Abstract
A series of spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles hybrid compounds were prepared in good yields by regioselective, three-component, 1,3-dipolar cycloaddition reactions between \(\alpha , \beta \)-unsaturated ketones with furanyl substituents and unstable azomethine ylides, which were generated in situ from isatin and various types of amino acids. The synthesized compounds were screened for their antibacterial activities against a spectrum of pathogens. Preliminary studies identified compound 5c as a potent antimicrobial agent against drug-resistant bacteria. In addition, molecular docking studies indicated that compound 5c showed strong interactions with the active sites of lanosterol demethylase, dihydrofolate reductase, and topoisomerase II. This study provides an effective entry to the rapidly construction of a chemical library of heterocycles and compound 5c is one potent antibacterial lead for subsequent optimization.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
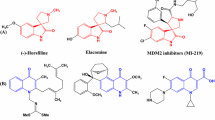
Spirooxindoles, such as functionalized spirooxindolo-pyrrolidine and pyrrolizidine, are important structural building blocks found in many natural products and they represent attractive targets in organic synthesis because of their highly pronounced biological properties as well as wide-ranging utility as synthetic intermediates for alkaloids, drug candidates, and clinical pharmaceuticals [1–3]. Spirooxindoles have been reported to exhibit biological activities such as antimicrobial [4], antitumoral [5], anti-inflammatory [6], anti-HIV [7] and potent non-peptide inhibition of the p53–MDM2 interaction [8, 9]. For instance, compound 1a, coerulescine, the simplest spirooxindole–pyrrolidine alkaloid in nature, displays a local anesthetic effect [10]; compound 1b, spirotryprostatin A, isolated from the fermentation broth of Aspergillus fumigatus, has been identified as a novel inhibitor of microtubule assembly and serotonin receptor inhibitor [11]; compound 2a, pteropodine and its analog compound 2b, rychnophylline are in advanced preclinical development for antigenotoxic, antioxidant therapeutics [12] (Fig. 1).
Although numerous impressive successes have been recorded for the synthesis of diversely structured spirooxindoles over the past years, the conventional one-pot, multi-component reactions are one of the most significant strategies for the synthesis [13–19]. In this regard, 1,3-dipolar cycloaddition could provide an efficient approach for the construction of nitrogen-containing five-membered ring heterocycles [20, 21]. At the same time, many research groups worked on the application of multi-component, domino 1,3-dipolar cycloaddition reactions to constitute a versatile protocol for the construction of poly functionalized spiroheterocycles viz. spiro-pyrrolidines and pyrrolizines, and their screening for antimicrobial activity. Some institutions and companies have unearthed several compounds with activities comparable to or superior to some of the currently employed first-line antibacterial drugs [22–30]. After large-scale synthesis, the preliminary results exhibited that screening hit compounds generally had different heterocyclic functional groups, such as nitrophenyl [23], beta-lactam [24], and thiophene [28]. Among them, the furanyl group was an active moiety present in widely used antibiotics such as furazolidone, furacilin, and nitrofurantoin, which were also used as chemotherapeutic agents for treating microbial diseases. Hence, there is a renewed interest in the synthesis of furanyl-containing spirooxindoles with potential antibacterial applications.
Prompted by these considerations and as a part of our own interest in cycloaddition reactions, we report herein the preliminary studies about the efficient synthesis of novel regiosteselective spiropyrrolidine pyrrolidines, pyrrolizidines, and pyrrolothiazole frameworks containing a furanyl moiety via the one-pot, multicomponent condensation of azomethine ylides (generated in situ from amino acids and isatin) with the Knoevenagel adduct derivatives (preformed from the reaction of 2-acetyl furan with substituted benzaldehydes). A library of compounds with spirooxindole structures was synthesized and screened for potential antimicrobial activities. This study formed a part of our researches initiated on the construction of novel heterocycles via 1,3-dipolar cycloaddition reactions to discover new lead compounds with antibacterial activities.
Results and discussions
In our initial endeavour, the Knoevenagel adducts 3a–g were synthesized via a classic conventional method by condensing commercially available 2-acetyl furan and substituted benzaldehydes in ethanol using NaOH as catalyst. Crude intermediates were purified by recrystallization in ethanol with 63–99 % total yields. Melting point, NMR and mass spectrometry data were consistent with those reported in the literature [31, 32].
From a mechanistic perspective and in combination with our experience in this field, we envisaged that an azomethine ylide could be generated in situ from isatin and l-proline, then trapped with 3-phenyl-1-(furan-2-yl)prop-2-enone (3d) acting as dipolarophile to afford spiropyrrolizidine oxindole 5d. Therefore, we started our investigation using a one-pot multicomponent synthesis with isatin, l-proline, and 3-phenyl-1-(furan-2-yl) prop-2-enone (3d) as a model reaction.
The reaction parameters including solvents and reaction temperatures were preliminary screened. Several solvents including 1,4-dioxane, THF, toluene, \({\text{ CH}}_{3}{\text{ CN}}, {\text{ CH}}_{3}{\text{ OH}}\), EtOH and \({\text{ H}}_{2}{\text{ O}}\) were explored (Table 1, entries 1–7). As it can be seen in Table 1, the reaction in methanol gave the desired product as a single regioisomer almost quantitatively (95 %) (Table 1, entry 9) while toluene gave the desired product in only 61 % yield (Table 1, entry 3). In general, the yields of reactions in protic solvents were higher than those in aprotic solvents. Furthermore, nearly no product was observed when \({\text{ H}}_{2}{\text{ O}}\) was employed as a solvent (Table 1, entry 5) and it might be caused by the poor solubility of isatin and compound 3d in water. Elevating the reaction temperature could lead to a higher reactivity (Table 1, entries 7–9). The best result was observed by refluxing the reaction mixture in methanol where 5d was obtained in high yield (95 %) as shown in Table 1, entry 9 and the reaction time was shortened to 1.5 h. Consequently, we chose these conditions for the rest of our studies.
With the optimized reaction conditions in hand, the scope of the new multi-component reaction was investigated for compounds 3a–g and different amino acids. Gratifyingly, as shown in Table 2, aryl groups with either an electron-donating or an electron-withdrawing group at the meta or para positon of the phenyl gave their corresponding products 5a–g, 6a–g, and 7a–g in good yields ranging from 81 to 90 %. In addition, heterocyclis substituents such as thiophene also proceeded smoothly to generate the desired products 5g, 6g, and 7g in good yields. It can be seen in Table 2 that the nature of the substituents in aryl groups on the 3-phenyl-1-(furan-2-yl)prop-2-enones and different amino acids had no significant effect on the reaction. The corresponding spiropyrrolidine oxindoles products 5a–g and 6a–g were obtained as single diastereoisomers in good yields with excellent diastereoselectivities (up to \(>\) 99:1) (Table 2, entry 1–14). For compounds 7a–g, the reaction took place with moderate-to-good yields ranging from 65 to 88 %, although the diastereoselectivity ratio decreased significantly (Table 2, entry 15–21).
