Abstract
The CO2 emission rates have been continuously incremented during the last decades. To mitigate it, a method to store carbon in terrestrial ecosystems is the addition of biochar to soil. After its application to soil, biochar suffers an ageing process, able to deteriorate its functional properties as soil improver. However, at present, it is not clear how to evaluate biochar ageing. The main aim of this study is to evaluate biochar ageing by determination of temporal changes on (a) soil respiration after biochar addition and (b) the relationship between CO2 adsorption capacity and wettability of biochar as measurable parameters indicating biochar ageing. Results show that 1 month after biochar addition, soil respiration decreased when poplar and pine biochars were applied to bare soils, in the absence of vegetation. One year after biochar addition, this reduction on soil respiration disappeared, evidencing biochar ageing due to decrements on its CO2 adsorption capacity. Compared with fresh biochar, decreased CO2 adsorption capacity of biochar corresponded with enhanced biochar wettability for both biochar types. Its means that poplar and pine biochars, while initially hydrophobic, became hydrophilic after 1 year of its application to soil. It is concluded that changes of biochar CO2 adsorption capacity in time go along with improved wettability as mutually opposed processes. Globally, pine biochar tends to adsorb a higher quantity of CO2 than poplar biochar. The absence of CO2 adsorption of soil without biochar demonstrates the remarkable capacity of both biochars to adsorb carbon dioxide and promote carbon storage in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, scientific and public interest and awareness about climate change and its effects on human life, ecosystem services and biodiversity in general is increasing. In December 2015, the Paris Agreement (194 countries and EU) was signed to enforce the global transformation to a low-carbon and climate-resilient society (Olivier et al. 2015). However, in 2017, more than 60 scientists stated that we had less than 3 years to safeguard our climate because, if global CO2 emission rates continue to rise beyond 2020 or even remain on the present level, the temperature goals set in Paris will become almost unattainable (Figueres et al. 2017). Unfortunately, the forecasts have worsened because recently highest CO2 emission rates were registered, increasing the CO2 level from approximately 280 ppm before the industrial revolution period to a maximum of CO2 concentration of 415 ppm in June 2021 (Zeebe et al. 2016; Tans and Keeling 2021). It is hypothesised that anthropogenic emissions of CO2 are the main driver to climate change (Friedlingstein et al. 2010) and its concentration in the atmosphere may increase summer temperatures, heat stress and precipitation extremes in many climate zones (Baker et al. 2018). High CO2 levels are also bringing other unexpected consequences around the world, such as the impoverishment of human nutrition because crop growth under elevated CO2 level decrease plant protein, iron and zinc concentrations (Smith and Myers 2018). Without any doubt, atmospheric CO2 emission levels are one of the major concerns today.
Soil respiration, as important source for CO2 emissions from soils, is composed by autotrophic respiration from plant roots and associated microorganisms and by heterotrophic respiration via microbial decomposition of soil organic matter (SOM) (Vargas et al 2011). Soil CO2 emissions are regulated by properties such as soil-water contents, local soil-water availability, soil pore size, pH and nutrient status and local meteorological factors such as air temperature, humidity and wind speed, which influence the movement of CO2 through and out of soil (Orchard and Cook 1983; Raich 1992). There are serious indications that the global increase in temperature is promoting a greater emission of CO2 from the soils, due to enhanced SOC decomposition in temperate and tropical soils (Bond-Lamberty and Thomson 2010). Consequently, CO2 capture and storage are crucial to combat excessive atmospheric CO2 concentrations (Alcalde et al. 2018). Lal (2008) indicated that a strategy to reduce global CO2 emission rates is C capturing from atmosphere through natural and engineering techniques such as biochar (stable or low degradable carbon obtained from pyrolysed biomass) addition to soil. This strategy to deploy biochar on a large scale would divert a portion of global carbon flux as biomass residues stored in soil lead finally to less carbon emissions back to the atmosphere (Wang et al. 2023). The use of biochar as soil amendment successfully incremented carbon storage and improved soil quality and fertility of different soil types, in different regions and climates (Bolan et al. 2022). Biochar could help to accumulate additional carbon into soils, since its use promotes increments on vegetal biomass (Yu et al. 2019). On the other hand, biochar is considered a material able to increase soil-water contents in soils (Adhikari et al. 2022) and physically and chemically adsorb greenhouse gases (Thomazini et al. 2015). Unfortunately, biochar could contain combustion-driven toxic organic compounds such as polycyclic aromatic hydrocarbons (PAHs), chlorinated hydrocarbons or dioxins (Kookana et al. 2011). Specifically, the release of PAHs resulting from cellulose pyrolysis is a potential drawback regarding biochar usage (Ojeda et al. 2016) because PAHs are considered toxic for organisms due to their carcinogenic and mutagenic properties (Wang et al. 2017). Although biochar was successfully used to reduce CO2 emissions from SOM decomposition in soils (Li et al. 2018), in some cases, its application either promoted (Smith et al. 2010) or had no influence on soil respiration (Liu et al. 2016). In conclusion, it is important to quantify advantages and disadvantages of biochar use as organic amendment under consideration of soil CO2 emissions, based on the hypothesis that biochar application to soil could produce both: increase or decrease in soil respiration which determines the long-term soil carbon budget.
However, biochar ageing effects (Mia et al. 2017) after application to soil are currently ignored, especially the consideration of time-dependent alteration effects of physical and chemical soil properties. Biochar ageing processes could be related to changes on its elemental composition, hydrophilicity and amphotericity (Cheng and Lehmann 2009), caused by biotic (microbial activity) or abiotic (chemisorption of oxygen) oxidation (Cheng et al. 2006). After oxidation, surface hydrophilicity potentially increases on aged biochar surfaces (Joseph et al 2010). More specifically, fresh biochar, initially hydrophobic before its application to soil, could become a highly hydrophilic material (Ojeda et al 2015). Since gas adsorption and liquid adsorption are specific characteristics of porous materials (Shafawi et al. 2021), it is meaningful to establish a relationship between carbon and water adsorption for biochar-enriched soil. However, studies about the biochar ageing process and its impact on soil carbon sequestration dynamics are scarce. Hence, the main objectives of this study were (i) to determine the biochar ageing effects on soil CO2 emissions by relating soil properties to heterotrophic respiration intensity and (ii) to evaluate the biochar ageing by analysing the relationship between carbon sequestration capacity vs. biochar-water contact angles. Data from Ojeda et al. (2015) and Marks et al. (2014a, 2014b), which are referred to the same experiment, were taken into account to support the observed results in soil respiration and CO2 adsorption capacity in soils amended with slow-pyrolysed biochars.
2 Materials and methods
2.1 Site description
The experiment was developed in the greenhouses of the Autonomous University of Barcelona (Cerdanyola del Vallès, Spain) (Ojeda et al. 2015; Marks et al. 2014a). The soil samples were collected from the topsoil of a Fluventic Haploxerept (Soil Survey Staff 2010). The sampling site was located in the experimental fields of the Institute of Agro-Food Research and Technology (IRTA) (Marimón Tower, Caldes de Montbui, Catalonia, Spain), and after collection, soil was air-dried for 1 week and sieved < 5 mm (Ojeda et al. 2015). Table 1 shows the main soil physical and chemical properties.
