Evaporation of steel smelting dust components with a different content of reducing agent within it is studied by experiment. Evaporation takes place in the workspace of a tubular furnace in an inert gas stream with material density in the container of 1 g/cm3 in the temperature range 650–1200°C. The grain size composition of the original dust is studied. The elemental and phase composition of specimens (original steel smelting dust and after tests) is determined by means of an x-ray phase analyzer. The period of the end of evaporation is determined. Conditions are established for evaporation of volatile components, and the degree of metal removal is determined. Results obtained agree with those of thermodynamic calculations for reaction of components of steel smelting dust with carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Steel smelting dust is a multicomponent material formed during steel production and captured with a steel smelting unit in the gas cleaning system. The following chemical compounds may be found within dust from an arc steel smelting furnace (ASF): ZnFe2O4, Fe3O4, MgFe2O4, FeCr2O4, MgO, Mn3O4, SiO2, ZnO [1]. According to data in [2], coarse ASF dust consists of particles of coal and lime of irregular shape with a size of 20–500 μm; spherical particles with a size of 20–200 μm have a chemical composition corresponding to slag; coarse agglomerates of fine particles with a size up to 1000 μm. The main part of ASF dust is fine particles with a size less than 20 μm: zinc oxide single crystals whose size is not more than several nanometers; metal and slag particles of spherical shape, and their size varies from 0.2 to 20 μm.
In this work, the object studied was dust from an ASF gas cleaner, formed during smelting of carbon steel. In the course of preparation for studying, the original dust was screened to fractions less than 400 μm in order to exclude coarse lime and carbon particles. The screened dust was dried at 150°C for 2 h in order to exclude the effect of moisture (0.78%) on volatile component evaporation kinetics.
The grain size composition of the test dust was studied using a Carl Zeiss Axiovert 200 MAT microscope. Dust has the following fractions: 62% of particles with a size of less than 5 μm; 31% more than 5 μm and less than 10 μm; 7% more than 10 μm.
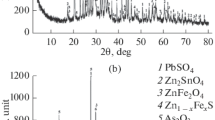
The elemental (Table 1) and phase composition of dust was determined in an ARL 9900 Work Station analyzer. This instrument has low sensitivity towards light elements and does not capture oxygen, carbon, and fluorine present in steel smelting dust. From the start, it was prescribed that metal in dust is in the form of oxides, since the furnace free space and gas conduit have an atmosphere of a predominantly oxidizing nature. Carbon concentration was studied by infra-red absorption analysis and comprised 1.5%. The presence of the following phases was established in dust: Fe3O4, ZnO, ZnFe2O4, PbO, NaCl, KCl, SiO2. Thus, with respect to grain size, elemental, and phase composition the test dust is typical for an ASF gas cleaner.
Experiments for evaporation of steel smelting dust volatile components were performed in a unit operating on the basis of a tubular resistance furnace (Fig. 1). In the course of a test, dust or a mixture of dust with graphite powder was molded with a prescribed density in a ceramic container. A specimen was placed in the furnace working area after furnace warmup and establishment of a prescribed constant temperature (±5°C). Nitrogen was fed to the reaction tube with a prescribed flow rate (250–950 ml/min under normal conditions. The average gas velocity was determined for the reaction tube cross sectional area. After exposure of a specimen within the furnace for a prescribed time, it was extracted and immediately placed in a desiccator for cooling to room temperature. The total amount of evaporated substance was determined from specimen weight difference with the container before and after heat treatment.
According to test data on the heating dynamics in the lower, central, and surface layers of a specimen, cooling of the central part from the surface on reaching the test temperature was insignificant (not more than 5°C), and subsequently during experiments the specimen surface temperature was monitored. The period of specimen warm-up to a test temperature was 5–6 min.
Study results. Primary data for the change in specimen weight over time with different experiment conditions (Figs. 2 and 3) were approximated by a fractional rational function:
where Δ m is the specimen’s weight change, g; τ is the specimen’s exposure time in a furnace, min; and A, B are constant actors.
At up to 1200°C during reaction of carbon there may be reduction of Fe, Zn, Pb, Na, K, Cd, Cu oxides [3]. The carbon content required stoichiometrically for oxide reduction is achieved by adding 9% graphite powder to dust.
With the addition of reducing agent, the weight of substance removed is markedly greater, particularly at high temperature.
Graphite powder added to a sample is on average 0.675 g, and the difference in weight loss at high temperature reaches 2.680 g. Consequently, an increase in the amount of substance removed from dust is achieved not only as a result of removing a considerable amount of carbon, but mainly due to the considerable amount of reduced and evaporated metals.
It is seen from Figs. 2 and 3 that after exposure for about 120 min the specimen’s weight change ceases within the limits of measurement accuracy, i.e., the time of the end of exposure may be considered that for almost complete metal reduction and evaporation in a specimen under the given measurement conditions.