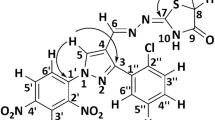
To obtain creditable structure–activity relationships, a careful structural study was needed for the potential of regio- and diastereoisomers. The structures proposed for all products 5–7 were in agreement with their NMR spectra as discussed next for compound 7b. In the \(^{1}{\text{ H}}\) NMR spectra of 7b, the pyrrolidine ring proton of C-5 exhibited a doublet at \(\delta \) 5.12 (\(J = 10.5\,{\text{ Hz}}\)). The proton of C-4 which was attached to the aryl appeared as a triplet at \(\delta \) 4.23 (\(J = 10.6\,{\text{ Hz}}\)). The pyrrolidine ring proton of C-3 which was attached to the furan moiety exhibited a doublet at \(\delta \) 4.59 (\(J = 10.7\,{\text{ Hz}}\)). The aromatic protons were distributed in the region \(\delta \) 7.41–6.24. The –NH proton of the oxindole moiety appeared as a singlet at \(\delta \)7.81. These chemical shift assignments confirmed the proposed structure of 7b. Based on the calculation of the coupling constant (\(J\)-based configuration analysis, \({{J}}>10\,\text{ Hz}\)), the relative configuration of this structure should be as same as compound 7b shown in Fig. 2 and the configuration was further confirmed by the X-ray study of a single crystal of compound 7b (Fig. 2). The results revealed that the pyrrolidine ring adopted an envelope form with the spiro carbon being out of plane. The \(^{13}{\text{ C}}\) NMR of compound 7b supported the proposed structure as well. The pyrrolidine ring carbons resonated in the region \(\delta \) 68.54–54.53 ppm. The oxindole carbonyl and the carbonyl carbons resonated at \(\delta \)182.32 and \(\delta \)185.04, respectively (Fig. 2). The HMQC and NOESY 2D spectra data of compound 5b were obtained. The protons of the chiral center carbons could be identified by HMQC and NOESY analysis. The proton exhibiting a doublet at \(\delta \)4.58 was not related to the proton recorded as a triplet at \(\delta \)3.86. This meant that the two protons are located on different sides of the ring. On the other hand, the proton at \(\delta \)4.58 and the proton at \(\delta 4.22\) have weak remote correlations indicating that they are located on the same side of the ring. Furthermore, the molecular weights of the desired target structures were confirmed by ESI-TOF high resolution mass spectrum (HRMS). Each spectra displayed a very prominent signal corresponding to the compound combined with one proton or one sodium cation. All analytical data is documented in the supplementary data.
a Selected \({1}^{\text{ H}}\) and \(^{13}{\text{ C}}\) NMR chemical shifts of 7b. b Single crystal X-ray diffraction study of compound 7b [Crystallographic data of compound 7b reported in this manuscript have been deposited with Cambridge Crystallographic Data Centre as supplement ary publication no. CCDC-897479. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK; fax: +44 1223 336033; or deposit@ccdc.cam.ac.uk)]
A plausible mechanism for the formation of regio- and diastereoisomers could be explained as follows: the azomethine ylides, generated from the reaction of isatin and the amino acids, have two nucleophilic carbons, potentially resulting in two regioisomers. The regioselectivity observed in the formation of the product is explained on the basis that the transition state (Ts-1) leading to the observed products is likely to be more stable than the other possible one (Ts-2), which would be destabilized by interactions between the aryl ring from the styrene derivative and the amino acid chain (Fig. 3). The aryl ring attached to the pyrrolidone ring is cis to the carbonyl of the oxindole moiety and trans to the benzo group. This diastereoselectivity suggested that the transition state Ts-3 led to the minor diastereoisomers by unfavorable interactions between the phenyl ring and the benzo group of the oxindole moiety.
The 21-compound library was screened for preliminary in vitro antibacterial activity against our five ATCC-bacterial strain panel (Staphylococcus aureus ATCC 29213, Methicillin-resistant Staphylococcus aureus ATCC 43300, E. coli ATCC 25922, Pseudomonas aeruginos ATCC 27853 and Klebsiella pneumoniae ATCC 700603) using the standard broth dilution method [33]. As shown in Table 3, products 5–7-exhibited antibacterial activities with MIC (minimum inhibitory concentration) ranging from 16 to 128 \(\upmu {\text{ g/mL}}\). From the initial results, we could see that most compounds were only effective against Gram-negative bacteria, especially P. aeruginos. The spiro compound with pyrrolizidines scaffold, compound 5c, showed good antibacterial activity against P. aeruginos at lower concentrations (\(16\,\upmu {\text{ g/mL}}\)) and when the methoxy group was replaced by halogens (compound 5b) there was a decrease in activity against P. aeruginos.
For the further determination of the antibacterial spectrum of our compounds, the most promising agent 5c was tested against many important clinical isolates. Norfloxacin, levofloxacin, and ciprofloxacin, all frequently used as antimicrobial therapeutics, were chosen as positive control. Among P. aeruginos, compound 5c was active against both susceptible and multidrug-resistant isolates, with MICs of 16–32 mg/L, respectively. Though compounds 5c showed higher MICs against the susceptible strains than the clinical used antibiotics norfloxacin, levofloxacin, and ciprofloxacin, the activity of compound 5c was not affected in the screening of drug-resistant strains. This meant that compound 5c exhibited much better activity than norfloxacin, levofloxacin and ciprofloxacin in MDRP Table 4.
Although the cellular target was not defined in the experimental antimicrobial investigations of these molecules, it was desirable to investigate the interactions between the active compounds and the enzymes, such as lanosterol demethylase, dihydrofolate reductase and topoisomerase II, which are targeted by clinically used antimicrobial drugs (Fig. 4) [34]. Docking of compound 5c showed that the compound interacted with the active sites of the enzymes through H-bonds between its furanyl oxygen and Ser64, Ile375, Asn74, Ser128, Gly140, Lys147, Thr126 amino-acid residues, respectively. These docking results support the potential of these compounds to interfere with sterol biosynthesis and DNA replication in P. aeruginos ultimately leading to bacterial death [35].
Conclusion
In summary, the aforementioned strategy presented a direct route to prepare biologically relevant spirooxindoles scaffolds by one-pot, multi-component 1,3-dipolar cycloaddition reaction via azomethine ylides. The advantages of this process included high bond-forming efficiency, good to high yields, simple work-up procedure, mild reaction conditions, and regio- and diastereo selectivies. The method provided rapid access to a library of spirooxindoles containing the furanyl moiety and these spiroheterocycles displayed good in vitro antibacterial activities against Gram-negative bacteria. For our study compound 5c exhibited better activity on the multidrug resistance bacteria particularly P. aeruginos. The presence of the spirooxindoles, broadened the potential for further transformation of these compounds into therapeutically useful drug candidates.
Experimental
General
Thin layer chromatography (TLC) (silica gel plates \({\text{ GF}}_{254})\) was used to monitor the reaction progress and the purity of the compounds: compounds were visualized by irradiation with UV light and/or by treatment with a solution of phosphomolybdic acid (20 % wt. in ethanol) followed by heating. The melting points were recorded on a SGW X-4 micro melting point apparatus and are uncorrected. The \(^{1}{\text{ H}}\) and \(^{13}{\text{ C}}\) NMR spectra were recorded at 400 and 100 MHz, respectively, on a Bruker Avance III 400 MHz instrument in \({\text{ CDCl}}_{3}\) using TMS as an internal standard. Chemical shifts are reported as \(\delta \) values (ppm). Mass spectra were recorded on a Bruker amaZon SL spectrometer. Chemicals and solvents were either A.R. grade or purified by standard techniques.
