Leaching residues of oxidized nickel ores is studied. According to the results of chemical analysis, it is found that with 62% extraction of nickel into the solution residues contain, wt.%: 0.58 Ni, 0.016 Co, 14.35 Fe, 9.08 Mg, 1.96 Al, and 21.16 Si. With 89% extraction residues contain, wt.%: 0.26 Ni, 0.001 Co, 6.5 Fe, 9.12 Mg, 1.46 Al, and 26.45 Si. The grain size composition of leaching residues is studied. It is found that the amount of fine fraction (– 2.5 + 0 mm) in the residues with 89% extraction almost doubles compared with the initial ore, and the amount of coarse fraction (– 21.5 + 10 mm) is halved. X-ray phase and X-ray microanalysis methods are used in the work to study residues from leaching oxidized nickel ores (ONO). With extraction of 62% of nickel from ONO nickel is detected predominantly in talc, iron oxides, and siliceous iron oxides. Nickel is not detected in leaching residues with 89% recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
According to various estimates oxidized nickel ores (ONO) are currently ≈ 70% world reserves of nickel [1, 2]. For this type of ore there is typically a relatively low, compared with sulfide copper-nickel ores, nickel content, not constant with respect to composition and absence of dissemination of nickel-containing minerals within a lump of ore. In view of the above mentioned features enrichment of ONO is not possible.
Until recently ONO treatment within Russia has been accomplished by shaft melting. However, with a drop in world price for saleable nickel shaft melting became unprofitable. In this connection the South Ural Nickel and Ural Nickel Plants were closed [3, 4].
Hydrometallurgical methods may be used as an alternative to shaft melting in order to process ONO, and their use makes it possible to resolve this problem.
Within the world autoclave and ammonium carbonate technologies have been introduced for processing rich ONO. Agitation nitric, hydrochloric, and sulfuric acid methods have been studied for leaching [5,6,7]. There is information about a lump method for processing ONO [8, 9]. Research is known for obtaining nickel by means Alyssum morale special growths, capable of selective accumulation of nickel with subsequent leaching from them. Nonetheless, not one of the published methods has industrial application for processing ONO.
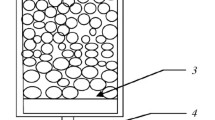
Previously in [14] a study was made for percolation leaching of oxidized nickel ore of the Serov deposit. The object selected for study was iron-magnesium ONO. Processing of this type of ore by pyrometallurgical and also hydrometallurgical methods was difficult in view of the high content of iron and magnesium. Ore with a size of – 21.5 + 0 mm was leached with aqueous solution of sulfuric acid in percolators with a volume of 3800 (percolator 1) and 7500 cm3 (percolator 2) (Fig. 1) with T = 25°C, S:L = 1.7, and a pause between irrigation of 1–3 days. As a result of leaching for 440 days in percolator 1 nickel extraction of 89% was achieved, and percolator it was 62% in 350 days.
Residues were obtained in the course of leaching in percolators with a different degree of extraction. The residues obtained with respect to composition are similar to those obtained in overseas enterprises. However, in overseas hydrometallurgical enterprises, i.e., Moa Bay, Murrin-Murrin, and Bulong (Australia) solid residues are not treated, but stored in dumps and tailing stores [1]. This in turn has an unfavorable effect on the ecological situation in the region of mining and processing.
Solution of the problem of treating solid restudies of ONO leaching will make it possible to increase the completeness of use of leached ore and the ecological nature of its treatment. In view of this a study was made in this work of the composition of solid residues from percolation leaching of iron-magnesium oxidized nickel ore.
The grain size composition of solid residues was studied with a different degree of nickel extraction. It was concluded that during leaching there is self-refinement of ore (Table 1). The output of the coarse fraction itself – 21.5 + 10 mm with extraction of 89% nickel in percolator 1 decreased by a factor of two, and correspondingly there was an increase by a factor of two in the yield of the fine fraction itself – 21.5 + 0 mm. This affected the rate of solution percolation, which at the start of leaching was 1–1/5 m/h, and at the end ≈ 0.2–0.3 m/h.