2.2 Biochar characterisation
The soil was amended with two types of biochar, obtained from poplar and pine wood splinters subjected to slow pyrolysis technique. Physical and chemical data are presented in Table 2 (Ojeda et al. 2015, Marks et al. 2014b). With respect to biochar manufacturing, slow pyrolysis of poplar and pine wood splinters was conducted in a low oxygen chamber at 500–550°C for 15 min. Production was conducted at the laboratories of the Chemical and Environmental Engineering Group of the University of León (León, Spain) (Ojeda et al. 2015). A general characterisation of both biochar types is given in Table 2 (data from Ojeda et al. 2015; Marks et al. 2014b).
2.3 Experimental setup
The soil treatments tested were as follows: C: control, i.e. soil without biochar; PoS: soil + slow-pyrolysed poplar wood; and PiS: soil + slow-pyrolysed pine wood. The biochar doses applied were as follows: 11.6 g of slow-pyrolysed poplar by kg of soil and 10.9 g of slow-pyrolysed pine per kg of soil were added at the beginning of the experiment. These biochar doses were equivalent to 1% of C, mixing 10 g C of biochar per 1000 g of dry soil. Twelve plastic containers (4 replicates by treatment, 12 samples by sampling time) of 2 L were prepared. The experiment included two sampling dates to evaluate biochar and soil properties: 1 month (S1) and 1 year (S2) after biochar addition to soil. In order to avoid carbon inputs from vegetation, soil respiration was analysed without vegetation cover, simulating fallow scenarios. At each container, soil and a gravel layer at the bottom to facilitate drainage were placed. Soil treatments (with or without slow-pyrolysed biochar) were mechanically homogenised by cement mixer. The material was then placed into the plastic containers, with a layer of gravel at the bottom to facilitate drainage. All plastic containers were placed in a plastic semi-cylindrical walk-in tunnel, partly open (without plastic) laterally during the experiment. All soil samples were irrigated every 3 days (spring, summer) or weekly (autumn, winter) during a year, with a constant water amount equal to 50% of field capacity (FC) (see next section). At each sampling time (destructive sampling), soil samples were air-dried at 25°C and 50% relative air humidity, sieved at 5 mm and stored at 4°C in the dark, before being analysed for soil respiration measurements. From these samples, biochar particles were removed manually, one by one, carefully, using metal tweezers until obtain 0.1 g (in agree with the sample volume required for CO2 adsorption analysis), 1 month and 1 year after biochar application to soil, in order to analyse changes in biochar wettability and CO2 adsorption capacity. Weekly, the growing vegetation was manually removed during the entire experimental period (for more details, see Ojeda et al. (2015)).
2.4 Soil-water retention and soil CO2 emissions or soil respiration
Soil-water contents at field capacity of subsamples with and without biochar were estimated gravimetrically weighing the PVC soil cores (height: 3.4 cm, inner diameter: 1.7 cm) using an electronic balance (0.001 g precision). After 24 h of soil saturation, followed by 24 h of free drainage, in a room at 20°C, the drained soil cores were placed over a sand box (Eijkelkamp®) at a soil suction of − 0.03 MPa to establish field capacity conditions. Soil core weight was recorded until equilibrium (usually during 5 days) (Ojeda et al. 2015). With respect to soil respiration, soil CO2 emissions of fresh soil subsamples (with and without biochar) taken from containers were adjusted to a soil-water content equal to 50% field capacity and incubated at 20°C during 5 days. The measurement of carbon mineralised to CO2, trapped in 1 M NaOH, was performed by titration against 0.5 M HCl (Anderson 1982). After incubation, the CO2 trapped in NaOH were compared to those observed in containers without soil (blanks). All sodium hydroxide lost during the sample incubation time was finally attributed to the CO2 emitted from soil samples during soil organic matter (SOM) decomposition.
2.5 CO2 adsorption in soils and biochar
The CO2 adsorption capacity of soil aggregates (< 2mm, 2g) and particles of fresh biochar (not applied to soil, categorised as S0, < 2mm, 0.1g) and particles of aged biochar particles (removed from soil subsamples manually after 1 month (S1) and 1 year (S2) after its application, < 2mm, 0.1g) was evaluated by an in-house built volumetric Sievert system (Silva et al. 2013). Two volumes—reference volume (27.2 cm3) and sample chamber volume (3.2 cm3)—were measured with high precision before the CO2 adsorption measurements. Initially, the sample chamber was evacuated, and the reference volume was filled with a defined amount of CO2. Then, a valve was opened to let the gas expand to the combined volume of the two chambers. The final pressure value after its stabilisation (10 min, approximately), under constant temperature (20°C), was recorded. Consequently, the present experiment evaluates the CO2 adsorption capacity of biochar during 10 min. The difference between the initial and final volume and pressure values defined the variation of the number of moles adsorbed by porous materials (soil, biochar), calculated using Benedict-Webb-Rubin (BWR) equation of state by the software GS2013 (Domingos 2013). Six successive expansions gave the total quantity of gas adsorbed at each equilibrium, obtaining a curve between 0 and 5 bars calculated from a CO2 adsorption rates vs. pressure values. This procedure included calibration corrections of the pressure transducer. At each sample, the CO2 adsorption capacity at atmospheric pressure was estimated from plots of CO2 adsorption vs. pressure values. All the CO2 adsorption experiments carried out were preceded by the determination of the void volume of the respective sample chambers containing the soil or biochar samples, respectively, by using helium gas as testing agent.
2.6 Biochar wettability
To assess the ageing process of biochar particles applied to soil, biochar-water contact angles were determined by the sessile drop method to indicate modifications in wettability. An optical contact angle measuring and contour analysis system (OCA 15, DataPhysics, Filderstadt, Germany) was used to determine soil-water contact angles on crushed and uncrushed biochar particles, previously removed from soil subsamples. A double-sided adhesive tape was fixed to a flexible tissue, and then, biochar particles (< 2 mm) were adhered, obtaining a homogeneous grain cover on the tape surface. After the tissue with biochar particles was placed under a syringe with distilled water (fixed vertically at OCA 15), a drop of 1 µg of water was placed on the surface of several biochar particles and the formation of the water drop contour line was recorded with a video camera, by a CCD-equipped contact angle microscope (OCA 15, DataPhysics, Filderstadt, Germany). The direct measurement of contact angles at the solid-liquid interphase as provided by the sessile drop method could be considered the best option to evaluate biochar wettability and respective modifications compared to other tests such as the indirect capillary rise method (Bachmann et al. 2003), where contact angles are estimated by an equation that compares the adsorption of two different liquids (e.g. water and hexane).
