quinoline derivatives. J Heterocycl Chem 55(1):240–245. https://doi.org/10.1002/jhet.3031
Rahangdale PK, Inam F, Chourasia SS (2018) Quantitative structure activity relationship and biological activity studies of 4-methyl-2-(4-substituted phenyl)quinoline derivatives. Asian J Chem 30(3):479–482. https://doi.org/10.14233/ajchem.2018.20811
Banu S, Bollu R, Naseema M, Gomedhika PM, Nagarapu L, Sirisha K, Kumar CG, Gundasw SK (2018) A novel templates of piperazinyl-1,2-dihydroquinoline-3-carboxylates: synthesis, anti-microbial evaluation and molecular docking studies. Bioorg Med Chem Lett 28(7):1166–1170. https://doi.org/10.1016/j.bmcl.2018.03.007
Salve PS, Alegaon SG, Sriram D (2017) Three-component, one-pot synthesis of anthranilamide Schiff bases bearing 4-aminoquinoline moiety as Mycobacterium tuberculosis gyrase inhibitors. Bioorg Med Chem Lett 27(8):1859–1866. https://doi.org/10.1016/j.bmcl.2017.02.031
Vausselin T, Seron K, Lavie M, Mesalam AA, Lemasson M, Belouzard S, Feneant L, Danneels A, Rouille Y, Cocquerel L, Foquet L, Rosenberg AR, Wychowski C, Meuleman P, Melnyk P, Dubuisson J (2016) Identification of a new benzimidazole derivative as an antiviral against hepatitis C virus. J Virol 90(19):8422–8434. https://doi.org/10.1128/JVI.00404-16
Das P, Deng X, Zhang L, Roth MG, Fontoura BMA, Phillips MA, De Brabander JK (2013) SAR-based optimization of a 4-quinoline carboxylic acid analogue with potent antiviral activity. ACS Med Chem Lett 4(6):517–521. https://doi.org/10.1021/ml300464h
Singh K, Kaur H, Chibale K, Balzarini J (2013) Synthesis of 4-aminoquinoline–pyrimidine hybrids as potent antimalarials and their mode of action studies. Eur J Med Chem 66:314–323. https://doi.org/10.1016/j.ejmech.2013.05.046
Bhat HR, Singh UP, Gahtori P, Ghosh SK, Gogoi K, Prakash A, Singh RK (2013) 4-Aminoquinoline-1,3,5-triazine: design, synthesis, in vitro antimalarial activity and docking studies. New J Chem 37(9):2654–2662. https://doi.org/10.1039/c3nj00317e
Marvania B, Kakadiya R, Christian W, Chen TL, Wu MH, Suman S, Tala K, Lee TC, Shah A, Su TL (2014) The synthesis and biological evaluation of new DNA-directed alkylating agents, phenyl N-mustard-4-anilinoquinoline conjugates containing a urea linker. Eur J Med Chem 83:695–708. https://doi.org/10.1016/j.ejmech.2014.06.066
Abbas SH, Abd El-Hafeez AA, Shoman ME, Montano MM, Hassan HA (2019) New quinoline/chalcone hybrids as anti-cancer agents: design, synthesis, and evaluations of cytotoxicity and PI3 K inhibitory activity. Bioorg Chem 82:360–377. https://doi.org/10.1016/j.bioorg.2018.10.064
Meléndez Gómez CM, Kouznetsov VV, Sortino MA, Álvarez SL, Zacchino SA (2008) In vitro antifungal activity of polyfunctionalized 2-(hetero)arylquinolines prepared through imino Diels-Alder reactions. Bioorg Med Chem 16(17):7908–7920. https://doi.org/10.1016/j.bmc.2008.07.079
Liberto NA, Simoes JB, Silva SdP, da Silva CJ, Modolo LV, de Fatima A, Silva LM, Derita M, Zacchino S, Zuniga OMP, Romanelli GP, Fernandes SA (2017) Quinolines: microwave-assisted synthesis and their antifungal, anticancer and radical scavenger properties. Bioorg Med Chem 25(3):1153–1162. https://doi.org/10.1016/j.bmc.2016.12.023
Mudaliar S, Chikhalia KH, Shah NK (2016) Synthesis of 2-, 3- or 4-phenylsubtituted chalcones based on 4-phenylamino-6-nitro-2-[(E)-2-phenylvinyl]quinoline, evaluation of their antimicrobial and antifungal activity. Lett Drug Des Discovery 13(8):818–823. https://doi.org/10.2174/1570180812666151016205033
Montoya A, Quiroga J, Abonia R, Derita M, Sortino M, Ornelas A, Zacchino S, Insuasty B (2016) Hybrid molecules containing a 7-chloro-4-aminoquinoline nucleus and a substituted 2-pyrazoline with antiproliferative and antifungal activity. Molecules 21(8):969/1–969/19. https://doi.org/10.3390/molecules21080969
Yang R, Ma YN, Huang T, Xie W, Zhang X, Huang GS, Liu XD (2018) Synthesis and antifungal activities of 4-thioquinoline compounds. Chin J Org Chem 38(8):2143–2150. https://doi.org/10.6023/cjoc201801024
Tsai JY, Chang CS, Huang YF, Chen HS, Lin SK, Wong FF, Huang LJ, Kuo SC (2008) Investigation of amination in 4-chloro-2-phenylquinoline derivatives with amide solvents. Tetrahedron 64(51):11751–11755. https://doi.org/10.1016/j.tet.2008.09.100
Pickard AJ, Liu F, Bartenstein TF, Haines LG, Levine KE, Kucera GL, Bierbach U (2014) Redesigning the DNA-targeted chromophore in platinum-acridine anticancer agents: a structure-activity relationship study. Chemistry 20(49):16174–16187. https://doi.org/10.1002/chem.201404845
Yang R, Gao ZF, Zhao JY, Li WB, Zhou L, Miao F (2015) New class of 2-aryl-6-chloro-3,4-dihydroisoquinolinium salts as potential antifungal agents for plant protection: synthesis, bioactivity and structure-activity relationships. J Agric Food Chem 63(7):1906–1914. https://doi.org/10.1021/jf505609z
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 31601670) and the Foundation of Education Department of Sichuan Province (No. 18ZB0079).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, R., Du, W., Yuan, H. et al. Synthesis and biological evaluation of 2-phenyl-4-aminoquinolines as potential antifungal agents. Mol Divers 24, 1065–1075 (2020). https://doi.org/10.1007/s11030-019-10012-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-10012-1