The chemical composition is given in Table 2 for solid residues. With an increase during nickel extraction in an amount of 89% into solution in percolator 1 a reduction was observed in the weight of residues by 50.8% (from 3.68 to 1.81 kg). Associated elements were extracted into solution, wt.%: 99.5 Со, 76.3 Mg, 68.4 Al, 78.4 Fe, 96.6 Mn, 69 Cu. 25.6Si, and 76.9 Ca. The content of sulfur increased as a result of forming sulfates.
It should be noted that extraction of iron into solution of percolator 1 was 78.4%. This is explained by the fact that in the initial leaching stages (extraction of nickel ≤ 40%) there was a change in the leaching solution from pH 0.5–0.8 and the production solution was with pH = 1.5.
During nickel extraction into solution (62%) in percolator 2 the yield of solid residue decreased by 18.7% (from 7.5 to 6.1 kg). Extraction of associated elements into solution comprised, wt.%: 66.7 Со, 60.9 Mg, 47.7 Al, 21.3 Fe, 94.3 Mg, 63,4 Cu, 1 Si. 69.3 C, + 3.30 S. In percolator 2 production solution emerged with pH 2.5–3 and as a result of hydrolysis of Fe3+ ions and its subsequent precipitation iron extraction from ore was 21.3% in total. Mainly this amount of iron was derived from production solution with pH 1–2 in the first and second stages of leaching. At the same time, it is well known that iron in solutions derived from leaching ore with pH = 2.5–3 remains in a dump in the form of insoluble compounds, for example iron hydroxide (Fe(OH)3), basic iron sulfate (Fe2 (SO4)3), sodium jarosite (NaFe3(OH)6 (SO4)2), or carphosiderite (H3O·Fe3 (OH)6 (SO4)2) [4, 5].
Results of X-ray phase studies of solid residues obtained in an Bruker AXS diffractometer are given in Table 3. Extraction of minerals from ore into solution (in percolators 1 and 2 with nickel extraction of 89 and 62%) comprised correspondingly, wt.%: quartz 52.4 and 46.2; antigorite and lizardite 82.04 and 60.4, talc 30.16 and 9.23, actinolite 17.4 and 17.29; clinochlore 45.53 and 56.1. the most active leaching of lizardite and antigorite is observed, which are probably the main sources of nickel, cobalt, and magnesium. It is noted that the most complete nickel extraction from ore (72–89%) in percolator 1 is observed with formation of a new phase, jarosite.
The increase in solid ore leaching residue content of iron oxide by factors of 10 and 15 is most marked. This occurs because during leaching ores iron oxide from lizardite, antigorite, actinolite, and clinochlore is transferred into sulfuric acid solutions in the form of sulfates (Fe3SO4·nH2O), which at pH = 3 hydrolyse with formation of iron hydroxide and remains in solid residues.
Data are given in Table 4 for microanalysis of solid residues of ONO leaching in percolator 2 with extraction of 52% nickel from ore. In iron oxides, siliceous oxides of iron and talc the is unleachable nickel (see Table 4, positions 1–3) whose content is correspondingly, wt.%: 1.03, 1.21, and 1.44, 0.78, and 0.69.
It should be noted that the nickel content in ONO talc is 0.46 wt.%, and in solid leaching residues it is 0.78 and 0.69 wt.%, which probably points to the more complex leaching of nickel from talc. In other samples (see Table 4, positions 4–6) nickel is not detected.
Results are provided in Table 5 for microanalysis of solid residues leaching ONO in percolator 1 with extraction of 89% nickel from ore.
The main component of solid residues is quartz (it is detected in six talc phases). In one phase iron oxides were detected, in three phases there was siliceous iron oxide, and in one phase there was actinolite, for which presence of calcium is typical up to 9.58 wt.%.
Nickel was not detected in any of the phases (even in talc) since in using a percolator less nickel extraction is achieved, i.e., 89%.
From results of chemical and X-ray microstructural analyses it has been established that the residue consists predominantly of actinolite, iron oxide, and talc. Subsequently after adding aluminum and calcium oxides solid residues may be used as a raw material for preparing cement [13].
Conclusions
-
1.
During leaching of oxidized nickel ore (ONO) to a level of 89% nickel extraction there is a change in the grain size composition of solid residues with an increase in fine phase – 21.5 + 0 mm by a factor of two compared with the original ONO composition, which is connected with “self grinding” of ore during leaching. As a result of this the rate of solution percolation thigh a layer of ore decreases from 1–1.5 to 0.3 m/h, but remains suitable under production conditions.