General procedure for the synthesis of 5a–g
A mixtrue of compound 3 (1 mmol), isatin (1 mmol), and proline (1 mmol) in methanol (12 mL) was refluxed for 1.5 h, until completion of the reaction as indicated by TLC. The solvent was removed under reduced pressure and the resulting residue was purified by column chromatography using petroleum ether/ethyl acetate (4:1) as an eluent to afford desired product.
\(1^{\prime }\)-(2,4-Dichlorophenyl)-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5a)
Yellow solid, yield 83 %, mp 174–176 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3})\,\delta \) 8.08 (s, 1H), 7.60 (d, \(J\) = 8.5 Hz, 1H), 7.39 (d, \(J\) = 2.1 Hz, 1H), 7.32 (dd, \(J\) = 12.9, 4.2 Hz, 2H), 7.27–7.11 (m, 2H), 7.06–6.94 (m, 2H), 6.73 (d, \(J\) = 7.7 Hz, 1H), 6.28 (dd, \(J\) = 3.5, 1.6 Hz, 1H), 4.74 (d, \(J\) = 11.5 Hz, 1H), 4.66–4.55 (m, 1H), 4.19–4.05 (m, 1H), 2.79 (m, 1H), 2.70–2.55 (m, 1H), 2.17–2.98 (m, 2H), 2.01–1.88 (m, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3})\,\delta \) 183.98, 180.75, 152.29, 146.72, 140.58, 135.88, 135.51, 132.95, 129.69, 129.61, 129.36, 127.61, 124.80, 122.57, 117.95, 112.32, 109.99, 73.48, 72.30, 63.89, 48.37, 47.10, 29.65, 26.75. ESI-MS (m/z): calcd. for 466, obsd.465 (\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 467.0929 (\({\text{[M+H]}}^{+}\)), obsd.467.0937.
\(1^{\prime }\)-(3,4-Dichlorophenyl)-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5b)
White solid, yield 82 %, mp 131–132\(\,^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3})\,\delta \) 7.94 (s, 1H), 7.60 (d, \(J\) = 1.7 Hz, 1H), 7.39–7.30 (m, 2H), 7.23 (d, \(J\) = 7.5 Hz, 2H), 7.15 (t, \(J\) = 7.7 Hz, 1H), 6.99 (dd, \(J\) =8.0, 5.8 Hz, 2H), 6.72 (d, \(J\) = 7.7 Hz, 1H), 6.30 (dd, \(J\) = 3.6, 1.6 Hz, 1H), 4.58 (d, \(J\) = 11.4 Hz, 1H), 4.22 (m, 1H), 3.86 (t, \(J\) = 10.8 Hz, 1H), 2.76 (dd, \(J\) = 16.4, 7.9 Hz, 1H), 2.68–2.56 (m, 1H), 2.04–1.88 (m, 2H), 1.88–1.70 (m, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 184.04, 180.60, 152.19, 146.94, 140.51, 140.02, 132.67, 131.05, 130.65, 130.25, 129.65, 127.50, 127.56, 124.72, 122.45, 118.28, 112.33, 109.99, 73.42, 71.40, 64.46, 51.46, 48.31, 29.98, 26.83. ESI-MS (m/z): calcd. for 466.09, obsd.465(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 467.0929 (\({\text{[M+H]}}^{+}\)), obsd.467.0927.
\(1^{\prime }\)-(4-Methoxyphenyl)-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5c)
White solid, yield 88 %, mp 162–164 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 8.19 (s, 1H), 7.40 (d, \(J\) = 8.6 Hz, 2H), 7.31 (dd, \(J\) = 11.0, 4.2 Hz, 2H), 7.15 (t, \(J\) = 7.7 Hz, 1H), 7.00 (dd, \(J\) = 13.2, 5.6 Hz, 2H), 6.84 (d, \(J\) = 8.7 Hz, 2H), 6.73 (d, \(J\) = 7.7 Hz, 1H), 6.26 (dd, \(J\) = 3.6, 1.6 Hz, 1H), 4.63 (d, \(J\) = 11.6 Hz, 1H), 4.22 (dt, \(J\) = 10.0, 6.1 Hz, 1H), 3.99–3.82 (m, 1H), 3.76 (s, 3H), 2.73 (dd, \(J\) = 12.1, 4.4 Hz, 1H), 2.67–2.55 (m, 1H), 2.07–1.88 (m, 2H), 1.7–1.60 (m, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 184.26, 181.08, 158.60, 152.39, 146.78, 140.51, 131.42, 129.46, 129.10, 127.72, 125.08, 122.37, 118.18, 114.10, 112.15, 110.01, 73.77, 71.72, 64.47, 55.23, 51.67, 48.33, 30.30, 27.07. ESI-MS(m/z): calcd. for 428, obsd.427(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 429.1814 (\({\text{[M+H]}}^{+}\)), obsd.429.1820.
\(1^{\prime }\)-Phenyl-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5d)
White solid, yield 90 %, mp 185–187 \(^{\circ }{\text{ C}}. {1}^{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 8.34 (s, 1H), 7.46–7.38 (m, 2H), 7.24 (dd, \(J\) = 10.4, 4.9 Hz, 4H), 7.18–7.04 (m, 2H), 7.00–6.88 (m, 2H), 6.68 (d, \(J\) = 7.7 Hz, 1H), 6.24–6.16 (m, 1H), 4.62 (d, \(J\) = 11.6 Hz, 1H), 4.26 – 4.13 (m, 1H), 3.86 (dd, \(J\) = 11.3, 10.2 Hz, 1H), 2.76–2.62 (m, 1H), 2.62–2.50 (m, 1H), 2.15–1.78 (m, 3H), 1.78–1.13 (m, 1H). \({13}^{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \,\delta \) 183.16, 180.18, 151.32, 145.75, 139.56, 138.49, 128.46, 127.65, 127.13, 126.67, 125.97, 124.01, 121.35, 117.12, 111.14, 109.05, 72.79, 70.81, 63.33, 51.33, 47.31, 29.29, 26.03. ESI-MS(m/z): calcd. for 398, obsd.397(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 399.1709 (\({\text{[M+H]}}^{+}\)), obsd.399.1737.
\(1^{\prime }\)-(4-Bromophenyl)-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5e)
White solid, yield 85 %, mp 134–135 \(^{\circ }{\text{ C}}. {1}^{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 8.12 (s, 1H), 7.48–7.31 (m, 6H), 7.15 (t, \(J\) = 7.7 Hz, 1H), 6.99 (dd, \(J\) = 13.5, 5.6 Hz, 2H), 6.73 (d, \(J\) = 7.7 Hz, 1H), 6.28 (dd, \(J\) = 3.6, 1.6 Hz, 1H), 4.61 (d, \(J\) = 11.5 Hz, 1H), 4.26–4.16 (m, 1H), 3.94–3.83 (m, 1H), 2.75 (dd, \(J\) = 11.9, 4.6 Hz, 1H), 2.69–2.55 (m, 1H), 2.07–1.85 (m,3H), 1.84–1.49 (m, 1H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \,\delta \) 184.09, 180.86, 152.26, 146.90, 140.52, 138.60, 131.81, 129.92, 129.59, 127.63, 124.86, 122.43, 120.89, 118.23, 112.27, 110.04, 73.61, 71.55, 64.43, 51.81, 48.33, 30.13, 26.95. ESI-MS(m/z): calcd. for 476, obsd.395(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 477.0814 (\({\text{[M+H]}}^{+}\)), obsd.477.0809.