The mean solid-liquid contact angle (CA) value measured between biochar surface and the water drop was calculated after 30 ms, after drop placement by the SCA 20 programme (DataPhysics, Filderstadt, Germany) (Bachmann et al. 2013). The measurements were taken with fresh biochar particles (initial condition (S0)—not exposed to field conditions) and with biochar particles exposed to environmental conditions, after 1 month (S1) and after 1 year of its application (S2) A general description of biochar granulometry is presented in Table 2 (data from Ojeda et al. 2015).
2.7 Soil organic carbon
Total organic carbon (TOC) contents were estimated by a dichromate acid oxidation at 150°C in strong acid media (Nelson and Sommers 1982) in soil fraction < 2 mm.
2.8 Polycyclic aromatic hydrocarbons (PAHs)
The concentration of 13 different types of PAHs were determined in pine and poplar slow-pyrolysed biochars, after its extraction and purification by gas chromatography (Agilent GC system 7890A, Paris, France) coupled with a mass spectrometer (Agilent 5975C inert XL MSD, Paris, France) (Gateuille et al. 2014): naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a,h)anthracene, indeno(c,d)pyrene and benzo(g,h,i)perylene. Approximately 1 g of biochar was extracted by ASE 350TM (Thermo Scientific). The extraction consisted of 3 cycles of 5 min at 100°C with a dichloromethane: acetone mixture (50/50), followed by 3 cycles of 5 min at 100°C with a hexane/acetone mixture (50/50). The two extracts were then combined and concentrated by an EZ-2 evaporator, a rotary evaporator, or under nitrogen flow. The extracts were purified with copper (one night) and then on a Florisil Superclean column (MgO-SiOH) with two mixtures of solvents (hexane/dichloromethane 50/50 and hexane/acetone 50/50) according to the method developed by Sánchez-Avila et al. (2011). After a final concentration step, the samples were analysed by gas chromatography coupled with double mass spectrometry (GC/MS-MS) (Alliot et al. 2014).
2.9 Statistical analysis
To evaluate the significance of temporal changes on soil and biochar properties, a two-way ANOVA was applied among treatments (C: control, PoS: slow-pyrolysed poplar, PiS: slow-pyrolysed pine) for each sampling time (S0: fresh biochar not applied to soil, S1: after 1 month, and S2: after 1 year of biochar application to soil). When soil and biochar properties showed significant interaction among treatments and sampling time, a one-way ANOVA was conducted at each sampling, followed by Tukey’s post hoc tests to permit pairwise comparisons of means (p < 0.05). When data normality (Shapiro-Wilk test) or the equality of error variances (Levene test) was not confirmed per dataset, nonparametric test (Kruskal-Wallis test) were used, followed by a Dunn test to determine significant differences between treatments or sampling times. In terms of simple linear regressions, its statistical validity was verified by several tests available from R software42: (a) ANOVA of each model tested, (b) a mean of residues close to zero, (c) normality of unstandardised residues values (p > 0.05) by Shapiro-Wilk test, (d) the existence of potential outliers by Cook’s distance higher than 1 and (e) homoscedasticity by studentised Breusch-Pagan test. For all tests required to compare treatment-time interaction and to obtain simple regressions, the R software was used (R Core Team 2013).
3 Results
3.1 Soil CO2 emissions
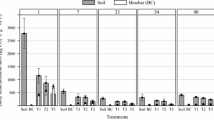
In terms of CO2 emitted during SOM decomposition (soil respiration (SR)), a significant interaction among treatments and sampling times was observed (Table 3). The SR values were reduced by slow-pyrolysed poplar and pine compared to the control treatment without biochar (Fig. 1a) by 11.1% and 13.4% respectively, 1 month after the starting of the experiment (S1). In contrast, the capacity of biochar to reduce CO2 emissions from soils disappeared 1 year after its addition to soils (S2) (Fig. 1a). Globally, regardless of the treatment type, the SR values decreased between sampling times S1 and S2 by 18.9%. In addition, SR values at S1 were higher than those observed at S2 in control and PoS treatments, while in the case of PiS treatment, SR values at S2 were higher than those observed at S1 (Fig. 1a).
Mean values of a soil respiration (SR) rates, b CO2 adsorption capacity (CO2 ads) and c total organic carbon (TOC). C: control treatment without biochar, PiS: slow-pyrolysed pine biochar, PoS: slow-pyrolysed poplar biochar. S1: sampling 1 month after the start of the experiment. S2: sampling 1 year after the start of the experiment. Different lowercase letters mean significant differences between treatments (p < 0.05)
3.2 Soil and biochar CO2adsorption capacity
Significant differences of CO2 adsorption among soil samples and poplar and pine biochar particles were observed (Table 3). Pieces of slow-pyrolysed pine and slow-pyrolysed poplar showed a remarkably high CO2 adsorption capacity, in comparison with the low CO2 desorption rates (negative values) observed in soil without biochar, i.e. 73.8- and 91.1-fold the soil desorption rate, respectively (Fig. 1b). In addition, irrespective of the evaluated material (soil, biochars), the CO2 adsorption capacities were higher at S1 than those at S2 (Fig. 1b).
3.3 Soil organic carbon
The application of slow-pyrolysed biochars made it possible to permanently modify soil carbon stock (Fig. 1c, data from Ojeda et al. (2015)) over the entire length of the experiment, as shown by the absence of significant interaction among treatments and sampling times (Table 3). The addition of biochar to soils increased the total organic carbon (TOC) contents around 32.4% by slow-pyrolysed poplar and around 29.1% by slow-pyrolysed pine. In total, without taking into account the type of treatment, TOC values at S1 were higher than those at S2 (Fig. 1c).
3.4 Biochar ageing influence on CO2adsorption and wettability
Comparing biochar particles before and after its application to soil, it was possible to observe that biochar capacity to adsorb CO2 (CO2 ads-b) changed significantly with time, with a significant interaction between treatments and sampling times (Table 3). The CO2 ads-b values of slow-pyrolysed poplar decreased 34.1% after 1 year (S2) of application to soil with respect to biochar particles which were not applied to soils (S0) as shown by Fig. 2a. Similarly, the CO2 ads-b values of slow-pyrolysed pine decreased by 61.9% 1 year (S2) after application regarding biochar particles that were not added to soil (S0). In contrast, the CO2 adsorption capacity of biochars (CO2ads-b) was higher in slow-pyrolysed pine than that in slow-pyrolysed poplar biochar particles (Fig. 2a), at S0 (28.5%) and at S1 (38.3%) (Fig. 2a). Interestingly, at sampling time S2, these differences of CO2ads-b values between biochar types disappeared (Fig. 2a). In summary, irrespective of the treatment method, CO2 adsorption capacity of slow-pyrolysed pine biochar was higher than that in slow-pyrolysed poplar biochar, and CO2 adsorption capacity at S0 and S1 was higher than that observed at S2 (Fig. 2a).
