Abstract
Alcohol misuse represents a serious health concern, especially during adolescence, with approximately 18% of high school students engaging in binge drinking. Despite widespread misuse of alcohol, its effects on how the brain functions is not fully understood. This study utilized a binge drinking model in adolescent rats to examine effects on brain function as measured by brain glucose metabolism (BGluM). Following an injection of [18 FDG] fluro-2-deoxy-D-glucose, rats had voluntary access to either water or various concentrations of ethanol to obtain the following targeted doses: water (no ethanol), low dose ethanol (0.29 ± 0.03 g/kg), moderate dose ethanol (0.98 ± 0.05), and high dose ethanol (2.19 ± 0.23 g/kg). Rats were subsequently scanned using positron emission tomography. All three doses of ethanol were found to decrease BGluM in the restrosplenial cortex, visual cortex, jaw region of the somatosensory cortex, and cerebellum. For both the LD and MD ethanol dose, decreased BGluM was seen in the superior colliculi. The MD ethanol dose also decreased BGluM in the subiculum, frontal association area, as well as the primary motor cortex. Lastly, the HD ethanol dose decreased BGluM in the hippocampus, thalamus, raphe nucleus, inferior colliculus, and the primary motor cortex. Similar decreases in the hippocampus were also seen in the LD group. Taken together, these results highlight the negative consequences of acute binge drinking on BGluM in many regions of the brain involved in sensory, motor, and cognitive processes. Future studies are needed to assess the long-term effects of alcohol binge drinking on brain function as well as its cessation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol binge drinking is a widespread problem among adolescents and young adults. The Substance Abuse and Mental Health Services Administration (SAMSHA) defines binge drinking as drinking five or more alcoholic drinks on the same occasion on at least one day in the past 30 days. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines binge drinking as a pattern of drinking that produces blood alcohol concentrations of greater than 0.08 g/dL. A blood alcohol content (BAC) of 0.08 g/dL usually occurs after four drinks for women and five drinks for men over a two hour period (SAMSHA 2015).
Binge drinking is a major public health problem that has serious risks in being associated with acute and chronic health problems. Physical consequences in the form of injury as well as cognitive consequences are problems associated acute with binge drinking (Krieger, 2018). Chronic consequences include alcohol dependence and alcohol use disorder (Kuntsche et al. 2017).
Binge exposure to alcohol produces more widespread damage to the brain in adolescents compared to adults (Crews et al. 2000) and binge drinking has been associated with increased likelihood of psychiatric problems among adolescence (Esser et al. 2017; Kandel et al. 1997; Miller et al. 2007). Though levels of alcohol use among youth in the United States have seen a decline in recent decades with past interventions, alcohol remains the most commonly used substance and its use is a leading cause of death and injury (Patrick and Schulenberg 2013). In the United States, 17.7% of high school students report binge drinking during the past 30 days and one in six high school students were binge drinkers (Esser et al. 2017). Binge drinking is also a global issue. According to the World Health Organization, 3 million deaths every year result from the harmful use of alcohol, which represents 5.3% of all deaths worldwide (WHO, 2018). Despite widespread use of alcohol, its impact on how the brain functions remains incomplete.
Positron emission tomography (PET) imaging and the use of the radiotracer 2-[18FDG] fluro-2-deoxy-D-glucose (FDG) is a powerful tool to examine changes in brain function. Since glucose is the main source of energy in the mammalian brain, we can assess brain glucose metabolism (BGluM) as an indicator of brain function. FDG PET is particularly strong because it characterizes BGluM non-invasively in the awake subject. FDG PET has been used extensively for studying alcohol abuse in humans (Thanos et al. 2008). Volkow and colleagues showed that alcohol administration (at 0.5 g/kg) in healthy human subjects substantially reduced glucose utilization that nearly parallels what is seen with anesthetics (Volkow et al. 2006). Given that these doses induced only mild intoxication, it raised the question whether alcohol intoxication changes the way the brain utilizes energy sources. The inhibitory effects of ethanol on glucose utilization was also seen to be greater in alcoholics (Volkow et al. 1990). It was later discovered that alcohol intoxication increased [1-11 C] acetate uptake in the brain in areas with the lowest glucose uptake (Volkow et al. 2013). This effect trended higher among heavy drinkers. The utilization of alternative energy source during alcohol intoxication clarifies the paradoxical findings from Volkow and colleagues (2006) showing profound decreases in glucose utilization with minimal impact on cognition.