\(1^{\prime }\)-(4-Fluorophenyl)-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5f)
White solid, yield 86 %, mp 161–162 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 8.21 (s, 1H), 7.45 (dd, \(J\) = 8.5, 5.4 Hz, 2H), 7.34–7.25(m, 2H), 7.15 (t, \(J\) = 7.6 Hz, 1H), 7.06–6.89 (m, 4H), 6.73 (d, \(J\) = 7.7 Hz, 1H), 6.27 (dd, \(J\) = 3.5, 1.5 Hz, 1H), 4.62 (d, \(J\) = 11.5 Hz, 1H), 4.26–4.13 (m, 1H), 3.97–3.82 (m, 1H), 2.83–2.66 (m, 1H), 2.66–2.51 (m, 1H), 2.06–1.61 (m, 4H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 184.18, 181.00, 160.70, 152.31, 146.87, 140.54, 135.17, 129.62, 127.64, 124.93, 122.42, 118.21, 115.65, 115.44, 112.24, 110.05, 73.65, 71.69, 64.55, 51.61, 48.33, 30.19, 26.99. ESI-MS(m/z): calcd. for 416, obsd.415(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 417.1614(\({\text{[M+H]}}^{+}\)), obsd.417.1617.
\(1^{\prime }\)-(Thiophen-2-yl)-\(2^{\prime }\)-(furan-2-carbonyl)-\(1^{\prime }\),\(2^{\prime }\),\(5^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-hexahydrospiro[indoline-3,\(3^{\prime }\)-pyrrolizin]-2-one (5g)
White solid, yield 89 %, mp 185–188 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \,\delta \) 8.48 (s, 1H), 7.36 (d, \(J\) = 1.0 Hz, 1H), 7.27 (m, 1H), 7.20–7.11 (m, 2H), 7.06–6.97 (m, 3H), 6.92 (dd, \(J\) = 5.1, 3.5 Hz, 1H), 6.75 (d, \(J\) = 7.7 Hz, 1H), 6.29 (dd, \(J\) = 3.6, 1.7 Hz, 1H), 4.62 (d, \(J\) = 11.2 Hz, 1H), 4.26 (m, 2H), 2.77–2.56 (m, 2H), 2.09 – 1.90 (m, 2H), 1.90–1.74 (m, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 183.79, 180.96, 152.22, 147.05, 142.53, 140.54, 129.61, 127.65, 126.96, 125.00, 124.74, 123.84, 122.42, 118.57, 112.26, 110.21, 73.88, 71.74, 64.93, 48.28, 47.37, 30.42, 27.12. ESI-MS(m/z): calcd. for 404, obsd.405(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 427.1092 (\({\text{[M+Na]}}^{+}\)), obsd.427.1094.
General procedure for the synthesis of 6a–g
A mixtrue of compound 3 (1 mmol), isatin (1 mmol) and thiaproline (1 mmol) in methanol (12 mL) was refluxed for 1.5 h, until completion of the reaction as indicated by TLC. The solvent was removed under reduced pressure and the resulting residue was purified by column chromatography using petroleum ether/ethyl acetate (4:1) as an eluent to afford desired product.
\(7^{\prime }\)-(2,4-Dichlorophenyl)-\(6^{\prime }\)-(furan-2-carbonyl)-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6a)
White solid, yield 82 %, mp 178–180 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 7.59 (d, \(J\) = 33.4 Hz, 1H), 7.55–7.45 (m, 1H), 7.33 (dd, \(J\) = 24.7, 5.7 Hz, 1H), 7.18–7.06 (m, 2H), 6.97 (t, \(J\) = 7.6 Hz, 1H), 6.88 (d, \(J\) = 3.5 Hz, 1H), 6.62 (d, \(J\) = 7.7 Hz, 1H), 6.45 (dd, \(J\) = 3.5, 1.5 Hz, 1H), 6.25 (d, \(J\) = 2.2 Hz, 1H), 4.60 (m, 2H), 4.21 (s, 1H), 3.83 (d, \(J\) = 10.4 Hz, 1H), 3.66 (d, \(J\) = 6.8 Hz, 1H), 3.08 (s, 1H), 3.06–2.89 (m, 1H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 178.5, 168.609,150.93, 148.95, 145.77, 139.30, 132.52, 129.18, 128.93, 128.57, 127.72, 126.68, 121.80, 121.62, 117.07, 116.49, 111.43, 108.47, 73.87, 73.03, 60.75, 53.35, 44.57, 35.01. ESI-MS(m/z): calcd. for 484.04, obsd.483(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 507.0313 (\({\text{[M+Na]}}^{+}\)), obsd.507.0313.
\(7^{\prime }\)-(3,4-Dichlorophenyl)-\(6^{\prime }\)-(furan-2-carbonyl)-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6b)
White solid, yield 83 %, mp 128–130 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 7.79 (s, 1H), 7.59 (dd, \(J\) = 23.7, 4.6 Hz, 2H), 7.48–7.31 (m, 3H), 7.18 (t, \(J\) = 7.7 Hz, 1H), 7.10–6.92 (m, 2H), 6.69 (d, \(J\) = 7.7 Hz, 1H), 6.32 (dd, \(J\) = 3.5, 1.6 Hz, 1H), 4.48 (d, \(J\) = 11.8 Hz, 1H), 4.42–4.25 (m, 1H), 4.01–3.84 (m, 2H), 3.52 (d, \(J\) = 10.3 Hz, 1H), 3.07 (dd, \(J\) = 11.7, 6.3 Hz, 1H), 2.97 (dd, \(J\) = 11.7, 1.9 Hz, 1H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 182.82, 179.83, 151.90, 146.97, 140.24, 139.08, 132.93, 131.62, 130.84, 130.30, 128.64, 127.81, 122.77, 118.38, 112.45, 109.75, 74.27, 62.66, 58.50, 54.26, 49.92, 36.25, 18.46. ESI-MS(m/z): calcd. for 484.04, obsd.483(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 507.0313 (\({\text{[M+Na]}}^{+}\)), obsd.507.0313.
\(6^{\prime }\)-(Furan-2-carbonyl)-\(7^{\prime }\)-(4-methoxyphenyl)-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6c)
Yellow solid, yield 89 %, mp 161–163 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.92 (s, 1H), 7.57 (d, \(J\) = 7.6 Hz, 1H), 7.35 (d, \(J\) = 8.7 Hz, 2H), 7.28 (d, \(J\) = 0.8 Hz, 1H), 7.10 (t, \(J\) = 7.4 Hz, 1H), 6.97 (t, \(J\) = 7.5 Hz, 1H), 6.89 (d, \(J\) = 3.5 Hz, 1H), 6.79 (d, \(J\) = 8.6 Hz, 2H), 6.62 (d, \(J\) = 7.7 Hz, 1H), 6.21 (dd, \(J\) = 3.5, 1.5 Hz, 1H), 4.45 (d, \(J\) = 12.0 Hz, 1H), 4.36–4.20 (m, 1H), 3.84 (dt, \(J\) = 9.9, 5.6 Hz, 2H), 3.68(s,3H), 3.45 (d, \(J\) = 10.4 Hz, 1H), 2.96 (qd, \(J\) = 11.7, 4.1 Hz, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 182.15, 179.25, 157.89, 151.13, 145.77, 139.22, 129.47, 128.98, 128.31, 127.86, 122.07, 121.61, 117.19, 113.25, 111.22, 108.67, 73.69, 73.45, 61.52, 57.46, 54.23, 53.58, 49.34, 35.43, 17.42. ESI-MS(m/z): calcd. for 446.13, obsd.445(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 469.1198 (\({\text{[M+Na]}}^{+}\)), obsd.469.1205.
