Abstract
A series of new chiral 1,3,4-thiadiazole-based bis-sulfonamides 4a–4w and tri-sulfonamide analogue 5 was synthesized and evaluated as anti-HIV agents. The reaction of chiral amino acids 1 with sulfonyl chlorides 2, followed by subsequent reaction of resultant N-protected amino acids 2a–2f with thiosemicarbazide in the presence of excess phosphorous oxychloride afforded N-(1-(5-amino-1,3,4-thiadiazol-2-yl)alkyl)-4-arylsulfonamides 3a–3f. Treatment of 2a–2f with substituted sulfonyl chlorides in portions furnished the target bis-sulfonamide analogues 4a–4w in good yields, together with the unexpected 5. The new compounds were assayed against HIV-1 and HIV-2 in MT-4 cells. Compounds 4s were the most active in inhibiting HIV-1 with IC50 = 9.5 μM (SI = 6.6), suggesting to be a new lead in the development of an antiviral agent. Interestingly, compound 5 exhibited significant cytotoxicity of > 4.09 μM and could be a promising antiproliferative agent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiadiazoles represent a class of compounds having immense importance in medicinal chemistry due to their mesoionic nature and good lipophilicity [1,2,3]. They are very useful intermediates/subunits for the development of molecules of pharmaceutical or biological interest including antibacterial [4, 5], antifungal [6,7,8], anti-inflammatory [9, 10], antimicrobial [10,11,12,13], antitubercular [14,15,16], anticancer [17,18,19,20,21,22], anti-helicobacter pylori [23, 24] and anticonvulsant [25] properties. In recent years, we have synthesized a series of new naphthalene derivatives bearing 1,3,4-thiadiaziole backbone as potetial anti-HIV agents [26], meanwhile Ijichi et al. [27] reported that 4-(2,6-dichlorophenyl)-1,2,5-thiadiazol-3-yl N-methyl-N-alkylcarbamates proved inhibitory to HIV-1 replication in the nanomolar concentration range. Many drugs containing 1,3,4-thiadiazole nucleus such as acetazolamide [28], methazolamide [29], megazol [30], and xanomeline [31] (Fig. 1) are available in the market.
Sulfonamides (sulfa drugs) were the first drugs largely employed and systematically used as preventive and chemotherapeutic agents against various diseases [32]. With the rapid progress in this field, more active and selective sulfonamide derivatives have been prepared by linking various heterocyclic moieties with sulfonamide core [33].
Based on these observations and as continuation of our research interests in sulfonamides and 1,3,4-thiadiazole derivatives [34,35,36], herein we report the synthesis of a new series of chiral 1,3,4-thiadiazole-based bis-sulfonamides (4a–4w and 5), their structure characterization and evaluation of their anti-HIV activities.
Results and discussion
Chemistry
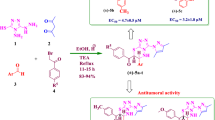
The synthesis of chiral 1,3,4-thiadiazole-based bis-sulfonamides was initiated by the reaction of chiral amino acids 1 with arylsulfonyl chlorides 2 to furnish the corresponding N-arylsulfonylated amino acids 2a–2f (79-9% yield), which were further reacted with thiosemicarbazide in the presence of excess phosphorous oxychloride to afford 2,5-disubstituted 1,3,4-thiadiazole-based arylsulfonamides 3a–3f. Initially, when the reaction was carried out at high temperature, it resulted in the formation of side products providing low yields of the desired target molecules. However, when the reaction was performed at 75–80 °C (optimizing reaction temperature starting from 0 °C), it provided good yields of the desired products. After successful synthesis of 2,5-disubstituted 1,3,4-thiadiazole-based arylsulfonamides 3a–3f in 65–76% yields, the synthesis of bis-arylsulfonamides 4a–4w was attempted. The reaction of 3a–3f with the respective arylsulfonyl chloride using pyridine as a base and solvent provided bis-arylsulfonamides 4a–4w in 67–82% yield (Scheme 1). It is pertinent to mention here that a reasonable amount of unreacted 3a–3f was recovered when the reaction was performed using equimolar quantities of reactants diminishing the yields of the desired products. Nevertheless, the gradual increase in arylsulfonyl chlorides (1:1.4) under inert argon atmosphere lead to the formation of desired products 4a–4w in good yields. Furthermore, addition of arylsulfonyl chloride to the reaction mixture in this ratio all at once resulted in the formation of N,N-disubstituted product 5 in 50% yield (Scheme 1). However, the addition of arylsulfonyl chloride in small portions led to the desired monosubstituted product.
The N-arylsulfonylation of amino acids to compounds 2a–2f was verified by the IR, 1H and 13C NMR spectra. The IR spectra were characterized by the appearance of bands for symmetric and asymmetric stretchings of the arylsulfonyl group in the regions 1162–1156 and 1328–1311 cm−1, respectively, meanwhile carbonyl groups appeared in the region of 1729–1717 cm−1. In the 1H NMR spectra of 2a–2f, NH proton appeared as broad singlets or doublets at the regions δ 5.13–6.77 ppm, meanwhile the carboxylic acid protons resonated at the regions δ 8.0–10.33 ppm, exchangeable with D2O. The structures of 3a–3f were confirmed by their IR, 1H, 13C NMR and mass spectra. The IR spectra showed peaks in the regions 3263–3156 cm−1 attributed to the NH stretchings of NH2 group, while the peaks at 1729–1717 cm−1 were attributed to the carbonyl group stretching. In the 1H NMR spectra of 3a–3f, the doublets or broad singlets at the regions δ 8.56–6.43 ppm were assigned to NH2 or secondary NH protons, exchangeable with D2O. In 13C-NMR spectra of 3a–3f, C-2 of the thiadiazole moiety appeared at the regions δ 161.3–162.3 ppm, while C-5 of the same ring resonated at the regions δ 168.9–169.8 ppm. Furthermore, the structures of 4a–4w and 5 were assigned on the basis of their IR, 1H, 13C NMR and mass spectra. The characteristic signals for secondary NH absorption at 3289–3255 cm−1 in the IR spectra indicated the formation of the desired products. In 1H-NMR spectra, the aromatic protons appeared as multiplets or doublets in regions δ 7.86–7.35 ppm, integrating to eight protons, while the aliphatic and substituents protons were fully analyzed (c.f. “Experimental section”). In the 13C-NMR spectra of 4a–4w, C-2 and C-5 of the thiazdiazole backbone resonated at the regions δ 159.3–161.7 and 162.8–169.2 ppm, respectively. The eight signals at the regions δ 114.0–144.0 ppm were assigned to the aromatic carbon atoms, while CHNH carbon atom resonated at the regions δ 49.0–59.8 ppm. The aromatic carbon atom C-OMe (of compounds 4d, 4h, 4l, 4p, 4s and 4w) appeared at the regions δ 158.8–162.8 ppm. The other aliphatic and substituent carbon atoms were fully assigned (c.f. “Experimental section”) Compound 4h was selected for further NMR experiments. The gradient heteronuclear multiple-bond correlation [37] NMR spectrum of 4h showed two 3JH,C couplings: C-2 carbon atom of the thiadiazole backbone at δC 161.1 ppm coupled with CHMe proton at δH 4.46 ppm as well as methyl protons of the same group at δH 1.47 ppm. Further, a 2JH,C coupling between methyl protons of methoxy substituent at δH 3.83 ppm and aromatic carbon atom C-OMe at δC 162.7 ppm was observed. Additionally, a 2JH,C coupling between CHMe protons at δH 4.46 ppm with CHMe carbon atom at δC 49.6 ppm was witnessed (Fig. 2).
In vitro anti-HIV activity
Compounds 3a–3f, 4a–4w and 5 were evaluated for their inhibitory activity against HIV-1 (strain IIIB) and HIV-2 (strain ROD) and monitored by the inhibition of the virus-induced cytopathic effect in the human T-lymphocyte (MT-4) cells, using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) method [38]. The results are summarized in Table 1, in which the data for nevirapin [39], azidothymidine (AZT) [40], and lamivudine (3TC) [41] are included for comparison. The cytotoxicity of the compounds was determined in parallel. None of the tested compounds were active against inhibition of HIV-1 and HIV-2, although they showed cytotoxicity against MT-4 cells at micromolar concentrations. However, 4s exhibited an IC50 value of 9.25 μM, with a selectivity index (SI) value of 6.6; however, compound 5 turned out cytotoxic for exponentially growing MT-4 cells (human CD4+ lymphocytes) in the low micromolar range (CC50 4.09 μM). This study revealed that compounds containing a branched-chain aliphatic group (leucine residue) together with a methoxy substituent at the arylsulfonamide moiety showed significant anti-HIV-1 activity, while the presence of three arylsulfonamido groups bearing a 1,3,4-thiadiazole ring would enhance the cytotoxicity of such molecules (e.g., compound 5).