Mean values of a CO2 adsorption capacity of biochars (CO2 ads-b), b contact angles of crushed biochar (CAc) and c contact angles of uncrushed biochar (CAuc). PiS: slow-pyrolysed pine biochar, PoS: slow-pyrolysed poplar biochar. S0: initial sampling (biochar before its application to soil). S1: sampling 1 month after the start of the experiment. S2: sampling 1 year after the start of the experiment. Different lowercase letters mean significant differences between treatments, while different capital letters mean significant differences between sampling times (p < 0.05)
On the other hand, the internal (crushed biochar particles) wettability and superficial (uncrushed biochar particles) wettability of poplar and pine biochars were evaluated by the measurement of biochar-water contact angles on crushed (CAc) and uncrushed (CAuc) biochar particles (Fig. 2b, c, data from Ojeda et al. 2015). Globally, regardless of the sampling times, two observations were made: on one hand, the mean CAc values of slow-pyrolysed poplar biochar were higher than those observed at slow-pyrolysed pine biochar, 8.4% (Fig. 2b). On the other hand, the mean CAc values at samplings S0 and S1 were 32.8% higher than those observed at sampling S2 1 year after biochar application to soil (Fig. 2b).
Finally, superficial wettability of biochar particles was evaluated by the measurement of biochar-water contact angles on uncrushed biochar particles (CAuc) (Fig. 2c, data from Ojeda et al. (2015)). Without taking into account the sampling times, CAuc values of both biochars were similar (Fig. 2c). However, examining the temporal variation of superficial wettability of biochars, it was observed that CAuc values at samplings S0 and S1 were higher than those observed at S2, 76.3% (Fig. 2c).
3.5 Interaction between respiration and CO2adsorption capacity of biochars
In terms of the relationship between soil respiration rates and CO2 adsorption capacity of the biochar particles, it was observed that increased CO2 adsorption capacity of biochar corresponded to decreased soil respiration rates, 1 month after biochar application to soil (S1) (Fig. 3a). In contrast, no significant relationship between soil respiration rates and CO2 adsorption capacity of biochars was observed, 1 year after biochar application to soil (S2) (Fig. 3b).
Biochar wettability was now related to changes in the CO2 adsorption capacity of biochars as a function of time; i.e. two relationships were evaluated (Fig. 4): (a) CO2ads vs. CAc and (b) CO2ads vs. CAuc. It was observed that increased biochar-water contact angles (CAc and CAuc), equivalent to reduced biochar wettability, were related to increased CO2 adsorption capacity of slow-pyrolysed poplar and also for pine biochars (Fig. 4a, b). Both relationships explained approximately a 30% of observed variability.
3.6 Polycyclic aromatic hydrocarbon (PAH) and metal contents in biochars
In terms of to the contaminants associated with biochars (EBC 2023), the slow-pyrolysed poplar and pine biochars may have the potential to elevate PAHs above permissible levels (Table 4). In terms of metal contents, slow-pyrolysed poplar and pine biochars presented Cr, Cu and Ni contents higher than the permissible limit, while Pb and Zn contents were lower than the mentioned limits (Table 4).
4 Discussion
4.1 Biochar effects on soil respiration
In general, soil respiration or CO2 emissions from soils occur when environmental conditions are able to promote SOM decomposition by microbial activity (Wang et al 2014). During this process, soil organic matter is partially transformed to CO2, which is released to the atmosphere reaching around 30 Pg C yr−1 of SOM-derived CO2 from tropical forest soils (Nottingham et al. 2022). The addition of biochar to soil is a practice addressed to replace easily-mineralised organic matter or fertilisers with a material able to improve soil fertility and resistant to the decomposition processes promoted by microbial activity (Tang et al. 2022), promoting carbon storage in soils and helping to mitigate climate change (Keith et al. 2015). The viability of this idea rose from the discovery of Terra Preta soils in Brazil (Sombroek 1966), where patches of high fertile soils were found surrounded by unfertile soils (Lal 2016), suggesting that the addition of stable carbon to soil improves significantly soil quality (Lorenz and Lal 2014). The benefits of biochar used as soil amendment include (a) increasing the number of beneficial bacteria on soil contaminated by microplastics (Ran et al. 2023), (b) decreasing cadmium contamination in crops (Wang et al. 2024), (c) improvement of crop adaptation to ambient conditions such as nutrient deficiency, aridity and water stress conditions (Zhang et al. 2024) and (d) reductions of pesticide residues (Sarker et al. 2023). With respect to the soil respiration (SR) rates observed in soil with and without biochar, two clear processes were observed: (a) a reduction of CO2 emissions from soil incubated at 50% of field capacity after 1 month of biochar addition (Fig. 1a) and (b) the disappearance of this effect of slow-pyrolysed poplar and pine biochars on soil respiration, after 1 year of biochar addition to soil (Fig. 1a). In the absence of a cover vegetation, a similar scenario to fallow lands without carbon inputs from plants, these consecutive events indicate that the effect of biochar on soil microbial activity is temporal, not permanent. The reduction of SR rates, in terms of soil microbial activity, could be due to (a) SOM encapsulation on biochar pores, physically protecting SOM to access of microbial activity (Zimmerman et al. 2011), (b) biochar-induced increments on soil-water storage capacity (Wong et al. 2022), able to decrease oxygen availability on soils (Or et al. 2007), (c) reduction in SOM decomposition due to an inhibition of carbohydrate catabolism by increments in bacterial and fungal diversity promoted after biochar application (Chen et al. 2019), (d) toxic effects of the biomass combustion products contained in biochars, such as polycyclic aromatic hydrocarbon (PAH) (Godlewska et al. 2021), and (e) physical adsorption of CO2 by biochar (Zhang et al. 2019) (Figs. 1b, 2a, and 3a). In addition, decrements in SR values after 1 year of continuous addition of water in soils without biochar and in soils with slow-pyrolysed poplar biochar probably indicated that the quantity of easily decomposable SOM was depleted after 1 year (Fig. 1a), in the absence of carbon inputs from vegetation cover (Fig. 1c).
As a consequence, the temporal influence of biochar on the reduction of SR rates (Fig. 1a) could suggest that it is necessary to repeatedly add biochar to soil to maintain the observed initial benefits of biochar application, i.e. in terms of the adsorption of CO2 emitted from soils. The CO2 adsorption capacity of biochar disappeared after 1 year of biochar application to soil (Fig. 3) probably due to biotic or abiotic oxidation processes able to transform biochar initially water-repellent biochar into hydrophilic biochar (Zimmerman 2010), especially on its surface (Fig. 4). However, it is also necessary to take into account that PAHs contained in slow-pyrolysed poplar and pine biochars (Table 4) are part of the 16 PAHs from the US EPA priority pollutant list (EBC 2023). Then, repeated applications of biochar to soil could include the risk of accumulating PAHs or other contaminants over advisable limits (Table 4) for human health (Wang et al. 2019), since biochar is able to adsorb and store a wide range of contaminants (e.g. PAHs and metals) (Abbas et al. 2018), with undesirable consequences. Specifically, PAH contents of both biochars could reduce soil microbial activity due to high contents of naphthalene (Table 4), considered the most abundant PAH in biochars, which may have the potential to reduce nitrogen transformations in soils amended with biochar (Chang et al. 2002), although its residence time on soil could be low due its volatility. Odinga et al. (2021) made several recommendations to ensure a safe application of biochar to soils, including (a) analytical studies of PAHs, polychlorinated biphenyls (PCBs), volatile organic compounds (VOCs), environmentally persistent free radicals (EPFRs) and metal contents, (b) optimal manufacturing temperatures to obtain biochars, able to avoid or reduce the precursor substances in biochars, and (c) ecotoxicological evaluation of biochar doses applied to soils. However, the technical challenge to obtain biochar with these quality requirements is still a goal worth to be achieved.