\(6^{\prime }\)-(Furan-2-carbonyl)-\(7^{\prime }\)-phenyl-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6d)
White solid, yield 88 %, mp 194–196 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 8.09 (s, 1H), 7.64 (d, \(J\) = 7.6 Hz, 1H), 7.51 (d, \(J\) = 7.3 Hz, 2H), 7.34 (dd, \(J\) = 10.5, 4.4 Hz, 3H), 7.29–7.13 (m, 1H), 7.04 (td, \(J\) = 7.6, 0.8 Hz, 1H), 6.96 (d, \(J\) = 3.5 Hz, 1H), 6.71 (d, \(J\) = 7.7 Hz, 1H), 6.28 (dd, \(J\) = 3.6, 1.6 Hz, 1H), 4.59 (d, \(J\) = 11.9 Hz, 1H), 4.44–4.32 (m, 1H), 4.02–3.86 (m, 2H), 3.53 (d, \(J\) = 10.4 Hz, 1H), 3.12–2.96 (m, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 183.10, 180.34, 152.12, 146.80, 140.30, 138.66, 130.06, 128.89, 128.37, 127.48, 123.09, 122.68, 118.19, 112.27, 109.76, 74.81, 74.50, 62.53, 54.50, 51.02, 36.47. ESI-MS(m/z): calcd. for 416.12, obsd.415(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 439.1092 (\({\text{[M+Na]}}^{+}\)), obsd.439.1113.
\(7^{\prime }\)-(4-Bromophenyl)-\(6^{\prime }\)-(furan-2-carbonyl)-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6e)
White solid, yield 81 %, mp 133–134 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.76 (s, 1H), 7.52 (d, \(J\) = 7.5 Hz, 1H), 7.47–7.24 (m, 4H), 7.10 (t, \(J\) = 7.3 Hz, 2H), 6.96 (t, \(J\) = 7.5 Hz, 1H), 6.87 (d, \(J\) = 3.5 Hz, 1H), 6.61 (d, \(J\) = 7.7 Hz, 1H), 6.23 (dd, \(J\) = 3.5, 1.6 Hz, 1H), 4.44 (d, \(J\) = 11.8 Hz, 1H), 4.38–4.17 (m, 1H), 3.95–3.76 (m, 2H), 3.45 (d, \(J\) = 10.3 Hz, 1H), 2.95 (ddd, \(J\) = 13.7, 11.7, 4.2 Hz, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 181.93, 178.96, 151.00, 145.86, 139.23, 136.73, 130.99, 129.10, 129.07, 127.71, 121.94, 121.69, 120.39, 117.23, 111.33, 108.69, 73.42, 73.22, 61.64, 57.46, 53.27, 49.33, 35.28, 17.43. ESI-MS(m/z): calcd. for 494.03, obsd.495(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 517.0197 (\({\text{[M+Na]}}^{+}\)), obsd.517.0199.
\(7^{\prime }\)-(4-Fluorophenyl)-\(6^{\prime }\)-(furan-2-carbonyl)-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6f)
White solid, yield 83 %, mp 201–202 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.94 (s, 1H), 7.61 (d, \(J\) = 7.6 Hz, 1H), 7.55–7.39 (m, 2H), 7.36 (d, \(J\) = 1.0 Hz, 1H), 7.18 (td, \(J\) = 7.7, 1.2 Hz, 1H), 7.08–6.92 (m, 4H), 6.70 (d, \(J\) = 7.7 Hz, 1H), 6.30 (dd, \(J\) = 3.6, 1.7 Hz, 1H), 4.52 (d, \(J\) = 11.9 Hz, 1H), 4.40–4.29 (m, 1H), 4.00–3.86 (m, 2H), 3.53 (d, \(J\) = 10.3 Hz, 1H), 3.03 (ddd, \(J\) = 13.9, 11.7, 4.3 Hz, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 183.05, 180.13, 152.07, 146.87, 140.26, 134.34, 130.11, 129.92, 129.84, 128.77, 123.00, 122.71, 118.23, 115.89, 112.33, 109.74, 74.64, 74.30, 62.73, 58.49, 54.39, 50.20, 36.34, 18.45. ESI-MS(m/z): calcd. for 434.11, obsd.435(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 457.0998 (\({\text{[M+Na]}}^{+}\)), obsd.457.0997.
\(6^{\prime }\)-(Furan-2-carbonyl)-\(7^{\prime }\)-(thiophen-2-yl)-\(3^{\prime }\),\(6^{\prime }\),\(7^{\prime }\),\(7a^{\prime }\)-tetrahydro-\(1^{\prime }\)H-spiro[indoline-3,\(5^{\prime }\)-pyrrolo[1,2-c]thiazol]-2-one (6g)
White solid, yield 87 %, mp 195–197 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.96 (s, 1H), 7.63 (d, \(J\) = 7.6 Hz, 1H), 7.39 (d, \(J\) = 1.0 Hz, 1H), 7.23–7.13 (m, 2H), 7.05 (ddd, \(J\) = 13.1, 11.4, 3.4 Hz, 3H), 6.94 (dd, \(J\) = 5.1, 3.5 Hz, 1H), 6.70 (d, \(J\) = 7.7 Hz, 1H), 6.32 (dd, \(J\) = 3.6, 1.6 Hz, 1H), 4.51 (d, \(J\) = 11.8 Hz, 1H), 4.47 – 4.35 (m, 1H), 4.27 (dd, \(J\) = 11.7, 9.9 Hz, 1H), 3.90 (d, \(J\) = 10.6 Hz, 1H), 3.52 (d, \(J\) = 10.6 Hz, 1H), 3.21–3.05 (m, 2H).\(^{ 13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 182.75, 179.93, 152.03, 147.07, 141.46, 140.20, 130.16, 128.93, 127.15, 125.77, 124.31, 122.81, 122.70, 118.62, 112.34, 109.80, 74.73, 74.66, 62.89, 54.72, 46.12, 36.54, 18.46. ESI-MS(m/z): calcd. for 422.07, obsd.421(\({\text{[M-H]}}^{-}\)). HRMS (m/z): calcd. for 445.0657 (\({\text{[M+Na]}}^{+}\)), obsd.445.0660.
General procedure for the synthesis of 7a–g
A mixtrue of compound 3 (1 mmol), isatin (1 mmol) and phenylglycine (1 mmol) in methanol (12 mL) was refluxed for 1.5 h, until completion of the reaction as indicated by TLC. The solvent was removed under reduced pressure and the resulting residue was purified by column chromatography using petroleum ether/ethyl acetate (4:1) as an eluent to afford desired product.