Conclusion
We have synthesized a series of new chiral 1,3,4-thiadiazole-based bis-arylsulfonamides 4a–4w and 5 in a multistep sequence starting from chiral amino acids. The new synthesized compounds were screened for their inhibitory activity against HIV-1 and HIV-2, where 4s showed significant inhibition of HIV-1 with IC50 value of 9.25 μM (SI = 6.6). The anti-HIV activity results suggested that 4s might act as a new candidate for reverse transcriptase inhibition. Furthermore, compound 5 exhibited significant cytotoxicity of > 4.09 μM against human T-lymphocyte (MT-4) cells and could be a promising antiproliferative agent.
Experimental section
Chemistry
Melting points were measured on a Gallenkamp melting point apparatus (MP-D) and are uncorrected. IR spectra were recorded on a Thermo Scientific Nicolet 6700 FTIR spectrophotometer using ATR (attenuated total reflectance) facility. NMR spectra were acquired on 300 MHz (1H) and on 100 MHz (13C) spectrometers (Bruker Avance, Germany) with TMS as internal standard and on the δ scale in ppm. The mass spectra were recorded on Agilent Technologies mass spectrometer (model: 5973) using ESI method. All the reactions were monitored using pre-coated silica gel-60 F254 TLC plates purchased from Merck (Germany), using CHCl3-MeOH 9:1 as eluent. Thiosemicarbazide was purchased from Sigma-Aldrich.
General procedure for the synthesis of N-(4-chloro/methylbenzenesulfonyl)amino acids 2a–2f
The respective amino acid (1.00 mmol) was dissolved in an aqueous solution of sodium carbonate (2.00 mmol, 212 mg in water (5 mL)) and a solution of arylsulfonyl chloride (1.20 mmol) in toluene/diethyl ether (7 mL) was added. The reaction mixture was stirred vigorously and monitored by TLC. After the completion of reaction (20–24 h), the organic layer was separated and the aqueous layer was acidified with dilute hydrochloric acid. The precipitated solid was filtered and recrystallized from aqueous EtOH.
N-(4-Methylbenzenesulfonyl)alanine (2a)
From l-alanine (74 mg). Yield: 223 mg (92%) as colorless; m.p.: 137–138 °C; Rf: 0.45; IR (υmax, neat, cm−1): 3400–2450 (OH), 3276 (NH), 1729 (C=O), 1572 (C=C), 1311, 1157 (2 × O = S = O), 1088 (C–O). 1H NMR (acetone-d6): δ 1.33 (d, 3H, J = 7.2 Hz, CH3CH), 2.42 (s, 3H, Ar-CH3), 3.97 (m, 1H, CHCH3), 6.77 (d, 1H, J = 8.4 Hz, NH), 7.37 (d, 2H, J = 8.1 Hz, Ar–H), 7.77 (d, 2H, J = 8.1 Hz, Ar–H), 10.33 (s, 1H, CO2H).
N-(4-Chlorobenzenesulfonyl)alanine (2b)
From l-alanine (74 mg). Yield: 235 mg (89%) as colorless; m.p.: 131–132 °C; Rf: 0.44; IR (υmax, neat, cm−1): 3450–2450 (OH), 3270 (NH), 1720 (C=O), 1574 (C=C), 1319, 1162 (2 × O = S = O), 1082 (C–O). 1H NMR (CDCl3): δ 1.46 (d, 3H, J = 7.2 Hz, CH3CH), 4.01 (m, 1H, CHCH3), 5.13 (bs, 1H, NH), 7.49 (d, 2H, J = 8.7 Hz, Ar–H), 7.82 (d, 2H, J = 8.7 Hz, Ar–H), 9.70 (s, 1H, CO2H).
N-(4-Methylbenzenesulfonyl)valine (2c)
From l-valine (117 mg). Yield: 184 mg (85%) as colorless crystals; m.p.: 151–153 °C; Rf: 0.45; IR (υmax, neat, cm−1): 3400–2400 (OH), 3280 (NH), 1729 (C=O), 1586 (C=C), 1318, 1160 (2 × O = S = O), 1081 (C–O). 1H NMR (CDCl3): δ 0.87 (d, 3H, J = 6.9 Hz, CH(CH3)2), 0.96 (d, 3H, J = 6.6 Hz, CH(CH3)2), 2.12 (m, 1H, CHCH(CH3)2), 2.42 (s, 3H, Ar-CH3), 3.80 (m, 1H, NHCH), 5.24 (d, 1H, J = 9.9 Hz, NH), 7.29 (d, 2H, J = 8.2 Hz, Ar–H), 7.73 (d, 2H, J = 8.4 Hz, Ar–H), 8.20 (s, 1H, CO2H).
N-(4-Chlorobenzenesulfonyl)valine (2d)
From l-valine (117 mg). Yield: 240 mg (82%) as colorless crystals; m.p.: 125–127 °C; Rf: 0.45; IR (υmax, neat, cm−1): 3450–2400 (OH), 3277 (NH), 1728 (C=O), 1578 (C=C), 1328, 1159 (2 × O = S = O), 1084 (C–O). 1H NMR (CDCl3): δ 0.76 (d, 3H, J = 6.9 Hz, CH(CH3)2), 0.88 (d, 3H, J = 6.9 Hz, CH(CH3)2), 2.14 (m, 1H, CHCH(CH3)2), 3.93 (m, 1H, NHCHCH), 5.65 (bs, 1H, NH), 7.66 (d, 2H, J = 8.4 Hz, Ar–H), 7.83 (d, 2H, J = 8.4 Hz, Ar–H), 8.60 (s, 1H, CO2H).
N-(4-Methylbenzenesulfonyl)leucine (2e)
From l-leucine (131 mg). Yield: 234 mg (82%) as colorless crystals; m.p.: 126–128 °C; Rf: 0.47; IR (υmax, neat, cm−1): 3400–2450 (OH), 3269 (NH), 1717 (C=O), 1576 (C=C), 1318, 1158 (2 × O = S = O), 1080 (C–O). 1H NMR (CDCl3): δ 0.83 (d, 3H, J = 6.6 Hz, CH2CH(CH3)2), 0.90 (d, 3H, J = 6.8 Hz, CH2CH(CH3)2), 1.51 (m, 2H, CH2CH(CH3)2), 1.79 (m, 1H, CH2CH(CH3)2), 2.43 (s, 3H, Ar-CH3), 3.93 (m, 1H, NHCHCH2), 5.25 (d, 1H, J = 9.6 Hz, NH), 7.29 (d, 2H, J = 8.4 Hz, Ar–H), 7.74 (d, 2H, J = 8.4 Hz, Ar–H), 8.60 (s, 1H, CO2H).
N-(4-Chlorobenzenesulfonyl)leucine (2f)
From l-leucine (131 mg). Yield: 242 mg (79%) as colorless crystals; m.p.: 111–114 °C; Rf: 0.48; IR (υmax, neat, cm−1): 3450–2450 (OH), 3275 (NH), 1728 (C=O), 1577 (C=C), 1319, 1156 (2×O = S = O), 1082 (C–O). 1H NMR (acetone-d6): δ 0.87 (d, 3H, J = 6.3 Hz, CH2CH(CH3)2), 0.93 (d, 3H, J = 6.5 Hz, CH2CH(CH3)2), 1.56 (m, 2H, CH2CH(CH3)2), 1.80 (m, 3H, m, CH2CH(CH3)2), 3.98 (m, 1H, NHCHCH2), 5.23 (d, 1H, J = 9.9 Hz, NH), 7.24 (d, 2H, J = 9.0 Hz, Ar–H), 7.80 (m, 1H, Ar–H), 10.02 (s, 1H, CO2H).
General procedure for the synthesis of N-(1-(5-amino-1,3,4-thiadiazol-2-yl)alkyl)-4-arylsulfonamides 3a–3f
To an ice-cooled mixture of thiosemicarbazide (165 mg, 1.00 mmol) and corresponding N-(4-chloro/methyl benzenesulfonyl)amino acid (1.0 mmol), an excess of phosphorus oxychloride (25 mL) was added slowly under continuous stirring. Subsequently, the temperature was raised gradually to 75–80 °C. The reaction was stirred at this temperature for 6 h, cooled and quenched with crushed ice. The resulting solution was refluxed for 4 h. The solution was then cooled and neutralized with solid KHCO3. The solid thus separated was filtered, washed with cold water and recrystallized from EtOH.