4.2 Biochar ageing effects on CO2 adsorption andwettability
Biochar ageing could be caused by interactions with soil mineral colloids (Ren et al. 2018), soil wetting-drying cycles, temperature changes and soil biological activity, whereby the latter is considered the most important factor in comparison to abiotic processes (Quan et al. 2020). In this study, biochar wettability and CO2 adsorption capacity were selected as analytical and easily accessible key properties to evaluate biochar surface ageing. A powerful tool to evaluate changes of wettability in solid and porous materials is the measurement of contact angles at the solid-liquid interphase (Woche et al. 2017). When biochar is applied to soil, probably a part of CO2 emissions produced by SOM decomposition can be adsorbed by biochar (Figs. 1a, b and 2a). The remarkable CO2 adsorption capacity of slow-pyrolysed biochars compared to CO2 desorption rates observed in soil (Fig. 1b) could be caused by (i) biochar aromaticity as observed by Marks et al. (2014b) on the same biochars used in this study, able to increase the van der Waals forces during biochar matrix and CO2 contact (Igalavithana et al. 2020), and (ii) high degree of hydrophobicity because water repellency pushes the competition between water and gas adsorption towards the gas phase and may also improve accessibility of small biochar pores for CO2 adsorption (Guo et al. 2022) (Figs. 2b, c and 4a, b). The main evidence that slow-pyrolysed biochars could suffer an ageing process was reflected by the fact that biochars increased its wettability, favouring water adsorption before CO2 in time (Fig. 2b, c). In contrast, the CO2 adsorption capacity of biochars decreased continuously with time (Figs. 2a and 4a, 4b), probably due to oxidation or leaching processes of biochar particles (Thomas 2021). Another possibility is that internal pores in biochar particles can be blocked at the particle surface through biofilm formation, hence reducing overall the capacity for physical and chemical adsorption of CO2 molecules (Amer et al. 2022).
To standardise the function of biochar in soil, categorisation of biochar wettability could follow the same standards as for soil; i.e. (i) a solid-liquid contact angles higher than 90° indicate hydrophobic material, (ii) solid-liquid contact angles less than 90° are indicators of still wettable but slightly to moderately water-repellent material and (iii) solid-liquid contact angles equal to zero indicate completely wettable material, as was observed for soil particles. Taking 90° as limit that differentiates hydrophobic materials from wettable materials, it was observed that the oxidation process of our biochars is more intensive on surfaces than inside the biochar particles (Fig. 2b, c). This is clear because the reduction of biochar-water contact angles was greater in uncrushed than in crushed biochar particles (Fig. 2b, c). This increment in biochar wettability could be due to (i) oxidation processes promoted by low molecular weight organic acids (LMWOAs) derived from microbial secretions and organic matter decomposition (Sun et al. 2016), (ii) the loss of organic surface coating of fresh biochar during leaching, exposing underlying micropores (He et al. 2019), and (iii) changes in bacterial cell surface properties (Karagulyan et al. 2022). In this study, around 30% of reduction of biochar CO2 adsorption capacity was explained by reduction in biochar-water contact angles (Fig. 4). It is possible that slow-pyrolysed biochars that initially promote CO2 adsorption turn after their transformation under ambient soil conditions to the hydrophilic state (Fig. 2b, c) which goes along with a reduction of physically adsorbed gaseous CO2. In addition, pore clogging due to clay contents and/or calcium carbonate leaching in soils with high carbonate contents (Table 1) could be another reason to explain the reductions on CO2 adsorption capacity of biochar in time (Sun et al. 2016).
As an outcome of the present investigation, the recent technological advances, addressed to improve biochar properties such as biochar activation (Sakhiya et al. 2020) or artificial pre-oxidation or post-oxidation methods (Nidheesh et al. 2021), need to take into account that probably CO2 adsorption and water storage in biochars change with time after application which should be subject of further investigations.
4.3 Limitations and recommendations
The present study was generally successful to demonstrate if and to what extend biochar ageing is able to reduce its capacity to adsorb CO2 in conjunction with increased wettability. However, some experimental limitations were observed during this study which could be improved: (a) CO2 adsorption rates on soil + biochar mixtures were not measured on the long term, (b) the manual removal of biochar is not efficient to remove the finest biochar particles and therefore one biochar fraction remains in soil not accessible for direct analysis, (c) the CO2 adsorption process was evaluated under limited conditions, i.e. during 10 min in a closed chamber, and (d) the measurement of soil organic carbon by a dichromate acid oxidation probably is not the best method to quantify biochar carbon because it could underestimate some of its components (Hammes et al. 2007). This was eventually observed in Fig. 1c, where the increment of 1% of soil carbon due to biochar addition was not reflected by this method. To overcome these limitations, it is necessary to evaluate CO2 adsorption rates on soils amended with biochar, using higher volumes of sample, at best also under field conditions for comparison. To improve the determination of biochar carbon on soils, the measurement of total carbon by combustion methods is recommended. With respect to the future use of biochar amendment, it is necessary to analyse the biochar CO2 adsorption capacity of different types of biochar considering the production mode as well as the origin material for biochar production. Finally, it is recommended to find a sustainable biochar dose, taking into account not only its potential benefits for carbon storage in soils but also its ecotoxicological effects on soil biota, evaluated by standard methods for micro-, meso- and microorganisms.