\(4^{\prime }\)-(2,4-Dichlorophenyl)-\(3^{\prime }\)-(furan-2-carbonyl)-\(5^{\prime }\)-phenylspiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7a)
Yellow solid, yield 65 %, mp 182–184 \(^{\circ }{\text{ C}}\). \(^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.78–7.73 (m, 2H), 7.37 –6.86 (m, 13H), 6.69 (d, \(J\) =7.8 Hz, 1H), 6.26 (t, \(J\) =1.6 Hz, 1H), 5.20 (d, \(J\) =10.4 Hz,1H), 4.88 (t, \(J\) = 28 Hz, 1H), 4.59 (d, \(J\) =10.4 Hz, 1H).\(^{ 13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 184.75, 182.09, 152.60, 146.29, 140.03, 139.17, 135.82, 135.42, 132.99, 130.18, 129.55, 129.40, 128.84, 128.39, 128.01, 127.49, 127.08, 126.13, 123.27, 116.89, 112.46, 109.34, 68.61, 62.22, 49.61. ESI-MS(m/z): calcd. for 502.09, obsd.525(\({\text{[M+Na]}}^{+}\)). HRMS (m/z): calcd. for 525.0749 (\({\text{[M+Na]}}^{+}\)), obsd.525.0755.
\(4^{\prime }\)-(3,4-Dichlorophenyl)-\(3^{\prime }\)-(furan-2-carbonyl)-\(5^{\prime }\)-phenylspiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7b)
Yellow solid, yield 71 %, mp 220–222 \(^{\circ }{\text{ C}}\). \(^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.81 (s, 1H), 7.46–7.20 (m, 5H), 7.07– 7.16 (m, 5H), 6.99 (t, \(J\) = 7.5 Hz, 1H), 6.99 (t, \(J\) = 7.5 Hz, 1H), 6.85 (d, \(J\) = 3.5 Hz, 1H), 6.66 (d, \(J\) = 8 Hz, 2H), 6.25 (d, \(J\) = 1.9 Hz, 1H), 5.12 (d, \(J\) = 10.5 Hz, 1H), 4.59 (d, \(J\) = 10.7 Hz, 1H), 4.23 (t, \(J\) = 10.6 Hz, 1H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 185.04, 182.33, 152.72, 146.34, 140.24, 140.01, 138.85, 129.45, 129.25, 128.55, 128.32, 127.71, 127.15, 127.07, 126.16, 123.22, 116.93, 112.30, 109.32, 68.54, 67.96, 62.67, 54.54. ESI-MS(m/z): calcd. for 502.09, obsd.503(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 525.0749 (\({\text{[M+Na]}}^{+}\)), obsd.525.0748.
\(3^{\prime }\)-(Furan-2-carbonyl)-\(4^{\prime }\)-(4-methoxyphenyl)-\(5^{\prime }\)-phenylspiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7c)
Yellow solid, yield 83 %, mp 181–182 \(^{\circ }{\text{ C}}\). \(^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.94 (s, 1H), 7.42–6.99 (m, 9H), 7.00 (d, \(J\) = 7.5 Hz, 1H), 7.04–6.82 (m, 3H), 6.78 (d, \(J\) = 8.3 Hz, 2H), 6.62 (dd, \(J\) = 27.7, 8.0 Hz, 1H), 6.28–6.21 (m, 1H), 5.06 (d, \(J\) = 10.5 Hz, 1H), 4.53 (t, \(J\) = 11.8 Hz, 1H), 4.17 (t, \(J\) = 10.6 Hz, 1H), 3.71 (s, 3H). \(^{ 13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 185.14, 182.48, 158.51, 152.75, 146.35, 140.38, 140.01, 130.76, 129.58, 129.48, 129.22, 128.30, 127.66, 127.16, 126.15, 123.21, 116.94, 113.94, 112.29, 109.36, 68.50, 67.90, 62.71, 55.15, 53.87. ESI-MS(m/z): calcd. for 464.17, obsd.465(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 487.1634 (\({\text{[M+Na]}}^{+}\)), obsd.487.1630.
\(3^{\prime }\)-(Furan-2-carbonyl)-\(4^{\prime }\),\(5^{\prime }\)-diphenylspiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7d)
Yellow solid, yield 88 %, mp 212–214 \(^{\circ }{\text{ C}}\). \(^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 8.00 (s, 1H), 7.52 (m, 2H), 7.45–7.29 (m, 6H), 7.29 – 7.05 (m, 6H), 7.05–6.84 (m, 2H), 6.68 (d, \(J\) = 7.7 Hz, 1H), 6.27 (d, \(J\) = 3.4 Hz, 1H), 5.07 (d, \(J\) = 10.4 Hz, 1H), 4.48 (d, \(J\) = 10.5 Hz, 1H), 4.17 (t, \(J\) = 10.5 Hz, 1H). \(^{ 13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 184.71, 182.29, 152.51, 146.51, 140.07, 139.56, 139.45, 132.53, 131.08, 130.53, 130.37, 129.43, 129.01, 128.53, 128.12, 128.07, 127.09, 126.00, 123.27, 117.13, 112.49, 109.48, 68.42, 67.65, 62.45, 53.62. ESI-MS(m/z): calcd. for 434.16, obsd.435(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 457.1528 (\({\text{[M+Na]}}^{+}\)), obsd.457.1537.
\(4^{\prime }\)-(4-Bromophenyl)-\(3^{\prime }\)-(furan-2-carbonyl)-\(5^{\prime }\)-phenylspiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7e)
Yellow solid, yield 78 %, mp 221–223 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 8.08 (s, 1H), 7.45–6.94 (m, 14H), 6.85 (d, \(J\) = 3.6 Hz, 1H), 6.67 (d, \(J\) = 7.7 Hz, 1H), 6.28–6.22 (m, 1H), 5.08 (d, \(J\) = 10.5 Hz, 1H), 4.51 (d, \(J\) = 10.7 Hz, 1H), 4.19 (t, \(J\) = 10.6 Hz, 1H). \(^{ 13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 184.86, 182.36, 152.59, 146.48, 140.09, 139.79, 137.97, 131.70, 130.29, 129.37, 129.17, 128.44, 127.93, 127.12, 126.06, 123.25, 120.95, 117.06, 112.43, 109.48, 68.47, 67.77, 62.51, 53.98. ESI-MS(m/z): calcd. for 512.07, obsd.513 (\({\text{[M+H]}}^{+}\)). calcd. for 535.0633 (\({\text{[M+Na]}}^{+}\)), obsd.535.0638.
\(4^{\prime }\)-(4-Fluorophenyl)-\(3^{\prime }\)-(furan-2-carbonyl)-\(5^{\prime }\)-phenylspiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7f)
Yellow solid, yield 82 %, mp 222–224 \(^{\circ }{\text{ C}}. ^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 7.79 (s, 1H), 7.45–7.28 (m, 5H), 7.25 (d, \(J\) = 7.0 Hz, 4H), 7.08 (t, \(J\) = 7.7 Hz, 1H), 6.93 (ddd, \(J\) = 30.8, 18.7, 5.5 Hz, 4H), 6.73 (d, \(J\) = 9.5 Hz, 1H), 6.66 (d, \(J\) = 7.7 Hz, 1H), 6.30–6.23 (m, 1H), 5.07 (d, \(J\) = 10.5 Hz, 1H), 4.52 (d, \(J\) = 10.7 Hz, 1H), 4.20 (t, \(J\) = 10.6 Hz, 1H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \, \delta \) 184.97, 182.26, 152.67, 146.41, 139.99, 134.55, 130.04, 129.96, 129.32, 128.38, 127.84, 127.11, 126.13, 123.25, 116.99, 115.53, 115.32, 112.37, 109.35, 68.39, 67.94, 62.60, 53.81. ESI-MS(m/z): calcd. for 452.15, obsd.453(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 475.1434 (\({\text{[M+Na]}}^{+}\)), obsd.475.1433.