N-(1-(5-Amino-1,3,4-thiadiazol-2-yl)ethyl)-4-methylbenzenesulfonamide (3a)
From 2a (243 mg). Yield: 226 mg (76%) as colorless crystals; m.p.: 230–232 °C; Rf: 0.34; IR (υmax, neat, cm−1): 3421, 3416 (2×NH, pri.), 3263 (NH, sec.), 1567 (C=C), 1321, 1162 (2 × O = S = O). 1H NMR (DMSO-d6): δ 1.24 (d, 3H, J = 6.9 Hz, CHCH3), 2.38 (s, 3H, Ar-CH3), 4.49 (m, 1H, CHCH3), 7.06 (s, 2H, NH2), 7.37 (d, 2H, J = 8.4 Hz, Ar-H3,3′), 7.67 (d, 2H, J = 8.4 Hz, Ar-H2,2′), 8.37 (d, 1H, J = 5.7 Hz, NHCH). 13C-NMR (DMSO-d6): δ 21.1 (CHCH3), 21.4 (Ar-CH3), 49.5 (CHCH3), 127.0 (Ar-C3,3′), 130.1 (Ar-C2,2′), 138.4 (Ar-C1), 143.4 (Ar-C4), 162.3 (C 2thiadiazole ), 169.8 (C 5thiadiazole ). ESI–MS: m/z 299 [M + H]+.
N-(1-(5-Amino-1,3,4-thiadiazol-2-yl)ethyl)-4-chlorobenzenesulfonamide (3b)
From 2b (264 mg). Yield: 222 mg (70%) as colorless crystals; m.p.: 207–209 °C; Rf: 0.36; IR (υmax, neat, cm−1): 3428, 3418 (2×NH, pri), 3244 (N–H, sec.), 1578 (C=C), 1311, 1157 (2 × O = S = O). 1H NMR (DMSO-d6): δ 1.28 (d, 3H, J = 6.9 Hz, CHCH3), 4.56 (m, 1H, CHCH3), 7.09 (s, 2H, NH2), 7.69 (d, 2H, J = 6.6 Hz, Ar-H3,3′), 7.76 (d, 2H, J = 6.6 Hz, Ar-H2,2′), 8.59 (d, 1H, J = 8.1 Hz, NHCH). 13C NMR (DMSO-d6): δ 21.3 (CHCH3), 49.6 (CHCH3), 128.9 (Ar-C2,2′), 129.7 (Ar-C3,3′), 137.8 (Ar-C4), 140.4 (Ar-C1), 161.3 (C 2thiadiazole ), 169. 7 (C 5thiadiazole ). ESI–MS: m/z 317/319 [M + H]+.
N-(1-(5-Amino-1,3,4-thiadiazol-2-yl)-2-methylpropyl)-4-methylbenzenesulfonamide (3c)
From 2c (271 mg). Yield: 213 mg (65%) as mauve solid; m.p.: 253–254 °C; Rf: 0.36; IR (υmax, neat, cm−1): 3358, 3350 (2×NH, pri), 3156 (NH, sec.), 1594 (C=C), 1328, 1159 (2 × O = S = O). 1H NMR (DMF-d7): δ 0.96 (3H, d, J = 6.6 Hz, CH(CH3)2), 1.16 (d, 3H, J = 6.6 Hz, CH(CH3)2), 2.22 (1H, m, CH(CH3)2), 2.56 (3H, s, Ar-CH3), 4.50 (1H, m, NHCH), 7.28 (s, 2H, NH2), 7.50 (d, 2H, J = 8.1 Hz, Ar-H3,3′), 7.84 (2H, d, J = 8.1 Hz, Ar-H2,2′), 8.24 (1H, d, J = 1.6 Hz, NHCH). 13C NMR (DMF-d7): δ 18.7 (CH(CH3)2), 18.9 (CH(CH3)2), 20.8 (CH(CH3)2), 33.6 (Ar-CH3), 60.1 (NHCH), 127.1 (Ar-C3,3′), 129.6 (Ar-C2,2′), 139.1 (Ar-C1), 143.0 (Ar-C4), 160.7 (C 2thiadiazole ), 169.6 (C 5thiadiazole ). ESI–MS: m/z 327 [M + H]+.
N-(1-(5-Amino-1,3,4-thiadiazol-2-yl)-2-methylpropyl)-4-chlorobenzenesulfonamide (3d)
From 2d (292 mg). Yield: 239 mg (69%) as light pink crystals; m.p.: 251–253 °C; Rf: 0.35; IR (υmax, neat, cm−1): 3457, 3448 (2×NH, pri), 3252 (NH, sec.), 1576 (C=C), 1318, 1160 (2 × O = S = O). 1H NMR (DMF-d7): δ 0.76 (d, 3H, J = 6.9 Hz, CH(CH3)2), 0.93 (d, 3H, J = 6.9 Hz, CH(CH3)2), 2.25 (m, 1H, CH(CH3)2), 4.33 (m, 1H, NHCH), 7.66 (d, 2H, J = 7.8 Hz, Ar-H3,3′), 7.83 (d, 2H, J = 8.1 Hz, Ar-H2,2′), 8.04 (bs, 2H, NH2), 8.65 (bs, 1H, NHCH). 13C NMR (DMF-d7): δ 18.4 (CH(CH3)2), 18.8 (CH(CH3)2), 21.1 (CH(CH3)2), 32.0 (NHCH), 128.8 (Ar-C2,2’), 129.6 (Ar-C3,3’), 138.0 (Ar-C4), 140.3 (Ar-C1), 161.6 (C 2thiadiazole ), 169.3 (C 5thiadiazole ). ESI–MS: m/z 345/347 [M + H]+.
N-(1-(5-Amino-1,3,4-thiadiazol-2-yl)-3-methylbutyl)-4-methylbenzenesulfonamide (3e)
From 2e (285 mg). Yield: 245 mg (72%) as colorless crystals; m.p.: 240–242 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3464, 3450 (2×NH, pri), 3263 (NH, sec.), 1572 (C=C), 1319, 1156 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.78 (d, 3H, J = 3.3 Hz, CH(CH3)2), 0.84 (d, 3H, J = 3.3 Hz, CH(CH3)2), 1.63 (m, 3H, CH2CH(CH3)2), 2.41 (s, 3H, Ar-CH3), 4.64 (m, 1H, NHCHCH2), 6.41 (s, 2H, NH2), 7.09 (d, 1H, J = 8.1 Hz, NHCH), 7.32 (d, 2H, J = 7.8 Hz, Ar-H3,3′), 7.68 (d, 2H, J = 8.4 Hz, Ar-H2,2′). 13C NMR (acetone-d6): δ 20.5 (CH(CH3)2), 21.2 (CH(CH3)2), 21.7 (CHCH2), 24.2 (CH(CH3)2), 44.8 (Ar-CH3), 52.2 (NHCHCH2), 127.1 (Ar-C3,3′), 129.4 (Ar-C2,2′), 138.5 (Ar-C1), 143.0 (Ar-C4), 161.9 (C 2thiadiazole ), 168.9 (C 5thiadiazole ). ESI–MS: m/z 341 [M + H]+.
N-(1-(5-Amino-1,3,4-thiadiazol-2-yl)-3-methylbutyl)-4-chlorobenzenesulfonamide (3f)
From 2f (306 mg). Yield: 267 mg (74%) as colorless crystals; Yield: 74%; m.p.: 227–229 °C; Rf: 0.44; IR (υmax, neat, cm−1): 3420, 3412 (2×NH, pri), 3212 (NH, sec.), 1576 (C=C), 1318, 1158 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.72 (d, 3H, J = 6.3 Hz, CH(CH3)2), 0.79 (d, 3H, J = 6.3 Hz, CH(CH3)2), 1.46 (m, 3H, CH2CH(CH3)2), 4.42 (m, 1H, NHCHCH2), 7.07 (s, 2H, NH2), 7.63 (m, 2H, Ar-H3,3′), 7.67 (m, 2H, -H2,2′), 8.54 (d, 1H, J = 8.1 Hz, NHCH). 13C NMR (acetone-d6): δ 21.6 (CH(CH3)2), 23.0 (CH(CH3)2), 24.4 (CHCH2), 34.5 (CH(CH3)2), 44.6 (NHCH), 128.6 (Ar-C2,2′), 129.2 (Ar-C3,3′), 137.3 (Ar-C4), 140.3 (Ar-C1), 161.4 (C 2thiadiazole ), 169.5 (C 5thiadiazole ). ESI–MS: m/z 359/361 [M + H]+.
General procedure for the synthesis of N-(1-(5-(N-arylsulfonyl)amino-1,3,4-thiadiazol-2-yl)alkyl)-4-arylsulfonamides 4a–4w and 5
To a stirred solution of N-(1-(5-amino-1,3,4-thiadiazol-2-yl)alkyl)-4-arylsulfonamide (1.0 mmol) in pyridine (15 mL), arylsulfonyl chloride (1.40 mmol) was added under argon at 0 °C in four equal portions. The reaction mixture was stirred at 0 °C for 30 min and then at ambient temperature for 36–48 h. After the completion of reaction (tlc), water was added and the product was extracted with ethyl acetate (2 × 50 mL). The organic extracts were washed with 2 N HCl, followed by brine and dried over anhyd. Na2SO4. The solvent was evaporated and the residue was recrystallized from EtOH to give the desired product.