-
2.
It has been established that the main sources of nickel in ONO are lizardite and antigorite minerals.
-
3.
Solid residues consist of antigorite, iron oxide, and talc, and subsequently after addition of aluminum oxide, which is obtained during reprocessing of leaching solutions, may be used as a raw material for preparing cement.
-
4.
According the X-ray microanalysis (XMRA) data ONO in percolator 2 during extraction of 62% Ni presence is established of nickel that cannot be leached from iron oxide, siliceous iron oxide, and in talc. This indicates that nickel is most difficult to leach from these minerals.
-
5.
Results of XMRA of ONO leaching residues in percolator 1 with extraction 89% nickel from ore did not show its presence in any of the phases. The main component of solid residues is quartz. It is detected in six phases of talc; in one phase iron oxide is detected, in three phases there is siliceous iron oxide, and in one phase there is actinolite for which there is typically presence of calcium up to 9.58 wt.%.
References
I. D. Reznik, G. P. Ermakov, and Ya. M. Shneerson, Nickel in 3 Vol., Vol. 2. Oxidation of Nickel Ores. Ore Properties. Pyrometallurgy and Hydrometallurgy of Oxidized Nickel Ores [in Russian], OOO Nauka Tekhnol., Moscow (2004).
A. P. Stavskii (editor), Mineral Raw material the Depths to Market, in 3 Vol. Vol. 2, Nonferrous Metals. Aluminum, Copper, Nickel, Tin, Lead, and Zinc [in Russian], Nauchnyi Mir, Moscow (2011).
Ural Nickel General Director: Nickel Production Soon to Cease [Electronic Source]; URL: http://www.metalinfo.ru/ru/news/94275 (access date 09.07.2018).
Rusnikel’ Discharges to the Street Thousands of Sverdlov and Chelyabinsk Workers [Electronic Source]; URL: http://pravdaurfo.ru/articles/149526-rusnikel-vykinul-na-ulicy-tysyachi-sverdlovchan-i (access date 04.20.2017).
I. I. Kalinichenko, V. V. Vaitner, A. S. Molodykh, and V. N. Shubin, RF Patent 2532871, МPК C22B 23/00, C22B 3/06. Method for Treating Oxidized Nickel Ores, Claim 04.23.2013; Publ. 11.10.2014. Bull. No. 31.
Ural Nickel. Development Path, Round Table Proceedings. UrFU, Ekaterinburg (2017).
V. I. Smirnov, Copper Hydrometallurgy [in Russian], Moscow (1947).
R. G. McDonald and B. I. Whittington, “Atmospheric acid leaching of nickel laterites review. Part I. Sulphuric acid technologies,” Hydrometallurgy, No. 91, 35–55 (2008).
R. G. McDonald and B. I. Whittington, “Atmospheric acid leaching of nickel laterites review. Part II. Chloride and biotechnologies,” Hydrometallurgy, No. 91, 56–69 (2008).
R. Barbaroux, G. Mercier, J. F. Blais, et al., “A new method for obtaining nickel from the hyper-accumulator plant Alyssum murale,” Separat. and Purific. Techn.,83. 57–65 (2011).
B. D. Khalezov, P. Yu. Chuvashov, and N. A. Vatolin, “Study of lump leaching of oxidized nickel ores of the Serov deposit,” in: Proc. XVI Internat. Sci. Tech. Conf., Ekaterinburg (2011).
B. D. Khalezov, Lump Leaching of Copper and Copper-Nickel Ores [in Russian], Ekaterinburg (2013).
Cement [Electronic source]. URL: https://ru.wikipedia.org/wiki/%D0%A6%D0%B5%D0%BC%D0%B5%D0%BD%D1%82 (access date 04.20.2017).
B. D. Khalezov, A. S. Gavrilov, S. A. Petrova, et al., “Lump leaching of oxidized nickel ores,” Metallurg., No. 1, 59–64 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 63, No. 8, pp. 77–82, August, 2019.
Rights and permissions
About this article
Cite this article
Khalezov, B.D., Gavrilov, A.S., Petrova, S.A. et al. Investigation of Solid Residues Obtained After Oxidized Nickel Ore Leaching. Metallurgist 63, 860–866 (2019). https://doi.org/10.1007/s11015-019-00900-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-019-00900-0