The impact of ethanol on brain activity has been examined in rodents using a variety of techniques as well as routes of administration. Williams-Hemby and Porrino found dose-dependent effects of acute intraperitoneally-injected (IP) ethanol administration on glucose utilization, as measured with [14 C] 2-deoxyglucose autoradiography (Williams-Hemby and Porrino 1994). Specifically, low (0.25 g/kg) and moderate (0.5 g/kg) doses of ethanol increased glucose utilization in several limbic, cortical, somatosensory, and midbrain regions, whereas their high dose (1.0 g/kg) promoted widespread decreases in glucose utilization. Using the same method for determining glucose utilization, acute intragastric administration of ethanol (at doses of 0.25–2 g/kg) increased glucose utilization in the mesocorticolimbic circuit (Williams-Hemby and Porrino 1997). In addition, rats maintained on stable oral self-administration of ethanol (0.5 g/kg) show increased glucose utilization in many striatal, cortical, and limbic areas (Porrino et al. 1998). As described, these changes following chronic oral self-administration differ from a similar dose given acutely via IP injection. Recently, Gispert and colleagues showed the effects of acute alcohol administration (1.5 g/kg via IP injection) differed depending on prior experience with alcohol in a rodent model of alcoholism with FDG PET imaging (Gispert et al. 2017). In alcohol-naïve rats, acute administration induced significant decreases in glucose uptake. This effect was attenuated in rats who were short-term alcohol drinkers (three months) and long-term alcohol drinkers (twelve months). Thus, alcohol differentially impacts glucose utilization in rats depending on the route that it is administered as well as prior history of alcohol use.
Although initial studies in adult humans have helped shape our understanding on the effects of alcohol on the brain, there is still much to be understood. In particular, there is a great lack of understanding on how alcohol changes brain function during adolescence, where there is great concern for potential developmental effects. Alcohol exposure during adolescence has detrimental and long-term consequences, including increased risk of violence and unintentional injuries (Hingson et al. 2009), increased risk for alcohol use disorder in adulthood (Grant and Dawson 1997), as well as a plethora of neurodevelopmental changes related to the structure of the brain as well as neurotransmitter systems (Crews et al. 2016; Lees et al. 2020). Moreover, the pattern of alcohol consumption on BGluM is something that hasn’t received much attention. Binge drinking is known to be a far more dangerous pattern of excessive alcohol use and increases the risk of alcohol use disorder (Chung et al. 2018). While adolescent binge drinking represents a serious health concern, there are challenges to directly studying its effect on BGluM in the human population. Since studying the effects of binge drinking is not feasible in adolescent humans, we use adolescent rats to model this effect.
The present study is the first to examine the effects of acute oral binge ethanol consumption in rats on brain glucose utilization and brain functional connectivity using FDG PET. Different doses of binge ethanol consumption were compared and selected based on the literature (McDowell et al. 2005). Our low dose (LD) treatment group was approximately 0.25 g/kg of ethanol or a 0.025 BAC (equivalent to 1.3 drinks in women and 1.5 drinks in men). Our moderate dose (MD) treatment group was 1 g/kg of ethanol [0.1 BAC is equivalent to 5 drinks for women and 6 drinks for men] (Duke et al. 2011). Our high dose (HD) treatment group was 2.2 g/kg of ethanol (0.22 BAC). Both the MD treatment as well as our HD treatment in our study fits the threshold definition of binge drinking of four drinks for women and five drinks for men over a two hour period (SAMSHA 2015).
Methods
Animals: Male, (n = 24) adolescent Sprague-Dawley rats (Taconic Inc., Germantown, NY) were housed individually with a 12-h reverse light-dark cycle (lights off at 0800 h) in a temperature and humidity-controlled room. Two weeks prior to the beginning of the PET imaging phase of the study, rats were gradually trained to drink daily their day’s water volume during a limited access 10-minute session. Food was always provided ad libitum. Animals were then randomly separated into four treatment groups prior to [F18] FDG µPET scans: (1) water control, (2) low dose (LD) ethanol (0.29 ± 0.03 g/kg), (3) moderate dose (MD) ethanol (0.98 ± 0.05 g/kg), and (4) high dose (HD) ethanol (2.19 ± 0.23 g/kg). Animals were scanned between 6 and 8 weeks of age. All experiments were conducted in conformity with the National Academy of Science Guide for Care and Use Laboratory Animals and approved by the University Institutional Animal Care and Use Committee.
Procedures: Binge Drinking. Two weeks prior to the first µPET scan, animals were gradually trained to daily limited access drinking of water for 10 min a day. This resulted in similar daily volume as ad libitum 24 h drinking, except that now it took place during the limited 10 min availability of the water bottle. During this two-week period, rats showed no signs of stress, as indicated by changes in porphyrin, coat appearance, or activity. All rats had to show stable water consumption for 2 consecutive days of less than 20% variance in the volume consumed before the day of PET scanning. The purpose of this design was to fit the timeframe associated with the dynamic uptake of the FDG tracer in the brain. The voluntary consumption of alcohol needed to occur between the point of FDG injection and before a plateau is seen with FDG uptake inside active brain cells.