\(3^{\prime }\)-(Furan-2-carbonyl)-\(5^{\prime }\)-phenyl-\(4^{\prime }\)-(thiophen-2-yl)spiro[indoline-3,\(2^{\prime }\)-pyrrolidin]-2-one (7g)
Yellow solid, yield 87 %, mp 203–205 \(^{\circ }{\text{ C}}.\,^{1}{\text{ H}}\) NMR (400 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 8.16 (s, 1H), 7.50 (m, 2H), 7.29–7.33(m, 6H), 7.09 (dd, \(J\) = 10.2, 6.2 Hz, 2H), 7.03–6.80 (m, 4H), 6.67 (d, \(J\) = 7.6 Hz, 1H), 6.31–6.24 (m, 1H), 5.14–4.99 (m, 1H), 4.57 (d, \(J\) = 4.9 Hz, 2H). \(^{13}{\text{ C}}\) NMR (100 MHz, \({\text{ CDCl}}_{3}) \quad \delta \) 184.64, 182.12, 152.57, 146.65, 141.74, 140.07, 139.80, 129.38, 129.31, 128.45, 128.04, 127.45, 126.85, 126.12, 125.70, 124.01, 123.26, 117.36, 112.41, 109.54, 68.52, 62.35, 63.13, 49.61. ESI-MS(m/z): calcd. for 440.12, obsd.441(\({\text{[M+H]}}^{+}\)). HRMS (m/z): calcd. for 463.1092 (\({\text{[M+Na]}}^{+}\)), obsd.463.1095.
In vitro minimum inhibitory concentration assay
MICs were determined by a microdilution method with MuellereHinton Broth (MHB) for staphylococci and Lennox Broth (LB) for Enterococci, following the National Committee for Clinical Laboratory Standards (NCCLS) (now called the Clinical Laboratory Standards Institute [CLSI]). The stock solutions of test compounds were diluted to give a serial 2-fold series, with final chemical concentrations ranging from 64 to 0.0125 \(\upmu \)g/mL. The MIC was defined as the lowest concentration of the chemical that inhibited the development of visible bacterial growth after an incubation for 16 h at \(37\,^{\circ }{\text{ C}}\). The MICs of standard antibiotics (norfloxacin, levofloxacin and ciprofloxacin) were determined by the same method.
Molecular docking procedure
Compounds were built using the builder toolkit of the software package Discover Studio 3.1 [36]. The crystal coordinates of lanosterol demethylase (pdb ID: 3JUS), dihydrofolate reductase (pdb ID: 3NZ6) and topoisomerase II (pdb ID: 1QZR) were downloaded from protein data bank, carrying econazole, 6-{3-[(2,4-diamino-5-methylpyrido[2,3-d]pyrimidin-6-yl)methyl]-4- methoxyphenoxy} hexanoic acid (a trimethoprim analogue) and dextrazoxazone as co-crystallized ligands, respectively. Structure of compound5c was pasted in the workspace carrying structures of the enzymes. The docking program implements an efficient grid based docking algorithm which approximates an exhaustive search within the free volume of the binding site cavity. The conformational space was explored by the geometry optimization of the flexible ligand (rings were treated as rigid) in combination with the incremental construction of the ligand torsions. Thus, docking occurs between the flexible ligand parts of the compound and enzyme. The ligand orientation was determined by a scoring function based on Ligscore and the final positions were ranked by lowest interaction energy values. H-bond and hydrophobic interactions between the compound and enzyme were explored.
References
Millemaggi A, Taylor RJK (2010) 3-Alkenyl-oxindoles: natural products, pharmaceuticals, and recent synthetic advances in tandem/telescoped approaches. Eur J Org Chem 2010(24): 4527–4547. doi:10.1002/ejoc.201000643
Edwankar CR, Edwankar RV, Namjoshi OA, Rallapalli SK, Yang J, Cook JM (2009) Recent progress in the total synthesis of indole alkaloids. Curr Opin Drug Discov Devel 12:752–771
Trost BM, Brennan MK (2009) Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis-Stuttgart 3003–3025. doi:10.1055/s-0029-1216975
Bhaskar G, Arun Y, Balachandran C, Saikumar C, Perumal PT (2012) Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem 51:79–91. doi:10.1016/j.ejmech.2012.02.024
Girgis AS, Stawinski J, Ismail NSM, Farag H (2012) Synthesis and QSAR study of novel cytotoxic spiro 3\(H\)-indole-3,2\(^{\prime }\)(1\(^{\prime }\)H)-pyrrolo 3,4-c pyrrole-2,3\(^{\prime }\),5\(^{\prime }\)(1H,2\(^{\prime }\)aH,4\(^{\prime }\)H)-triones. Eur J Med Chem 47:312–322. doi:10.1016/j.ejmech.2011.10.058
Anisetti R, Reddy MS (2012) Synthesis, antimicrobial, anti-inflammatory and antioxidant activity of novel Spiro (imidazo 4\(^{\prime }\),5\(^{\prime }\):4,5\(^{\prime }\)benzo 1,2-e 1,4 thiazepine)-9,3\(^{\prime }\)-indolines. J Sulfur Chem 33:363–372. doi:10.1080/17415993.2012.683432
Nicolaou KC, Sanchini S, Sarlah D, Lu G, Wu TR, Nomura DK, Cravatt BF, Cubitt B, de la Torre JC, Hessell AJ, Burton DR (2011) Design, synthesis, and biological evaluation of a biyouyanagin compound library. P Natl Acad Sci USA 108:6715–6720. doi:10.1073/pnas.1015258108
Millard M, Pathania D, Grande F, Xu SL, Neamati N (2011) Small-molecule inhibitors of p53-MDM2 interaction: the 2006–2010 update. Curr Pharm Design 17:536–559
Gomez-Monterrey I, Bertamino A, Porta A, Carotenuto A, Musella S, Aquino C, Granata I, Sala M, Brancaccio D, Picone D, Ercole C, Stiuso P, Campiglia P, Grieco P, Ianelli P, Maresca B, Novellino E (2010) Identification of the spiro(oxindole-3,3’-thiazolidine)-based derivatives as potential p53 activity modulators. J Med Chem 53:8319–8329. doi:10.1021/jm100838z
Anderton N, Cockrum PA, Colegate SM, Edgar JA, Flower K, Vit I, Willing RI (1998) Oxindoles from Phalaris coerulescens. Phytochemistry 48:437–439. doi:10.1016/s0031-9422(97)00946-1
Edmondson S, Danishefsky SJ, Sepp-Lorenzino L, Rosen N (1999) Total synthesis of spirotryprostatin A, leading to the discovery of some biologically promising analogues. J Am Chem Soc 121:2147–2155. doi:10.1021/ja983788i
Paniagua-Perez R, Madrigal-Bujaidar E, Molina-Jasso D, Reyes-Cadena S, Alvarez-Gonzalez I, Sanchez-Chapul L, Perez-Gallaga J (2009) Antigenotoxic, antioxidant and lymphocyte induction effects produced by pteropodine. Basic Clin Pharmacol 104:222–227. doi:10.1111/j.1742-7843.2008.00366.x
Domling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. doi:10.1021/cr0505728
Hulme C, Gore V (2003) Multi-component reactions: emerging chemistry in drug discovery. From xylocain to crixivan. Curr Med Chem 10:51–80. doi:10.2174/0929867033368600