4-Methyl-N-(1-(5-(4-methylphenylsulfonamido)-1,3,4-thiadiazol-2-yl)ethyl)benzenesulfonamide (4a)
From 3a (298 mg). Yield: 372 mg (82%) as colorless crystals; m.p.: 228–230 °C; Rf: 0.44; IR (υmax, neat, cm−1): 3262 (NH, sec.), 1592 (C=C), 1321, 1167 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.44 (d, 3H, J = 6.9 Hz, CH3CH), 2.38 (s, 3H, Ar-CH3), 2.42 (s, 3H, Ar-CH3), 4.67 (m, 1H, CH3CH), 7.35 (s, 1H, NHCH), 7.40–7.37 (m, 2H, Ar–H), 7.75–7.71 (m, 4H, Ar–H), 12.60 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.6 (CH3CH), 20.5, 20.6 (2xAr-CH3), 49.9 (CH3CH), 126.1, 127.1, 129.4, 129.68, 138.0, 139.8, 143.0, 143.7 (8×Carom.), 161.1 (C 2thiadiazole ), 167.6 (C 5thiadiazole ). ESI–MS: m/z 453 [M + H]+.
4-Chloro-N-(5-(1-(4-methylphenylsulfonamido)ethyl)-1,3,4-thiadiazol-2-yl)benzenesulfonamide (4b)
From 3a (298 mg). Yield: 378 mg (80%) as colorless crystals; m.p.: 199–201 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3270 (NH, sec.), 1578 (C=C), 1311, 1152 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.43 (d, 3H, J = 7.8 Hz, CH3CH), 2.39 (s, 3H, Ar-CH3), 4.69 (m, 1H, CH3CH), 7.36 (bs, 1H, NHCH), 7.40 (m, 2H, Ar–H), 7.64 (m, 2H, Ar–H), 7.75 (d, 2H, J = 8.3 Hz, Ar–H), 7.86 (m, 2H, Ar–H), 12.73 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.6 (CH3CH), 20.6 (Ar-CH3), 49.9 (CH3CH), 127.1, 127.9, 129.1, 129.7, 137.8, 137.9, 141.4, 143.7 (8×Carom.), 161.7 (C 2thiadiazole ), 168.1 (C 5thiadiazole ). ESI–MS: m/z 472/474 [M + H]+.
4-Bromo-N-(5-(1-(4-methylphenylsulfonamido)ethyl)-1,3,4-thiadiazol-2-yl)benzenesulfonamide (4c)
From 3a (298 mg). Yield: 372 mg (72%) as colorless crystals; m.p.: 202–204 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3280 (NH, sec.), 1586 (C=C), 1328, 1160 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.44 (d, 3H, J = 6.8 Hz, CH3CH), 2.40 (s, 3H, Ar-CH3), 4.68 (m, 1H, CH3CH), 7.40 (s, 1H, NHCH), 7.79–7.74 (m, 4H, Ar–H), 7.39–7.36 (m, 4H, Ar–H), 12.73 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.6 (CH3CH), 20.6 (Ar-CH3), 49.8 (CH3CH), 126.3, 127.1, 128.1, 129.7, 132.1, 138.0, 141.9, 143.7 (8×Ar-Carom.), 161.6 (C 2thiadiazole ), 168.1 (C 5thiadiazole ). ESI–MS: m/z 516/518 [M + H]+.
4-Methoxy-N-(5-(1-(4-methylphenylsulfonamido)ethyl)-1,3,4-thiadiazol-2-yl)benzenesulfonamide (4d)
From 3a (298 mg). Yield: 328 mg (70%) as white powder; m.p.: 229–231 °C; Rf: 0.44; IR (υmax, neat, cm−1): 3287 (NH, sec.), 1576 (C=C), 1348, 1151 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.43 (d, 3H, J = 7.2 Hz, CH3CH), 2.39 (s, 3H, Ar-CH3), 3.89 (s, 3H, OCH3) 4.67 (m, 1H, CH3CH), 7.11–7.01 (m, 2H, Ar–H), 7.31 (bs, 1H, NHCH), 7.37 (m, 2H, Ar–H), 7.81–7.68 (m, 2H, Ar–H), 12.56 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.5 (CH3CH), 20.6 (Ar-CH3), 49.8 (CH3CH), 55.2 (OCH3), 114.0, 127.1, 128.2, 128.5, 129.7, 134.5, 143.7 (7×Carom.), 160.9 (C 2thiadiazole ), 162.8 (Carom.-OMe), 167.3 (C 5thiadiazole ). ESI–MS: m/z 469 [M + H]+.
4-Chloro-N-(1-(5-(4-methylphenylsulfonamido)-1,3,4-thiadiazol-2-yl)ethyl)benzenesulfonamide (4e)
From 3b (319 mg). Yield: 326 mg (69%) as colorless crystals; m.p.: 212–213 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3269 (NH, sec.), 1585 (C=C), 1321, 1142 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.47 (d, 3H, J = 6.9 Hz, CH3CH), 2.42 (s, 3H, Ar-CH3), 4.74 (m, 1H, CH3CH), 7.38 (d, 2H, J = 8.1 Hz, Ar–H), 7.55 (bs, 1H, NHCH), 7.61–7.57 (m, 2H, Ar–H), 7.75–7.72 (m, 2H, Ar–H), 7.38 (d, 2H, J = 8.1 Hz, Ar–H), 12.62 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.7 (CH3CH), 20.5 (Ar-CH3), 49.9 (CH3CH), 126.1, 128.8, 129.2, 129.4, 138.5, 139.7, 139.7, 143.0 (8×Carom.), 160.8 (C 2thiadiazole ), 167.5 (C 5thiadiazole ). ESI–MS: m/z 472/474 [M + H]+.
4-Chloro-N-(1-(5-(4-chlorophenylsulfonamido)-1,3,4-thiadiazol-2-yl)ethyl)benzenesulfonamide (4f)
From 3b (319 mg). Yield: 350 mg (71%) as colorless crystals %; m.p.: 221–223 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3275 (NH, sec.), 1567 (C=C), 1329, 1146 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.47 (d, 3H, J = 6.9 Hz, CH3CH), 4.76 (m, 1H, CH3CH), 7.64 (bs, 1H, NHCH), 7.91–7.84 (m, 4H, Ar–H), 7.63–7.59 (m, 4H, Ar–H), 12.69 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.7 (CH3CH), 49.9 (CH3CH), 127.9, 128.9, 129.1, 129.4, 137.9, 138.6, 139.7, 141.3 (8×Carom.), 161.2 (C 2thiadiazole ), 168.0 (C 5thiadiazole ). ESI–MS: m/z 492/494 [M + H]+.
4-Bromo-N-(5-(1-(4-chlorophenylsulfonamido)ethyl)-1,3,4-thiadiazol-2-yl)benzenesulfonamide (4g)
From 3b (319 mg). Yield: 364 mg (68%) as colorless crystals; m.p.: 233–235 °C; Rf: 0.43; IR (υmax, neat, cm−1): 3278 (NH, sec.), 1580 (C=C), 1320, 1145 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.44 (d, 3H, J = 7.8 Hz, CH3CH), 4.64 (m, 1H, CH3CH), 7.21 (bs, 1H, NHCH), 7.35 (m 2H, Ar–H), 7.38 (n, 2H, Ar–H), 7.77–7.73 (m, 4H, m, Ar–H), 12.05 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.6 (CH3CH), 49.9 (CH3CH), 126.4, 128.0, 128.9, 129.4, 132.2, 138.5, 139.7, 141.8 (8×Carom.), 161.2 (C 2thiadiazole ), 168.0 (C 5thiadiazole ). ESI–MS: m/z 536/538 [M + H]+.
4-Chloro-N-(1-(5-(4-methoxyphenylsulfonamido)-1,3,4-thiadiazol-2-yl)ethyl)benzenesulfonamide (4h)
From 3b (319 mg). Yield: 365 mg (75%) as white powder; m.p.: 222–224 °C; Rf: 0.45; IR (υmax, neat, cm−1): 3259 (NH, sec.), 1578 (C=C), 1326, 1126 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.47 (d, 3H, J = 6.9 Hz, CH3CH), 3.83 (s, 3H, OCH3), 4.56 (m, 1H, CH3CH), 7.12–7.08 (m, 2H, Ar–H), 7.64–7.60 (m, 2H, Ar–H), 7.71–7.67 (m, 4H, Ar–H), 8.78 (d, 1H, J = 8.1 Hz, NHCH), 13.96 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.2 (CH3CH), 49.6 (CH3CH), 56.1 (OCH3), 114.8, 128.3, 129.0, 129.8, 134.0, 138.2, 139.9 (7 × Carom.), 161.1 (C 2thiadiazole ), 162.7 (Carom.-OMe), 167.5 (C 5thiadiazole ). ESI–MS: m/z 488/490 [M + H]+.