µPET Scans. Rats were given a bolus injection of ~ 400 µCi of [F18] FDG, T = 0 min. A blood sample was taken from the tail vein and a blood glucose measurement was taken to ensure similar baseline blood glucose levels prior to FDG injection. Ten minutes after the initial FDG injection, rats were presented with their daily drinking bottle, this time containing one of the following: water, low dose, moderate dose, or high dose of ethanol, T = 10 min. Rats were given 10 min of limited access to drink in their home cage after which point the bottle was removed (T = 20 min). After the solution was removed, rats were anesthetized with isoflurane (T = 30 min). At this point, rats were scanned for a total of 20 min in a µPET R4 scanner. The rat was placed in a stereotaxic head holder (David Kopf, Instruments; CA, USA) in a prone position on the bed of the scanner. A second blood sample was taken from the tail vein and glucose measurement was taken after administration of FDG to ensure proper blood glucose levels after tracer injection, T = 40 (Fig. 1).
µPET imaging. An R4 microPET tomograph (transaxial resolution = 2.0 mm full width at half maximum, field of view = 1 1.5 mm was used for µPET imaging (Concorde Microsystems, Knoxville, TN). Animals were placed in the center field of view and were scanned under a static FDG for 20 min using a ramp filter with cutoff at Nyquist frequency.
Image Analysis. Images were reconstructed using a MAP algorithm (15 iterations, 0.01 smoothing value, 245 × 256 resolution) and manually co-registered onto an MRI template of the Paxinos and Watson stereotaxic coordinates (Schweinhardt et al. 2003) using PMOD version 3.9 software (PMOD Technologies, Zurich, Switzerland). A region of interest (ROI) template in the PMOD 3.9 software was used to determine percent changes in BGLuM at a regional level. In addition, statistical Parametric Software (SPM R2018a) was used to identify changes in BGluM for ethanol treated rats compared to the control group with clusters of voxel threshold K > 50 and p < 0.001 set as significant, based on prior recommendations (Woo et al. 2014). A one-way ANOVA was performed to identify significant contrasts. These clusters were then overlaid onto an MRI template using AMIDE software to generate images.
Results
A one-way ANOVA was conducted and all contrasts reported fulfilled conservative criteria of p < 0.001 and a minimum significant cluster of K ≥ 50 voxels. The significant clusters resulting from between groups were compiled (Table 1) and regions of the brain that contained significant clusters were identified (Fig. 2). Functional imaging results were also summarized in a sagittal view brain circuit showing the activation and inhibition circuits of the brain associated with binge ethanol consumption (Fig. 3).
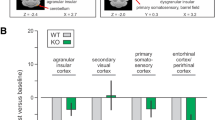
Coronal mPET images showing surviving significant clusters (p < 0.001 at K > 50) that were greater in the water drinking rats compared to rats binge drinking low dose ethanol. The blue clusters represent areas of the brain associated with inhibition or a decrease in BGluM associated with each dose of ethanol. Values on the color bar represent maximum t value seen in the comparison. (Top panel: Water > LD Ethanol) A-Somatosensory cortex jaw region (SJ), B Retrosplenial cortex (Rs), Hippocampus (HP), C Primary visual cortex (V1), Secondary visual cortex (V2), Retrosplenial cortex (Rs), Superior colliculus (Sc), D—6a cerebellar lobule (6aCb). (Middle panel: Water > MD Ethanol) A- Frontal Association Cortex (FrA), B- Primary Motor Cortex (M1), C- Primary visual cortex (V1), Secondary visual cortex (V2), Retrosplenial cortex (Rs), Superior colliculus (Sc), Subiculum (S), D-8th cerebellar lobule (8Cb). (Bottom panel: Water > MD Ethanol) A-Primary Motor Cortex (M1), Somatosensory cortex jaw region (SJ), B-Hippocampus (HP), Thalamus (Th), C-Retrosplenial cortex (Rs), Secondary Visual Cortex (V2), Inferior Colliculus (BIC), D-Raphe nucleus (RN), E-Cerebellum (Cb), F-8th cerebellar lobule (8Cb).