5 Conclusions
Biochar ageing is an important challenge in terms of the use of biochar as organic amendment. The measurement of CO2 adsorption capacity and wettability in biochar particles could be considered useful to evaluate biochar ageing. The observed relationship between biochar contact angles and CO2 adsorption capacity could explain partially the role of temporal changes of biochar wettability on soil organic matter decomposition process and, consequently, on the persistence of carbon storage in the soil. In general, slow-pyrolysed biochars could be considered an interesting aspect to mitigate greenhouse gas emissions, at least temporarily, due to an unexpected great capacity to adsorb CO2, at short term in soil. The future improvements on biochar technology could be addressed to obtain biochars that are more resistant to natural oxidation processes, in terms of preserving its CO2 adsorption capacity and its impact on reduced soil respiration along with low contents of contaminants produced during biomass pyrolysis. It is also very important to determine what percentage of the yearly soil respiration rate can be adsorbed by biochar and for how long this material could maintain its maximum adsorption capacity, i.e. to keep CO2 in soil for further transformation processes such as carbonate formation (Guo et al. 2022), promoting long-term stability of CO2 adsorption process at large scale. On the other hand, it is recommended to determine if hydrophobicity has further positive attributes or if negative effects on carbon storage in soils occur with time.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abbas Z, Ali S, Rizwan M, Zaheer I, Malik A, Riaz M, Shahid M, Rehman M, Al-Wabel M (2018) A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab J Geonsci 11:448. https://doi.org/10.1007/s12517-018-3790-1
Adhikari S, Timms W, Parvez MA (2022) Optimising water holding capacity and hydrophobicity of biochar for soil amendment – a review. Sci Total Environ 851(1):158043. https://doi.org/10.1016/j.scitotenv.2022.158043
Alcalde J, Flude S, Wilkinson M, Johnson G, Edlmann K, Bond C, Scott V, Gilfillan SMV, Ogaya X, Haszeldine RS (2018) Estimating geological CO2 storage security to deliver on climate mitigation. Nat Commun 9:2201. https://doi.org/10.1038/s41467-018-04423-1
Alliot F, Moreau-Guigon E, Bourges C, Desportes A, Teil M, Blanchard M, Chevreuil M (2014) A multi-residue method for characterization of endocrine disruptors in gaseous and particulate phases of ambient air. Atmos Environ 92:1–8. https://doi.org/10.1016/j.atmosenv.2014.02.044
Amer N, Lahijani P, Mohammadi M, Mohamed A (2022) Modification of biomass-derived biochar: a practical approach towards development of sustainable CO2 adsorbent. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02905-3
Anderson JPE (1982) Soil respiration. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. American Society of Agronomy Inc, Soil Sci Soc Am Inc, Madison, WI, USA, pp 831–902
Bachmann J, Woche SK, Goebel MO (2013) Small-scale contact angle mapping on undisturbed soil surfaces. J Hydrol Hydromech 61:3–8. https://doi.org/10.2478/johh-2013-0002
Bachmann J, Woche SK, Goebel MO, Kirkham MB, Horton R (2003) Extended methodology for determining wetting properties of porous media. Water Resour Res 39:1353–1366. https://doi.org/10.1029/2003WR002143
Baker H, Millar RJ, Karoly DJ, Beyerle U, Guillod BP, Mitchell D, Shiogama H, Sparrow S, Woollings T, Allen MR (2018) Higher CO2 concentrations increase extreme event risk in a 1.5 °C world. Nat Clim Change 8:604–608. https://doi.org/10.1038/s41558-018-0190-1
Bolan N, Hoang S, Beiyuan J, Gupta S, Hou D, Karakoti A, Joseph S, Jung S, Kim K-H, Kirkham M, Kua H, Kumar M, Kwon E, Ok Y, Perera V, Rinklebe J, Shaheen S, Sarkar B, Sarmah A, Singh B, Singh G, Tsang D, Vikrant K, Vithanage M, Vinu A, Wang H, Wijesekara H, Yan Y, Van Zwieten Younis S, L (2022) Multifunctional applications of biochar beyond carbon storage. Int Mater Rev 67(2):150–200. https://doi.org/10.1080/09506608.2021.1922047
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582. https://doi.org/10.1038/nature08930
Chang S, Hyman M, Williamson K (2002) Cooxidation of naphthalene and other polycyclic aromatic hydrocarbons by the nitrifying bacterium, Nitrosomonas europaea. Biodegradation 13:373–381. https://doi.org/10.1023/A:1022811430030
Chen L, Jiang Y, Liang C, Luo Y, Xu Q, Han C, Zhao Q, Sun B (2019) Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7:77. https://doi.org/10.1186/s40168-019-0693-7
Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488. https://doi.org/10.1016/j.orggeochem.2006.06.022
Cheng CH, Lehmann J (2009) Ageing of black carbon along a temperature gradient. Chemosphere 75:1021–1027. https://doi.org/10.1016/j.chemosphere.2009.01.045
Domingos VF (2013) Software GS2013 (Gas Sorption 2013). Department of Physics, University of Coimbra, Coimbra, Portugal, CEMDRX
EBC (2023) European biochar certificate - guidelines for a sustainable production of biochar. Carbon Standards International (CSI), Frick, Switzerland. (http://european-biochar.org). Version 10.3 from 5th Apr 2022. https://www.european-biochar.org/en/ct/2-EBC-and-WBC-guidelines-documents (updated on 5th April 2023).
Figueres C, Schellnhuber HJ, Whiteman G, Rockström J, Hobley A, Rahmstorf S (2017) Three years to safeguard our climate. Nature 546:593–595. https://doi.org/10.1038/546593a
Friedlingstein P, Houghton RA, Marland G, Hackler J, Boden TA, Conway TJ, Canadell JG, Raupach MR, Ciais P, Le Quéré C (2010) Update on CO2 emissions. Nat Geosci 3:811–812. https://doi.org/10.1038/ngeo1022
Gateuille D, Evrard O, Lefevre I, Moreau-Guigon E, Alliot F, Chevreuil M, Mouchel JM (2014) Mass balance and decontamination times of polycyclic aromatic hydrocarbons in rural nested catchments of an early industrialized region (Seine River basin, France). Sci Total Environ 470–471:608–617. https://doi.org/10.1016/j.scitotenv.2013.10.009
Godlewska P, Ok YS, Oleszczuk P (2021) The dark side of black gold: ecotoxicological aspects of biochar and biochar-amended soils. J Hazard Mater 403:123833. https://doi.org/10.1016/j.jhazmat.2020.123833
Guo S, Li Y, Wang Y, Wang L, Sun Y, Liu L (2022) Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capt Sci Technol 4:100059. https://doi.org/10.1016/j.ccst.2022.100059
Hammes K, Schmidt M, Smernik R, Currie L, Ball W, Nguyen T, Louchouarn P, Houel S, Gustafsson O, Elmquist M, Cornelissen G, Skjemstad J, Masiello C, Song J, Peng P, Mitra S, Dunn J, Hatcher P, Hockaday W, Smith D, Hartkopf-Fröder C, Böhmer A, Lüer B, Huebert B, Amelung W, Brodowski S, Huang L, Zhang W, Gschwend P, Flores-Cervantes D, Largeau C, Rouzaud J, Rumpel C, Guggenberger G, Kaiser K, Rodionov A, Gonzalez-Vila F, Gonzalez-Perez J, de la Rosa J, Manning D, López-Capél E, Ding L (2007) Comparison of quantification methods to measure fire derived (black/elemental) carbon in soils and sediments using reference materials from soil, water, sediment and the atmosphere. Glob Biogeochem Cycles 21:GB3016. https://doi.org/10.1029/2006GB002914