4-Methyl-N-(5-(2-methyl-1-(4-methylphenylsulfonamido)propyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4i)
From 3c (326 mg). Yield: 331 mg (69%) as colorless crystals; m.p.: 220–221 °C; Rf: 0.49; IR (υmax, neat, cm−1): 3286 (NH, sec.), 1586 (C=C), 1323, 1160 (2 × O = S = O). 1H NMR (acetone-d6): δ 1.04 (d, 3H, J = 6.9 Hz, CH(CH3)2), 1.12 (d, 3H, J = 6.6 Hz, CH(CH3)2), 2.24 (m, 1H, CH(CH3)2), 2.38 (s, 3H, Ar-CH3), 2.41 (s, 3H, Ar-CH3), 4.67 (m, 1H, NHCH), 7.35 (s, 1H, NHCH), 7.64–7.59 (m, 4H, Ar–H), 7.86–7.74 (m, 4H, Ar–H), 12.48 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 21.0 (CH(CH3)2), 24.1, 24.3 (2xAr-CH3), 39.4 (CH(CH3)2), 52.2 (NHCH), 126.4, 128.8, 129.4, 130.0, 136.4, 137.3, 143.0, 144.0 (8×Carom.), 159.5 (C 2thiadiazole ), 165.2 (C 5thiadiazole ). ESI–MS: m/z 481 [M + H]+.
4-Chloro-N-(5-(2-methyl-1-(4-methylphenylsulfonamido)propyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4j)
From 3c (326 mg). Yield: 336 mg (67%) as light pink powder; m.p.: 212–214 °C; Rf: 0.47; IR (υmax, neat, cm−1): 3276 (NH, sec.), 1575 (C=C), 1322, 1148 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.92 (d, 3H, J = 6.9 Hz, CH(CH3)2), 1.04 (d, 3H, J = 6.6 Hz, CH(CH3)2), 2.28 (m, 1H, CH(CH3)2), 2.38 (s, 3H, Ar-CH3), 4.27 (m, 1H, NHCH), 7.23 (s, 2H, J = 8.1 Hz, Ar–H), 7.35 (br, 1H, NHCH), 7.66–7.62 (m, 2H, Ar–H), 7.86–7.82 (m, 4H, Ar–H) 12.60 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.0 (CH(CH3)2), 20.5 (Ar-CH3), 43.4 (CH(CH3)2), 59.8 (NHCH), 127.1, 128.0, 129.3, 132.1, 138.0, 138.0, 142.1, 143.3 (8×Carom.), 161.2 (C 2thiadiazole ), 167.7 (C 5thiadiazole ). ESI–MS: m/z 500/502 [M + H]+.
4-Bromo-N-(5-(2-methyl-1-(4-methylphenylsulfonamido)propyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4k)
From 3c (326 mg). Yield: 365 mg (67%) as colorless crystals; m.p.: 209–211 °C; Rf: 0.49; IR (υmax, neat, cm−1): 3274 (NH, sec.), 1586 (C=C), 1325,1150 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.88 (d, 3H, J = 6.9 Hz, CH(CH3)2), 1.03 (d, 3H, J = 6.9 Hz, CH(CH3)2), 2.22 (m, 1H, CH(CH3)2), 2.29 (s, 3H, Ar-CH3), 4.32 (m, 1H, NHCH), 7.21 (d, 2H, J = 8.1 Hz, Ar–H), 7.25 (s, 1H, NHCH), 7.65 (d, 2H, J = 8.2 Hz, Ar–H), 7.82–7.75 (m, 4H, Ar–H), 12.88 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 18.4 (CH(CH3)2), 20.5 (Ar-CH3), 42.7 (CH(CH3)2), 59.8 (NHCH), 126.2, 127.1, 128.0, 129.3, 132.1, 138.0, 142.1, 143.3 (8×Carom.), 160.1 (C 2thiadiazole ), 167.7 (C 5thiadiazole ). ESI–MS: m/z 544/546 [M + H]+.
4-Methoxy-N-(5-(2-methyl-1-(4-methylphenylsulfonamido)propyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4l)
From 3c (326 mg). Yield: 342 mg (69%) as colorless crystals; m.p.: 230–231 °C; Rf: 0.48; IR (υmax, neat, cm−1): 3255 (NH, sec.), 1575 (C=C), 1328, 1158 (2 × O = S = O). 1H-NMR (acetone-d6): δ 0.88 (d, 3H, J = 6.9 Hz, CH(CH3)2), 1.03 (d, 3H, J = 6.6 Hz, CH(CH3)2), 2.15 (m, 1H, CH(CH3)2), 2.38 (s, 3H, Ar-CH3), 3.90 (s, 3H, OCH3), 4.25 (m, 1H, NHCH), 7.31 (bs, 1H, NHCH), 7.13–7.09 (m, 2H, Ar–H), 7.20 (d, 2H, J = 7.8 Hz, Ar–H), 7.79–7.76 (m, 4H, Ar–H), 12.60 (s, 1H, NHSO2). 13C-NMR (acetone-d6): δ 21.8 (CH(CH3)2), 21.9 (Ar-CH3), 48.9 (CH(CH3)2), 52.5 (NHCH), 55.5 (OCH3), 113.2, 128.0, 128.9, 129.4, 139.2, 139.8, 141.2 (7×Carom.), 159.3 (C 2thiadiazole ), 160.3 (Carom.-OMe), 167.7 (C 5thiadiazole ). ESI–MS: m/z 497 [M + H]+.
4-Chloro-N-(2-methyl-1-(5-(4-methylphenylsulfonamido)-1,3,4-thiadiazol-2-yl)propyl)benzene sulfonamide (4m)
From 3d (347 mg). Yield: 351 mg (70%) as colorless crystals; m.p.: 244–246 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3258 (NH, sec.), 1578 (C=C), 1320, 1146 (2 × O = S = O). 1H NMR (DMSO-d6): δ 0.73 (d, 3H, J = 6.6 Hz, CH(CH3)2), 0.89 (d, 3H, J = 6.7 Hz, CH(CH3)2), 1.95 (m, 1H, CH(CH3)2), 2.38 (s, 3H, Ar-CH3), 4.20 (m, 1H, NHCH), 8.71 (d, 1H, J = 8.7 Hz, NHCH), 7.46–7.39 (m, 4H, Ar–H), 7.69–7.63 (m, 4H, Ar–H), 13.94 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 19.9 (CH(CH3)2), 21.5 (Ar-CH3), 32.6 (CH(CH3)2), 59.7 (NHCH), 126.2, 128.9, 129.9, 130.1, 137.9, 139.8, 139.4, 143.3 (8×Carom.), 159.4 (C 2thiadiazole ), 167.4 (C 5thiadiazole ). ESI–MS: m/z 500/502 [M + H]+.
4-Chloro-N-(1-(5-(4-chlorophenylsulfonamido)-1,3,4-thiadiazol-2-yl)-2-methylpropyl)benzene sulfonamide (4n)
From 3d (347 mg). Yield: 359 mg (69%) as colorless crystals; m.p.: 229–231 °C; Rf: 0.43; IR (υmax, neat, cm−1): 3278 (NH, sec.), 1574 (C=C), 1322, 1143 (2 × O = S = O). 1H NMR (DMSO-d6): δ 0.75 (d, 3H, J = 6.8 Hz, CH(CH3)2), 0.82 (d, 3H, J = 6.6 Hz, CH(CH3)2), 1.95 (m, 1H, CH(CH3)2), 4.10 (m, 1H, NHCH), 7.23 (d, 1H, J = 8.8 Hz, NHCH), 7.44–7.39 (m, 4H, Ar–H), 7.84–7.79 (m, 4H, Ar–H) 12.20 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 21.8 (CH(CH3)2), 34.2 (CH(CH3)2), 52.4 (NHCH), 127.0, 127.3, 128.9, 129.3, 137.9, 138.7, 140.6, 141.4 (8×Carom.), 160.3 (C 2thiadiazole ), 167.6 (C 5thiadiazole ). ESI–MS: m/z 520/522 [M + H]+.
4-Bromo-N-(5-(1-(4-chlorophenylsulfonamido)-2-methylpropyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4o)
From 3d (347 mg). Yield: 407 mg (72%) as pale yellow powder; m.p.: 221–223 °C; Rf: 0.43; IR (υmax, neat, cm−1): 3289 (NH, sec.), 1587 (C=C), 1329, 1158 (2 × O = S = O). 1H NMR (DMSO-d6): δ 0.65 (d, 3H, J = 6.9 Hz, CH(CH3)2), 0.81 (d, 3H, J = 6.6 Hz, CH(CH3)2), 1.90 (m, 1H, CH(CH3)2), 4.10 (m, 1H, NHCH), 7.42–7.36 (m, 4H, Ar–H), 7.69 (d, 1H, J = 9.0 Hz, NHCH), 7.80–7.75 (m, 4H, Ar–H) 12.27 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.5 (CH(CH3)2), 32.5 (CH(CH3)2), 55.0 (NHCH), 126.4, 127.3, 128.3, 129.2, 132.2, 138.4, 140.7, 141.8 (8×Carom.), 160.5 (C 2thiadiazole ), 167.6 (C 5thiadiazole ). ESI–MS: m/z 564/566 [M + H]+.