The specimens’ composition was studied by x-ray fluorescence analysis after the end of the process. The reduction in relative element concentration (X i /X Fe, Fig. 4) points to a reduction in weight of a specific element in the specimen in the course of an experiment, since iron has a low vapor pressure [4] and cannot evaporate during all experiment conditions (m Fe = const).
Curves for the dependence (see Fig 4) make it possible to conclude that such elements as Al, Si, Cr, Ca, Mn, Mg, Cu are not removed from specimens in the whole test temperature range. Cadmium evaporates at 850°C and above, and at 1200° it is almost entirely removed from specimens. Lead and potassium evaporate over the whole temperature range. Removal of chlorine is marked, starting from 750°C, and at high temperature (1150–1200°C) chlorine is removed entirely. Removal of sodium commences at 750 and 850°C with a natural and stoichiometric carbon content in an original mixture, respectively.
The following differences are observed in order to remove zinc: for dust specimens of natural compositions insignificant zinc evaporation is observed only at high temperature (1150–1200°C); evaporation of zinc from dust in a mixture with solid carbon occurs at 950°C and above, and at the maximum test temperature it is almost entirely removed from dust and transferred to a gas phase.
The actual composition of carbon in specimens and equilibrium carbon content in dust according to calculated data, carried out by a TERRA [5] thermodynamic calculation program for phase composition, is presented in Fig. 5.
Without adding a reducing agent, the carbon existing within dust is almost entirely consumed in reducing oxides at low temperatures (its concentration decreases to 0.1% even at 650°C), meaning that for total reduction and removal of volatile metals (mainly zinc) the amount of reducing agent in dust of natural composition is inadequate. During heat treatment of dust with added graphite powder, carbon is present in specimens up to 1100°C and reacts with metal compounds reduced at high temperatures.
Results of the experiments agree with data obtained previously for thermodynamics of reaction of steel smelting dust components with carbon [3].
On the basis of data for the change in relative element concentration, proceeding from the constancy of the weight of iron in specimens, it is possible to calculate the degree of their removal (Table 2) by the equation
where R i is the degree of element removal from a specimen in the course of heat treatment; X 0 i , X i , X 0Fe , and X Fe are weight fractions of elements and iron in the original dust and in the specimen after testing, respectively, according to x-ray fluorescence analysis data.
It follows from Table 2 that with heat treatment of dust of natural composition to 1200°C it is possible to remove a considerable part of the following metals: Cd, Pb, K, and Na, and zinc is removed by not more than 7%. The carbon content has a marked effect on the degree of metal removal, and primarily zinc. At 1100°C and above, with a stoichiometric carbon content almost all zinc is removed from dust, and also the main amount of Cd, Pb, K, and Na, i.e., the dust heat treatment product contains all of the main volatile components.
Conclusion. From the results of the studies, it has been established that at about 1000°C without adding any reducing agent and other materials it is possible to remove from steel smelting dust a considerable amount of alkali metals (predominantly in the form of chlorides), lead, cadmium, and a significant amount of zinc. At the same time, in order to reduce iron and zinc with simultaneous evaporation of zinc it is necessary to add carbon in the amount not lower than the stoichiometric value at a temperature above 1000°C, and this is also confirmed by theoretical studies. The separation of zinc and other component extraction from steel smelting dust is possible by controlling the heat treatment regime and the carbon content.
Thus, in selecting optimum heat treatment conditions and the amount of added reducing agent it is possible to accomplish selective extraction of steel smelting dust volatile components and obtain a rich zinc-containing material within the product, which may serve as raw material for zinc production, and within the solid residue there is formation of an iron-containing product with a high degree of metallization.
References
G. M. S. J. Machado, F. A. Brehm, C. A. Mendes Moraes, et al., “Chemical, physical, structural and morphological characterization of the electric arc furnace dust,” J. Hazard. Mater., 136, 953–960 (2006).
A.-G. Guézennec, J.-C. Huber, F. Patisson, et al., “Dust formation in electric arc furnace: birth of the particles,” Powder Technol., 157, 2–11 (2005).
I. E. Doronin and A. G. Svyazhin, “Thermodynamic study of carbon reaction with steel melting dust components,” Metallurg, No. 1, 52–57 (2013).
D. F. Eliot, M. Glazer, and R. Ramakrishna, Thermodynamics of Steelmaking Processes [Russian translation], Metallurgiya, Moscow (1969).
B. G. Trusov, “Program system TERRA for modeling phase and chemical equilibria,” Proc. 14th Int. Conf. on Chemical Thermodynamics, St. Petersburg (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 10, pp. 37–41, October, 2014.
Rights and permissions
About this article
Cite this article
Doronin, I.E., Svyazhin, A.G. Experimental Study of Steel Smelting Dust Component Evaporation. Metallurgist 58, 866–871 (2015). https://doi.org/10.1007/s11015-015-0009-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-015-0009-5