He Y, Liu C, Tang X, Xian Q, Zhang J, Guan Z (2019) Biochar impacts on sorption-desorption of oxytetracycline and florfenicol in an alkaline farmland soil as affected by field ageing. Sci Total Environ 671:928–936. https://doi.org/10.1016/j.scitotenv.2019.03.414
Igalavithana D, Choi S, Shang J, Hanif A, Dissanayake P, Tsang D, Kwon J-H, Lee K, Ok YS (2020) Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: effect of porous structure and surface chemistry. Sci Total Environ 739:139845. https://doi.org/10.1016/j.scitotenv.2020.139845
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, van Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N, Smernik RJ, Amonette JE (2010) An investigation into the reactions of biochar in soil. Aust J Soil Res 48:501–515. https://doi.org/10.1071/SR10009
Karagulyan M, Goebel M, Diehl D, Quba A, Kästner M, Bachmann J, Wick L, Schaumann G, Miltner A (2022) Water stress-driven changes in bacterial cell surface properties. Appl Environ Microb 88:21. https://doi.org/10.1128/aem.00732-22
Keith A, Singh B, Dijkstra F (2015) Biochar reduces the rhizosphere priming effect on soil organic carbon. Soil Biol Biochem 88:372–379. https://doi.org/10.1016/j.soilbio.2015.06.007
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011) Biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–143. https://doi.org/10.1016/B978-0-12-385538-1.00003-2
Lal R (2008) Carbon sequestration. Phil Trans R Soc B 363:815–830. https://doi.org/10.1098/rstb.2007.2185
Lal R (2016) Biochar and soil carbon sequestration. In: Agricultural and Environmental Applications of Biochar: Advances and Barriers. SSSA Special Publication 63. M. Guo, Z. He, and S.M. Uchimiya. SSSA, 5585 Guilford Rd., Madison, WI 53711, USA, pp 175–197. https://doi.org/10.2136/sssaspecpub63.2014.0042.5
Li Y, Li Y, Chang S, Yang Y, Fu S, Jiang P, Luo Y, Yang M, Chen Z, Hu S, Zhao M, Liang X, Xu Q, Zhou G, Zhou J (2018) Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol Biochem 122:173–185. https://doi.org/10.1016/j.soilbio.2018.04.019
Liu X, Zheng J, Zhang D, Cheng K, Zhou H, Zhang A, Li L, Joseph S, Smith P, Crowley D, Kuzyakov Y, Pan G (2016) Biochar has no effect on soil respiration across Chinese agricultural soils. Sci Total Environ 554–555:259–265. https://doi.org/10.1016/j.scitotenv.2016.02.179
Lorenz K, Lal R (2014) Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J Plant Nutr and Soil Sc 177:651–670. https://doi.org/10.1002/jpln.201400058
Marks EAN, Alcañiz JM, Domene X (2014a) Unintended effects of biochars on shortterm plant growth in a calcareous soil. Plant Soil 385:87–105. https://doi.org/10.1007/s11104-014-2198-2
Marks E, Mattana S, Alcañiz JM, Domene X (2014b) Biochars provoke diverse soil mesofauna reproductive responses in laboratory bioassays. Eur J Soil Biol 60:104–111. https://doi.org/10.1016/j.ejsobi.2013.12.002
Mia S, Dijkstra FA, Singh B (2017) Long-term aging of biochar: a molecular understanding with agricultural and environmental implications. Adv Agron 141:1–51. https://doi.org/10.1016/bs.agron.2016.10.001
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller H, Keeney DR (eds) Method of soil analysis part 2: chemical and microbiological properties. ASA Monograph number 9. Agronomy series. Soil Science Society of America, Madison, pp 539–579
Nidheesh P, Gopinath A, Ranjith N, Akre A, Sreedharan V, Kumar M (2021) Potential role of biochar in advanced oxidation processes: a sustainable approach. Chem Eng J 405:126582. https://doi.org/10.1016/j.cej.2020.126582
Nottingham A, Gloor E, Bååth E, Meir P (2022) Soil carbon and microbes in the warming tropics. Funct Ecol 36:1338–1354. https://doi.org/10.1111/1365-2435.1405
Odinga ES, Gudda FO, Waigi MG, Wang J, Gao Y (2021) Occurrence, formation and environmental fate of polycyclic aromatic hydrocarbons in biochars. Fundamental Res 1:296–305. https://doi.org/10.1016/j.fmre.2021.03.003
Ojeda G, Mattana S, Avila A, Alcaniz JM, Volkmann M, Bachmann J (2015) Are soil-water functions affected by biochar application? Geoderma 249–250:1–11. https://doi.org/10.1016/j.geoderma.2015.02.014
Ojeda G, Patrício J, Mattana S, Sobral A (2016) Effects of biochar addition to estuarine sediments. J Soils Sediments 16:2482–2491. https://doi.org/10.1007/s11368-016-1493-3
Olivier JGJ, Janssens-Maenhout G, Muntean M, Peters JAHW (2015) Trends in global CO2 emissions, 2015 Report, 1st edn. PBL Netherlands Environmental Assessment Agency and European Commission, Joint Research Centre, The Hague, Netherlands
Or D, Smets BF, Wraith JM, Dechesne A, Friedman SP (2007) Physical constraints affecting bacterial habitats and activity in unsaturated porous media – a review. Adv Water Resour 30:1505–1527. https://doi.org/10.1016/j.advwatres.2006.05.025
Orchard VA, Cook FJ (1983) Relationship between soil respiration and soil moisture. Soil Biol Biochem 15:447–453. https://doi.org/10.1016/0038-0717(83)90010-X
Quan G, Fan Q, Zimmerman AR, Sun J, Cui L, Wang H, Gao B, Yan J (2020) Effects of laboratory biotic aging on the characteristics of biochar and its water–soluble organic products. J Hazardous Mater 382:121071. https://doi.org/10.1016/j.jhazmat.2019.121071
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Raich JW (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–99. https://doi.org/10.3402/tellusb.v44i2.15428
Ran T, Li J, Liao H, Zhao Y, Yang G, Long J (2023) Effects of biochar amendment on bacterial communities and their function predictions in a microplastic-contaminated Capsicum annuum L. soil. Environ Technol Innov 31:103174. https://doi.org/10.1016/j.eti.2023.103174
Ren XH, Sun HW, Wang F, Zhang P, Zhu HK (2018) Effect of aging in field soil on biochar’s properties and its sorption capacity. Environ Pollut 242:1880–1886. https://doi.org/10.1016/j.envpol.2018.07.078
Sakhiya AK, Anand A, Kaushal P (2020) Production, activation, and applications of biochar in recent times. Biochar 2:253–285. https://doi-org.ezproxy.unal.edu.co/https://doi.org/10.1007/s42773-020-00047-1
Sánchez-Avila J, Fernández-Sanjuan M, Vicente J, Lacrote S (2011) Development of a multi-residue method for the determination of organic micropollutants in water, sediment and mussels using gas chromatography–tandem mass spectrometry. J Chromatogr A 1218:6799–6811. https://doi.org/10.1016/j.chroma.2011.07.056
Sarker A, Yoo J-H, Jeong W-T (2023) Environmental fate and metabolic transformation of two non-ionic pesticides in soil: effect of biochar, moisture, and soil sterilization. Chemosphere 345:140458. https://doi.org/10.1016/j.chemosphere.2023.140458