4-Chloro-N-(1-(5-(4-methoxyphenylsulfonamido)-1,3,4-thiadiazol-2-yl)-2-methylpropyl)benzene sulfonamide (4p)
From 3d (347 mg). Yield: 362 mg (70%) as white powder; m.p.: 211–213 °C; Rf: 0.42; IR (υmax, neat, cm−1): 3268 (NH, sec.), 1582 (C=C), 1326, 1160 (2 × O = S = O). 1H NMR (DMSO-d6): δ 0.91 (d, 3H, J = 6.6 Hz, CH(CH3)2), 1.03 (d, 3H, J = 6.9 Hz, CH(CH3)2), 3.50 (m, 1H, CH(CH3)2), 3.90 (s, 3H, OCH3), 4.36 (m, 1H, NHCH), 7.10 (m, 2H, Ar–H), 7.43 (bs, 1H, NHCH), 7.45 (m, 2H, Ar–H), 7.80–7.75 (m, 4H, Ar–H), 12.48 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 18.5 (CH(CH3)2), 32.7 (CH(CH3)2), 55.2 (NHCH), 59.9 (OCH3), 114.0, 128.2, 128.7, 128.8, 134.4, 138.3, 139.8 (7×Carom.), 158.8 (Carom.-OMe), 162.8 (C 2thiadiazole ), 166.8 (C 5thiadiazole ). ESI–MS: m/z 516/518 [M + H]+.
4-Methyl-N-(5-(3-methyl-1-(4-methylphenylsulfonamido)butyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4q)
From 3e (340 mg). Yield: 371 mg (75%) as colorless crystals; m.p.: 234–236 °C; Rf: 0.52; IR (υmax, neat, cm−1): 3287 (NH, sec.), 1587 (C=C), 1328, 1164 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.88 (d, 3H, J = 6.6 Hz, CH(CH3)2), 0.90 (d, 3H, J = 6.6 Hz, CH(CH3)2), 1.77–1.69 (m, 3H, CH2CH(CH3)2), 2.48 (s, 3H, Ar-CH3), 4.53 (m, 1H, NHCH), 7.33 (d, 1H, J = 8.4 Hz, NHCH), 7.42–7.37 (m, 4H, Ar–H), 7.74–7.63 (m, 4H, Ar–H) 12.09 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.6 (CH(CH3)2), 21.7 (CH(CH3)2), 24.1 (Ar-CH3), 41.7 (CH2CH(CH3)2), 49.9 (NHCH), 126.1, 127.2, 129.4, 1297 137.9, 139.8, 143.0, 143.7 (8×Carom.), 161.2 (C 2thiadiazole ), 167.6 (C 5thiadiazole ). ESI–MS: m/z 495 [M + H]+.
4-Bromo-N-(5-(3-methyl-1-(4-methylphenylsulfonamido)butyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4r)
From 3e (340 mg). Yield: 436 mg (78%) as white powder; m.p.: 254–256 °C; Rf: 0.53; IR (υmax, neat, cm−1): 3269 (NH, sec.), 1572 (C=C), 1320, 1166 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.81 (d, 3H, J = 6.0 Hz, CH(CH3)2), 0.86 (d, 3H, J = 6.0 Hz, CH(CH3)2), 1.79–1.64 (m, 3H, CH2CH(CH3)2), 2.48 (s, 3H, Ar-CH3), 4.60 (m, 1H, NHCH), 7.27 (d, 2H, J = 8.1 Hz, Ar–H), 7.33 (d, 1H, J = 8.4 Hz, NHCH), 7.68 (d, 2H, J = 8.4 Hz, Ar–H), 7.82–7.75 (m, 4H, Ar–H), 12.09 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.9 (CH(CH3)2), 21.8 (CH(CH3)2), 24.1 (Ar-CH3) 43.5 (CH2CH(CH3)2), 52.5 (NHCH), 126.3, 127.5, 128.0, 129.5, 132.2, 137.9, 141.9, 143.6 (8×Carom.) 160.9 (C 2thiadiazole ), 167.7 (C 5thiadiazole ). ESI–MS: m/z 560/558[M + H]+.
4-Methoxy-N-(5-(3-methyl-1-(4-methylphenylsulfonamido)butyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4s)
From 3e (340 mg). Yield: 367 mg (72%) as colorless crystals; m.p.: 233–234 °C; Rf: 0.54; IR (υmax, neat, cm−1): 3281 (NH, sec.), 1587 (C=C), 1328, 1150 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.91 (d, 3H, J = 6.6 Hz, CH(CH3)2), 1.03 (d, 3H, J = 6.9 Hz, CH(CH3)2), 1.80–1.69 (m, 3H, CH2CH(CH3)2), 2.36 (s, 3H, Ar-CH3), 3.90 (s, 3H, OCH3), 4.52 (m, 1H, NHCH), 7.35 (m, 1H, NHCH), 7.18 (m, 2H, Ar–H), 7.49 (m, 2H, Ar–H), 7.79–7.67 (m, 4H, Ar–H), 12.05 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.1 (CH(CH3)2), 22.1 (CH(CH3)2), 24.2 (Ar-CH3) 41.2 (CH2CH(CH3)2), 53.1 (NHCH), 54.2 (OCH3), 116.1, 126.5, 128.2, 129.6, 133.2, 138.4, 139.7 (7×Carom.), 159.3 (C 2thiadiazole ), 160.1 (Carom.-OMe), 169.2 (C 5thiadiazole ). ESI–MS: m/z 511 [M + H]+.
4-Chloro-N-(3-methyl-1-(5-(4-methylphenylsulfonamido)-1,3,4-thiadiazol-2-yl)butyl)benzene sulfonamide (4t)
From 3e (340 mg). Yield: 386 mg (75%) as colorless crystals; m.p.: 215–217 °C; Rf: 0.55; IR (υmax, neat, cm−1): 3276 (NH, sec.), 1583 (C=C), 1329, 1153 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.85 (d, 3H, J = 6.2 Hz, CH(CH3)2), 0.89 (d, 3H, J = 6.2 Hz, CH(CH3)2), 1.78–1.69 (m, 3H, CH2CH(CH3)2), 2.48 (s, 3H, Ar-CH3), 4.66 (m, 1H, NHCH), 7.47–7.44 (m, 2H, Ar–H), 7.60 (s, 1H, NHCH), 7.63–7.53 (m, 2H, Ar–H), 7.87–7.71 (m, 4H, Ar–H), 12.62 (s, 1H, NHSO2). 13C NMR (acetone-d6): δ 20.9 (CH(CH3)2), 21.9 (CH(CH3)2), 24.2 (Ar-CH3), 43.5 (CH2CH(CH3)2), 52.7 (NHCH), 126.4, 128.8, 129.4, 130.0, 132.4, 138.4, 139.7, 144.0 (8×Carom.), 160.1 C 2thiadiazole ), 167.1 (C 5thiadiazole ). ESI–MS: m/z 514/516 [M + H]+.
4-Chloro-N-(1-(5-(4-chlorophenylsulfonamido)-1,3,4-thiadiazol-2-yl)-3-methylbutyl)benzene sulfonamide (4u)
From 3f (361 mg). Yield: 417 mg (78%) as colorless crystals; m.p.: 218–221 °C; Rf: 0.54; IR (υmax, neat, cm−1): 3279 (NH, sec.), 1574 (C=C), 1328, 1154 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.83 (d, 3H, J = 6.0 Hz, CH(CH3)2), 0.89 (d, 3H, J = 6.0 Hz, CH(CH3)2), 1.80–1.66 (m, 3H, CH2CH(CH3)2), 4.66 (m, 1H, NHCH), 7.58 (s, 1H, NHCH), 7.66–7.51 (m, 4H, Ar–H), 7.86–7.80 (m, 4H, Ar–H), 12.71 (s, 1H, NHSO2). 13C NMR (acetone-d6):δ 20.8 (CH(CH3)2), 24.1 (CH(CH3)2), 43.4 (CH2CH(CH3)2), 52.4 (NHCH), 127.0, 127.9, 128.9, 129.2, 137.9, 138.4, 139.7, 141.4 (8×Carom.), 160.5 (C 2thiadiazole ), 167.5 (C 5thiadiazole ). ESI–MS: m/z 534/536 [M + H]+.