Keshipour S, Shojaei S, Shaabani A (2012) A novel one-pot isocyanide-based four-component reaction: synthesis of highly functionalized 1\(H\)-pyrazolo 1,2-\(b\) phthalazine-1,2-dicarboxylates and 1\(H\)-pyrazolo 1,2-a pyridazine-1,2-dicarboxylates. Tetrahedron 68:6141–6145. doi: 10.1016/j.tet.2012.05.078
Naidu PS, Borah P, Bhuyan PJ (2012) Synthesis of some novel functionalized dihydropyrido 2,3-d pyrimidines via an one-pot three-component reaction catalysed by InCl3. Tetrahedron Lett 53: 4015–4017. doi:10.1016/j.tetlet.2012.05.102
Tisseh ZN, Ahmadi F, Dabiri M, Khavasi HR, Bazgir A (2012) A novel organocatalytic multi-component reaction: an efficient synthesis of polysubstituted pyrano-fused spirooxindoles. Tetrahedron Lett 53:3603–3606. doi:10.1016/j.tetlet.2012.05.019
Moulin E, Cormosw G, Giuseppone N (2012) Dynamic combinatorial chemistry as a tool for the design of functional materials and devices. Chem Soc Rev 41:1031–1049. doi:10.1039/c1cs15185a
Orru RVA, de Greef M (2003) Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis-Stuttgart 1471–1499: doi:10.1055/s-2003-40507
Liu J, Sun HB, Liu XJ, Ouyang L, Kang TR, Xie YM, Wang XY (2012) Direct construction of novel exo’-selective spiropyrrolidine bisoxindoles via a three-component 1,3-dipolar cycloaddition reaction. Tetrahedron Lett 53:2336–2340. doi:10.1016/j.tetlet.2012.02.099
Xie YM, Yao YQ, Sun HB, Yan TT, Liu J, Kang TR (2011) Facile synthesis of functionalized spiropyrrolizidine oxindoles via a three-component tandem cycloaddition reaction. Molecules 16:8745–8757. doi:10.3390/molecules16108745
Periyasami G, Raghunathan R, Surendiran G, Mathivanan N (2008) Synthesis of novel spiropyrrolizidines as potent antimicrobial agents for human and plant pathogens. Bioorg Med Chem Lett 18:2342–2345. doi:10.1016/j.bmcl.2008.02.065
Rajesh SM, Perumal S, Menendez JC, Yogeeswari P, Sriram D (2011) Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. MedChemComm 2:626–630. doi:10.1039/c0md00239a
Arumugam N, Periyasami G, Raghunathan R, Kamalraj S, Muthumary J (2011) Synthesis and antimicrobial activity of highly functionalised novel beta-lactam grafted spiropyrrolidines and pyrrolizidines. Eur J Med Chem 46:600–607. doi:10.1016/j.ejmech.2010.11.039
Kumar RR, Perumal S, Senthilkumar P, Yogeeswari P, Sriram D (2008) Discovery of antimycobacterial spiro-piperidin-4-ones: an atom economic, stereoselective synthesis, and biological intervention. J Med Chem 51:5731–5735. doi:10.1021/jm800545k
Kumar RR, Perumal S, Senthilkumar P, Yogeeswari P, Sriram D (2009) A facile synthesis and antimycobacterial evaluation of novel spiro-pyrido-pyrrolizines and pyrrolidines. Eur J Med Chem 44:3821–3829. doi:10.1016/j.ejmech.2009.05.010
Maheswari SU, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A facile 1,3-dipolar cycloaddition of azomethine ylides to 2-arylidene-1,3-indanediones: synthesis of dispiro-oxindolylpyrrolothiazoles and their antimycobacterial evaluation. Bioorg Med Chem Lett 20:7278–7282. doi:10.1016/j.bmcl.2010.10.080
Thangamani A (2010) Regiospecific synthesis and biological evaluation of spirooxindolopyrrolizidines via 3+2 cycloaddition of azomethine ylide. Eur J Med Chem 45:6120–6126. doi:10.1016/j.ejmech.2010.09.051
Prasanna P, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A regio- and stereoselective 1,3-dipolar cycloaddition for the synthesis of novel spiro-pyrrolothiazolyloxindoles and their antitubercular evaluation. Eur J Med Chem 45:5653–5661. doi:10.1016/j.ejmech.2010.09.019
Karthikeyan SV, Bala BD, Raja VPA, Perumal S, Yogeeswari P, Sriram D (2010) A highly atom economic, chemo-, regio- and stereoselective synthesis and evaluation of spiro-pyrrolothiazoles as antitubercular agents. Bioorg Med Chem Lett 20:350–353. doi:10.1016/j.bmcl.2009.10.107
Basnet A, Thapa P, Karki R, Na Y, Jahng Y, Jeong BS, Jeong TC, Lee CS, Lee ES (2007) 2,4,6-Trisubstituted pyridines: synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship. Bioorg Med Chem 15:4351–4359. doi:10.1016/j.bmc.2007.04.047
Nepali K, Singh G, Turan A, Agarwal A, Sapra S, Kumar R, Banerjee UC, Verma PK, Satti NK, Gupta MK, Suri OP, Dhar KL (2011) A rational approach for the design and synthesis of 1-acetyl-3,5-diaryl-4,5-dihydro(1\(H\))pyrazoles as a new class of potential non-purine xanthine oxidase inhibitors. Bioorg Med Chem 19:1950–1958. doi:10.1016/j.bmc.2011.01.058
Ouyang L, Huang YH, Zhao YW, He G, Xie YM, Liu J, He J, Liu B, Wei YQ (2012) Preparation, antibacterial evaluation and preliminary structure-activity relationship (SAR) study of benzothiazol- and benzoxazol-2-amine derivatives. Bioorg Med Chem Lett 22:3044–3049. doi:10.1016/j.bmcl.2012.03.079
Singh P, Verma P, Yadav B, Komath SS (2011) Synthesis and evaluation of indole-based new scaffolds for antimicrobial activities-identification of promising candidates. Bioorg Med Chem Lett 21:3367–3372. doi:10.1016/j.bmcl.2011.04.001
Singh N, Pandey SK, Anand N, Dwivedi R, Singh S, Sinha SK, Chaturvedi V, Jaiswal N, Srivastava AK, Shah P, Siddiqui MI, Tripathi RP (2011) Synthesis, molecular modeling and bio-evaluation of cycloalkyl fused 2-aminopyrimidines as antitubercular and antidiabetic agents. Bioorg Med Chem Lett 21:4404–4408. doi:10.1016/j.bmcl.2011.06.040
Discovery Studio, Version 3.1; Accelrys: San Diego, CA, 2011
Acknowledgments
We wish to thank Professor Xiaokang Liu and Dr. Xiaoli Ji (Sichuan University) for providing an assessment of pharmacological test for all the compounds in this program. We also thank Dr. Jianyou Shi (People’s Hospital of Sichuan province), Dr. Zhihua Mao (Sichuan University) and Professor Jie Li (Zhejiang University) for good suggestions on the NMR and X-ray structure analysis. Financial support from National Natural Science Foundation of China (Nos.81102325, 81001357 and 81273471) and China Postdoctoral Science Foundation (No. 2012T50781) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, G., Ouyang, L., Liu, J. et al. Synthesis of novel spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles via a regioselective three-component [3+2] cycloaddition and their preliminary antimicrobial evaluation. Mol Divers 17, 271–283 (2013). https://doi.org/10.1007/s11030-013-9432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9432-3