Shafawi A, Mohamed A, Lahijani P, Mohammadi M (2021) Recent advances in developing engineered biochar for CO2 capture: an insight into the biochar modification approaches. J Environ Chem Eng 9:106869. https://doi.org/10.1016/j.jece.2021.106869
Silva Jd Ae VF, Marto Domingos D, Costa LD, Marcos M, Silva MR, Gil JM, Sobral AJFN (2013) Reversible sequestering of CO2 on a multiporous crystalline framework of 2-quinolyl-porphyrin. Tetrahedron Lett 54(2449):2451. https://doi.org/10.1016/j.tetlet.2013.02.071
Smith J, Collins H, Bailey V (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347. https://doi.org/10.1016/j.soilbio.2010.09.013
Smith MR, Myers SS (2018) Impact of anthropogenic CO2 emissions on global human nutrition. Nat Clim Change 8:834–839. https://doi.org/10.1038/s41558-018-0253-3
Soil Survey Staff (2010) Keys of soil taxonomy, 11th edn. US Department of Agriculture and Natural Resources Conservation Service, USA
Sombroek WG (1966) Amazon soils: a reconnaissance of the soils of the Brazilian Amazon region. Wageningen Center for Agriculture Publications and Documentation, Wageningen, The Netherlands. https://library.wur.nl/WebQuery/wurpubs/421842. Accessed 10 Jan 2024
Sun B, Lian F, Bao Q, Liu Z, Song Z, Zhu L (2016) Impact of low molecular weight organic acids (LMWOAs) on biochar micropores and sorption properties for sulfamethoxazole. Environ Pollut 214:142–148. https://doi.org/10.1016/j.envpol.2016.04.017
Tang C, Yang F, Antonietti M (2022) Carbon Materials Advancing Microorganisms in Driving Soil Organic Carbon Regulation. Research 2022:9857374. https://doi.org/10.34133/2022/9857374
Tans P, Keeling R (2021) Earth system research laboratories (ESRL) Global monitoring laboratory–carbon cycle greenhouse gases, national oceanic and atmospheric administration (NOAA). US Department of Commerce. https://gml.noaa.gov/ccgg/. Accessed 10 Jan 2024
Thomas S (2021) Post-processing of biochars to enhance plant growth responses: a review and meta-analysis. Biochar 3:437–455. https://doi.org/10.1007/s42773-021-00115-0
Thomazini A, Spokas K, Hall K, Ippolito J, Lentz R, Novak J (2015) GHG impacts of biochar: predictability for the same biochar. Agr Ecosyst Environ 1:183–191. https://doi.org/10.1016/j.agee.2015.04.012
Vargas R, Carbone MS, Reichstein M, Baldocchi DD (2011) Frontiers and challenges in soil respiration research: from measurements to model-data integration. Biogeochemistry 102:1–13. https://doi.org/10.1007/s10533-010-9462-1
Wang C, Li J, Chen L, Huang X (2024) Distiller’s grain-derived biochar as novel soil amendment benefits growth and decreases Cd uptake of wheat by modifying Cd fractions and rhizospheric microbiota. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-023-01581-0
Wang C, Wanga Y, Herath HMSK (2017) Polycyclic aromatic hydrocarbons (PAHs) in biochar – their formation, occurrence and analysis: a review. Org Geochem 114:1–11. https://doi.org/10.1016/j.orggeochem.2017.09.001
Wang J, Odinga E, Zhang W, Zhou X, Yang B, Gatheru M, Gao Y (2019) Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: a synthesis study. Environ Int 130:104899. https://doi.org/10.1016/j.envint.2019.06.009
Wang L, Chen D, Zhu L (2023) Biochar carbon sequestration potential rectification in soils: synthesis effects of biochar on soil CO2, CH4 and N2O emissions. Sci Total Environ 904:167047. https://doi.org/10.1016/j.scitotenv.2023.167047
Wang Y, Hao Y, Cui X, Zhao H, Xu C, Zhou X, Xu Z (2014) Responses of soil respiration and its components to drought stress. J Soils Sediments 14:99–109. https://doi.org/10.1007/s11368-013-0799-7
Woche S, Goebel MO, Mikutta R, Schurig C, Kaestner M, Guggenberger G, Bachmann J (2017) Soil wettability can be explained by the chemical composition of particle interfaces - an XPS study. Sci Rep 7:42877. https://doi.org/10.1038/srep42877
Wong JTF, Chow KL, Chen XW, Wai C, Wong M (2022) Effects of biochar on soil water retention curves of compacted clay during wetting and drying. Biochar 4:4. https://doi.org/10.1007/s42773-021-00125-y
Yu H, Zou W, Chen J, Yu Chen H, Z, Huang J, Tang H, Wei X, Gao B, (2019) Biochar amendment improves crop production in problem soils: a review. J Environ Manag 232:8–21. https://doi.org/10.1016/j.jenvman.2018.10.117
Zeebe RE, Ridgwell A, Zachos JC (2016) Anthropogenic carbon release rate unprecedented during the past 66 million years. Nat Geosci 9:325–329. https://doi.org/10.1038/ngeo2681
Zhang C, Zeng G, Huang D, Lai C, Chen M, Cheng M, Tang W, Tang L, Dong H, Huang B, Tan X, Wang R (2019) Biochar for environmental management: mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem Eng J 373:902–922. https://doi.org/10.1016/j.cej.2019.05.139
Zhang WP, Surigaoge S, Yang H (2024) Diversified cropping systems with complementary root growth strategies improve crop adaptation to and remediation of hostile soils. Plant Soil. https://doi.org/10.1007/s11104-023-06464-y
Zimmerman A, Gao B, Ahn M (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179. https://doi.org/10.1016/j.soilbio.2011.02.005
Zimmerman A (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44(4):1295–1301. https://doi.org/10.1021/es903140c
Funding
This study was associated to the Colombian project PS-35-2020 (Universidad Nacional Abierta y a Distancia - UNAD). The authors thank also the Centro de Quimica de Coimbra supported by Fundação para a Ciencia e Tecnologia - FCT (Portugal). The work of CO2 adsorption performed at the CFisUC was supported by the Portuguese Science and Technology Foundation (FCT - Fundação para a Ciência e Tecnologia, I. P., Portugal) through projects UIDB/04564/2020 and UIDP/04564/2020. We want to thank all institutions involved in this study, for facilitating the access to their laboratories and giving support with the corresponding methodologies, equipment and funding resources. Biochar and soil respiration samples were provided from the SOCARRAT project (contract AGL2009-12343 of the Spanish Ministry of Science and Innovation, Spain).
Author information
Authors and Affiliations
Contributions
Gerardo Ojeda: methodology, investigation, statistical analysis, writing—original draft. João M. Gil: methodology and resources (CO2 adsorption). Stefania Mattana: methodology and resources (soil respiration) and review. Jörg Bachmann: methodology and resources (biochar-water contact angles). Katell Quenea: methodology and resources (PAH analysis). Abílio Sobral: formal analysis, review and editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ojeda, G., Gil, J.M., Mattana, S. et al. Biochar ageing effects on soil respiration, biochar wettability and gaseous CO2 adsorption. Mitig Adapt Strateg Glob Change 29, 11 (2024). https://doi.org/10.1007/s11027-024-10107-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11027-024-10107-7