4-Bromo-N-(5-(1-(4-chlorophenylsulfonamido)-3-methylbutyl)-1,3,4-thiadiazol-2-yl)benzene sulfonamide (4v)
From 3f (361 mg). Yield: 416 mg (72%) as white powder; m.p.: 218–220 °C; Rf: 0.54; IR (υmax, neat, cm−1): 3276 (NH, sec.), 1569 (C=C), 1326, 1159 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.82 (d, 3H, J = 6.0 Hz, CH(CH3)2), 0.89 (d, 3H, J = 6.2 Hz, CH(CH3)2), 1.80–1.69 (m, 3H, CH2CH(CH3)2), 4.65 (m, 1H, NHCH), 7.54–7.51 (4H, m, Ar–H), 7.57 (s, 1H, NHCH) 7.84–7.75 (m, 4H, Ar–H), 12.00 (1H, s, NHSO2). 13C NMR (acetone-d6): δ 21.8 (CH(CH3)2), 24.1 (CH(CH3)2), 43.5 (CH2CH(CH3)2), 52.5 (NHCH), 126.4, 128.0, 128.9, 129.8, 132.2, 138.4, 139.7, 141.8 (8×Carom.), 160.5 (C 2thiadiazole ), 167.6 (C 5thiadiazole ). ESI–MS: m/z 579/581 [M + H]+.
4-Chloro-N-(1-(5-(4-methoxyphenylsulfonamido)-1,3,4-thiadiazol-2-yl)-3-methylbutyl)benzene sulfonamide (4w)
From 3f (361 mg). Yield: 370 mg (70%) as colorless crystals; m.p.: 231–233 °C; Rf: 0.53; IR (υmax, neat, cm−1): 3259 (NH, sec.), 1566 (C=C), 1320, 1164 (2 × O = S = O), 1H NMR (acetone-d6): δ 0.85 (d, 3H, J = 6.0 Hz, CH(CH3)2), 0.89 (d, 3H, J = 6.0 Hz, CH(CH3)2), 1.78–1.64 (m, 3H, CH2CH(CH3)2), 3.90 (s, 3H, OCH3), 4.64 (m, 1H, NHCH), 7.12–7.07 (m, 2H, Ar–H), 7.52–7.48 (m, 2H, Ar–H), 7.55 (bs, 1H, NHCH), 7.84–7.75 (m, 4H, Ar–H), 12.50 (s, 1H, NHSO2); 13C NMR (acetone-d6): δ 21.8 (CH(CH3)2), 24.2 (CH(CH3)2), 48.9 (CH2CH(CH3)2), 52.5 (NHCH), 55.2 (OCH3), 114.0, 128.2, 128.9, 129.2, 134.3, 138.4, 139.7 (7×Carom.), 159.9 (C 2thiadiazole ), 162.8 (Carom.-OMe), 166.8 (C 5thiadiazole ). ESI–MS: m/z 530/532 [M + H]+.
N-(1-(5-(N,N-dichlorobenzenesulfonyl)amino-1,3,4-thiadiazol-2-yl)-3-methylbutyl)-4-methyl benzenesulfonamide (5)
From 3f (361 mg). Yield: 345 mg (50%) as colorless crystals; m.p.: 223–224 °C; Rf: 0.53; IR (υmax, neat, cm−1): 3283 (NH, sec.), 1715 (C = 0), 1520 (C=C), 1328, 1165 (2 × O = S = O). 1H NMR (acetone-d6): δ 0.88 (d, 3H, J = 6.9 Hz, CH(CH3)2), 0.90 (d, 3H, J = 6.9 Hz, CH(CH3)2), 2.05 (m, 3H, CH2CH(CH3)2), 2.32 (s, 3H, Ar-CH3), 4.64 (m, 1H, NHCH), 7.43 (s, 1H, NHCH), 8.01–7.27 (m, 12H, Ar–H). 13C NMR (acetone-d6): δ 20.5 (Ar-CH3), 21.4 (CH(CH3)2), 25.2 (CH(CH3)2), 43.0 (NHCH + CH2CH(CH3)2), 127.2, 128.1, 129.1, 129.4, 129.7, 131.2, 134.0, 137.6, 139.0, 139.6, 141.9, 143.9 (12×Carom.), 160.3 (C 2thiadiazole ), 168.2 (C 5thiadiazole ). ESI–MS: m/z 689/691 [M + H]+.
Biological activity assays
In vitro anti-HIV assay
Evaluation of the antiviral activity of 3a–3f, 4a–4w and 5 against the HIV-1 strain (IIIB) and the HIV-2 strain (ROD) in MT-4 cells was performed using an MTT assay as described previously [38]. In brief, stock solutions (10-times final concentration) of test compounds were added in 25-μL volumes to two series of triplicate wells to allow simultaneous evaluation of their effects on mock and HIV-infected cells at the beginning of each experiment. Serial fivefold dilutions of test compounds were made directly in flat-bottomed 96-well microtiter trays using a Biomek 3000 robot (Beckman instruments). Untreated control, HIV- and mock-infected cell samples were included for each sample. HIV-1 (IIIB) [42] or HIV-2 (ROD) [43] stock (50 μL) at 100–300 CCID50 (50% cell culture infectious dose) or culture medium was added to either of the infected or mock-infected wells of the microtiter tray. Mock-infected cells were used to evaluate the effect of test compound on uninfected cells in order to assess the cytotoxicity of the test compounds. Exponentially growing MT-4 cells [44] were centrifuged for 5 min at 1000 rpm (Minifuge T, rotor 2250; Heraeus, Germany), and the supernatant was discarded. The MT-4 cells were resuspended at 6 × 105 cells per mL, and volumes of 50 μL were transferred to the microtiter tray wells. Five days after infection, the viability of the mock- and HIV-infected cells was examined spectrophotometrically.
References
Li Y, Geng J, Liu Y, Yu S, Zhao G (2013) Thiadiazole a promising structure in medicinal chemistry. ChemMedChem 8:27–41. https://doi.org/10.1002/cmdc.201200355
Hu Y, Li C-Y, Wang X-M, Yang Y-H, Zhu H-L (2014) 1,3,4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem Rev 114:5572–5610. https://doi.org/10.1021/cr400131u
Jain AK, Sharma S, Vaidya A, Ravichandran V, Agrawal RK (2013) 1,3,4-Thiadiazole and its derivatives: a review on recent progress in biological activities. Chem Biol Drug Des 81:557–576. https://doi.org/10.1111/cbdd.12125
Ezabadi IR, Camoutsis C, Zoumpoulakis P, Geronikaki A, Soković M, Glamocilija J, Cirić A (2008) Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg Med Chem 16:1150–1161. https://doi.org/10.1016/j.bmc.2007.10.082
Kumura K, Wakiyama Y, Ueda K, Umemura E, Watanabe Yamamoto M, Yoshida T, Ajito K (2018) Synthesis and antibacterial activity of novel lincomycin derivatives. III. Optimization of a phenyl thiadiazole moiety. J Antibiot 71:104–112. https://doi.org/10.1038/ja.2017.59
Chen C, Song B, Yang S, Xu G, Bhadury PS, Jin L, Hu D, Li Q, Liu F, Xue W, Lu P, Chen Z (2007) Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg Med Chem 15:3981–3989. https://doi.org/10.1016/j.bmc.2007.04.014
Liu X-H, Shi Y-X, Ma Y, Zhang C-Y, Dong W-L, Pan L, Wang B-L, Li B-J, Li Z-M (2009) Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamides. Eur J Med Chem 44:2782–2786. https://doi.org/10.1016/j.ejmech.2009.01.012
Klip NT, Capan G, Gursoy A, Uzun M, Satana D (2010) Synthesis, structure, and antifungal evaluation of some novel 1,2,4-triazolylmercaptoacetylthiosemicarbazide and 1,2,4-triazolylmercaptomethyl-1,3,4-thiadiazole analogs. J Enz Inhib Med Chem 25:126–131. https://doi.org/10.3109/14756360903040439
Kadi AA, Al-Abdullah ES, Shehata IA, Habib EE, Ibrahim TM, El-Emam AA (2010) Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur J Med Chem 45:5006–5011. https://doi.org/10.1016/j.ejmech.2010.08.007
Tozkoparan B, Aytac SP, Gursoy S, Gunal S, Aktay G (2012) Novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives as dual analgesic/anti-inflammatory and antimicrobial agents. Lett Drug Des Discov 9:204–212. https://doi.org/10.2174/157018012799079626
Bhat AR, Tazeem Azam A, Choi I, Athar F (2011) 3-(1,3,4-Thiadiazole-2-yl)quinoline derivatives: synthesis, characterization and antimicrobial activity. Eur J Med Chem 46:3158–3166. https://doi.org/10.1016/j.ejmech.2011.04.013
Bansode S, Kamble R (2011) Synthesis of novel 2-(3′-aryl-sydnon-4′-ylidene)-5′-substituted-[1,3,4]-thiadiazolylamines and [1,3,4]-thiadiazol-2′-yl-3-oxo-[1,2,4]-triazoles as antimicrobial agents. Med Chem Res 21:867–873. https://doi.org/10.1007/s00044-011-9596-2
Onkol T, Doğruer DS, Uzun L, Adak S, Ozkan S, Sahin MF (2008) Synthesis and antimicrobial activity of new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. J Enzyme Inhib Med Chem 23:277–284. https://doi.org/10.1080/14756360701408697
Talath S, Gadad AK (2006) Synthesis, antibacterial and antitubercular activities of some 7-[4-(5-amino-[1,3,4]thiadiazole-2-sulfonyl)-piperazin-1-yl]fluoroquinolonic derivatives. Eur J Med Chem 41:918–924. https://doi.org/10.1016/j.ejmech.2006.03.027
Foroumadi A, Soltani F, Jabini R, Moshafi MH, Rasnani FM (2004) Antituberculosis agents X. Synthesis and evaluation of in vitro antituberculosis activity of 2-(5-nitro-2-furyl)- and 2-(1-methyl-5-nitro-lH-imidazol-2-yl)-1,3,4-thiadiazole derivatives. Arch Pharm Res 27:502–506. https://doi.org/10.1007/BF02980122
Chitra S, Paul N, Muthusubramanian S, Manisankar P, Yogeeswari P, Sriram D (2011) Synthesis of 3-heteroarylthioquinoline derivatives and their in vitro antituberculosis and cytotoxicity studies. Eur J Med Chem 46:4897–4903. https://doi.org/10.1016/j.ejmech.2011.07.046
Kumar D, Kumar NM, Chang K-H, Shah K (2010) Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur J Med Chem 45:4664–4668. https://doi.org/10.1016/j.ejmech.2010.07.023
Bhole RP, Bhusari KP (2010) Synthesis and antitumor activity of (4-hydroxyphenyl)[5-substituted alkyl/aryl)-2-thioxo-1,3,4-thiadiazol-3-yl]methanone and [(3,4-disubstituted)-1,3-thiazol-2ylidene]-4-hydroxybenzohyd-razide. Med Chem Res 20:695–704. https://doi.org/10.1007/s00044-010-9371-9
Chou J, Lai S, Pan S, Jow G, Chern J, Guh J (2003) Investigation of anticancer mechanism of thiadiazole-based compound in human non-small cell lung cancer A549 cells. Biochem Pharmacol 66:115–124. https://doi.org/10.1016/S0006-2952(03)00254-5
Matysiak J, Opolski A (2006) Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorg Med Chem 14:4483–4489. https://doi.org/10.1016/j.bmc.2006.02.027
Wei M, Feng L, Li X, Zhou X, Shao Z (2009) Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur J Med Chem 44:3340–3344. https://doi.org/10.1016/j.ejmech.2009.03.023
Sun S, Yang Y, Li W, Zhang Y, Wang X, Tang J, Zhu H (2011) Synthesis, biological evaluation and molecular docking studies of 1,3,4-thiadiazole derivatives containing 1,4-benzodioxan as potential antitumor agents. Bioorg Med Chem Lett 21:6116–6121. https://doi.org/10.1016/j.bmcl.2011.08.039
Moshaf MH, Sorkhi M, Emami S, Nakhjiri M, Yahya-Meymandi A, Negahbani AS, Siavoshi F, Omrani M, Alipour E, Vosooghi M, Shafiee A, Foroumadi A (2011) 5-Nitroimidazole-based 1,3,4-thiadiazoles: heterocyclic analogs of metronidazole as anti-helicobacter pylori agents. Arch Pharm Chem Life Sci 344:178–183. https://doi.org/10.1002/ardp.201000013
Mirzaei J, Siavoshi F, Emami S, Safari F, Khoshayand MR, Shafiee A, Foroumadi A (2008) Synthesis and in vitro anti-helicobacter pylori activity of N-[5-(5-nitro-2-heteroaryl)-1,3,4-thiadiazol-2-yl]thiomorpholines and related compounds. Eur J Med Chem 43:1575–1580. https://doi.org/10.1016/j.ejmech.2007.11.019
Rajak H, Deshmukh R, Aggarwal N, Kashaw S, Kharya MD, Mishra P (2009) Synthesis of novel 2,5-disubstituted 1,3,4-thiadiazoles for their potential anticonvulsant activity: pharmacophoric model studies. Arch Pharm Chem Life Sci 342:453–461. https://doi.org/10.1002/ardp.200800213
Hamad NS, Al-Haidery NH, Al-Masoudi IA, Sabri M, Sabri L, Al-Masoudi NA (2010) Amino acid derivatives, part 4: synthesis and anti-HIV activity of new naphthalene derivatives. Arch Pharm Chem Life Sci 343:397–403. https://doi.org/10.1002/ardp.200900293
Ijichi K, Fujiwara M, Nagano H, Matsumoto Y, Hanasaki Y, Ide T, Katsuura K, Takayama H, Shirakawa S, Aimi N, Shigeta S, Konno K, Matsushima M, Yokota T, Baba M (1996) Anti-HIV-1 activity of thiadiazole derivatives: structure-activity relationship, reverse transcriptase inhibition, and lipophilicity. Antivir Res 31:87–94. https://doi.org/10.1016/0166-3542(96)00950-3
Sneader W (2005) Drug discovery: a history. Wiley, West Sussex. https://doi.org/10.1002/0470015535
Iyer G, Bellantone R, Taft D (1999) In vitro characterization of the erythrocyte distribution of methazolamide: a model of erythrocyte transport and binding kinetics. J Pharmacokinet Biopharm 27:45–66. https://doi.org/10.1023/A:1020630712388
Hall BS, Wilkinson SR (2012) Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother 56:115–123. https://doi.org/10.1128/AAC.05135-11
Messer WS (2002) The utility of muscarinic agonists in the treatment of Alzheimer’s disease. J Mol Neurosci MN 19:187–193. https://doi.org/10.1007/s12031-002-0031-5
Hansch C, Sammes PG, Taylor JB (1990) Comprehensive medicinal chemistry, vol 2. Pergamon Press, Oxford, Chap 7.1
Supuran Claudiu T (2017) Special issue: sulfonamides. Molecules 22:1642–1646. https://doi.org/10.3390/molecules22101642
Iqbal Z, Hameed S, Ali S, Tehseen Y, Shahid M, Iqbal J (2015) Synthesis, characterization, hypoglycemic and aldose reductase inhibition activity of arylsulfonylspiro[fluorene-9,5′-imidazolidine]-2′,4′-diones. Eur J Med Chem 98:127–138. https://doi.org/10.1016/j.ejmech.2015.05.011
Abbas MA, Hameed S, Farman M, Kressler J, Mahmood N (2015) Conjugates of degraded and oxidized hydroxyethyl starch and sulfonylureas: synthesis, characterization, and in vivo antidiabetic Activity. Bioconj Chem 26:120–127. https://doi.org/10.1021/bc500509a
Khan MH, Hameed S, Farman M, Al-Masoudi NA, Stoeckli-Evans HZ (2015) Synthesis, anti-HIV activity and molecular modeling study of 3-aryl-6-adamantylmethyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Z Naturforsch 70b:609–616. https://doi.org/10.1515/znb-2015-0032
Willker W, Leibfritz D, Kerssebaum R, Bermel W (1993) Gradient selection in inverse heteronuclear correlation spectroscopy. Magn Reson Chem 31:287–292. https://doi.org/10.1002/mrc.1260310315
Pannecouque C, Daelemans D, De Clercq E (2008) Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors:revisited, 20 years later. Nat Protoc 3:427–434. https://doi.org/10.1038/nprot.2007.517
Hargrave KD, Proudfoot JR, Grozinger KG, Cullen E, Kapadia SR, Patel UR, Fuchs VU, Mauldin SC et al (1991) J Med Chem 34:2231–2241. https://doi.org/10.1021/jm00111a045
Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrmann SN, Gallo RC, Bolognesi D, Barry DW, Broder S (1985) 3′-Azido-3′-deoxythymidine (BW A509U), an antiviral agent that inihibits the ineffectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associates virus in vitro. Proc Natl Acad Sci USA 82:7096–7100
Coates JA, Cammack N, Jenkinson HJ, Jowett AJ, Pearson BA, Penn CR, Rouse PL, Viner KC, Cameron JM (1992) The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother 36:733–739. https://doi.org/10.1128/AAC.36.4.733
Popovic M, Sarngadharan MG, Read E, Gallo RC (1984) Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497–500. https://doi.org/10.1126/science.6200935
Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L (1983) Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871. https://doi.org/10.1126/science.6189183
Miyoshi I, Taguchi H, Kobonishi I, Yoshimoto S, Ohtsuki Y, Shiraishi Y, Akagi T (1982) Type C virus-producing cell lines derived from adult T cell leukemia. Gann Monogr Cancer Res 28:219–228
Acknowledgements
We thank Prof. C. Pannecouque of Rega Institute for Medical Research, Katholieke Universiteit, Leuven, Belgium, for the anti-HIV screening.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shafique, M., Hameed, S., Naseer, M.M. et al. Synthesis of new chiral 1,3,4-thiadiazole-based di- and tri-arylsulfonamide residues and evaluation of in vitro anti-HIV activity and cytotoxicity. Mol Divers 22, 957–968 (2018). https://doi.org/10.1007/s11030-018-9851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9851-2