Summary of the functional BGluM imaging results. Sagittal view showing the brain circuit and regions that showed significant BGluM inhibition (blue) in the rat brains in response to binge ethanol consumption by dose. Significant clusters identified for p < 0.001, voxel threshold K > 50. Circle diameter corresponds to cluster size
Rats binge drinking low dose ethanol, compared to control rats (drinking water), showed significant inhibition of BGluM in the primary and secondary visual cortices (V1, V2), retrosplenial cortex (Rs), primary somatosensory cortex (SJ), hippocampus (HP), superior colliculus (Sc), and 6a cerebellar lobule (6aCb). Similarly, rats binge drinking moderate dose ethanol, compared to control, showed inhibition in the primary and secondary visual cortices (V1, V2), the retrosplenial cortex (Rs), superior colliculus (Sc) and primary somatosensory cortex (SJ). The subiculum (S), primary motor cortex (M1), and frontal association cortex (FrA) also showed a significant decrease in activity in moderate dose treated rats compared to the control. Rats binge drinking high dose ethanol showed BGluM inhibition in the secondary visual cortex (V2), retrosplenial cortex (Rs), 8th cerebellar lobule (8Cb), and primary somatosensory cortex (SJ), inferior colliculus (BIC), hippocampus (HP), raphe nucleus (RN), and thalamus (Th) compared to rats drinking water.
Additionally, each uptake value from each ROI was divided by the injection dose of FDG and divided by the body weight to standardize the data. The result was a standardized uptake value for each region of interest for each animal and corresponding dose. A heat map, (Fig. 4), was created (Graphpad Software, San Diego, CA) to illustrate the percentage changes in SUV for each dose compared to the control. The negative values associated with decreases in BGluM are represented in blue and the positive values associated with increases in BGluM are represented in red.
Heat map showing the percent change in SUV values for each dose of ethanol compared to the control group for each region of interest. Each column from left to right corresponds to Water vs. Low Dose, Water vs. Moderate Dose, and Water vs. High Dose comparisons. The blue represents a decrease in brain glucose metabolism (inhibition) and the red represents an increase in brain glucose metabolism (activation)
Discussion
Binge drinking is a serious health concern, especially among the adolescent population. Despite widespread misuse of alcohol, the deleterious effects of this on brain function is lacking.
To help address this, the current study is the first to employ a binge drinking model in adolescent rats using clinically-relevant doses as well as a clinically-relevant route of administration to determine the impact on BGluM. We show dose-dependent effects of ethanol on BGluM in areas of the brain largely responsible for motor, sensory, and cognitive processes.
Decreased activity from the three ethanol doses were seen in the retrosplenial cortex and primary motor cortex. The retrosplenial cortex plays an important role in spatial cognition while the primary motor cortex aids in the activation of skeletal muscles and executing their movement (Jiang et al. 2018; Mitchell et al. 2018; Saab and Willis 2003). In addition, all doses of ethanol decreased BGluM in the primary somatosensory cortex and visual cortex, which play an important role in processing sensory information as well as executing motor functions, and the processing of visual information, respectively (Borich et al. 2015; Tootell et al. 1998). The LD and MD ethanol doses decreased BGluM in the superior colliculus, which is an important multi-sensory hub in the midbrain that is responsible for visual attention to environmental stimuli as well as defense behavior (Dean et al. 1988; Gitelman et al. 2002). The inhibition of the visual cortex, motor cortex, and the superior colliculi may represent an impaired ability to process visual information and respond according with motor movements following intoxication. This is consistent with fMRI data showing that alcohol administration reduces activation in the visual cortex following photic stimulation (Levin et al. 1998).
The cerebellum was inhibited by ethanol in the 6a cerebellar lobule for the LD group and the 8th cerebellar lobule for the MD and HD groups. The 6a cerebellar lobule plays a role in cognitive tasks while the 8th cerebellar lobule plays a role in sensory motor tasks (Stoodley et al. 2012). The decreased BGluM in these areas associated with motor cognition, processing, and navigation may indicate decreased motor cognition in behavior in each treatment dose of binge ethanol administration. This finding is supported by clinical data showing dramatic decreases in BGluM at a dose of 0.5 g/kg (Volkow et al. 2006).
The subiculum and the frontal association area are two regions that had decreased BGluM that were unique to the moderate dose binge ethanol rats. The hippocampus and subiculum are important for the formation, consolidation and retrieval of memories (Bohm et al. 2018), while the frontal association cortex is an area that has been associated with stimulus integration, memory formation, and other higher-order brain functions (Nakayama et al. 2015). Our findings of decreased BGluM in the frontal association area is consistent with the findings from Strother and colleagues, who showed reduced local cerebral glucose utilization after 1 g/kg dose of ethanol in the frontal cortex and posterior hippocampus among adolescent alcohol-preferring rats (Strother et al. 2008). Reduced BGluM in the hippocampus was found among the low dose and high dose ethanol binge rats. The decreased glucose metabolism in the hippocampus, subiculum, and frontal association area may be responsible for the associated memory loss known to occur during binge drinking (Hermens and Lagopoulos 2018).
In the high dose ethanol binge rats, the inferior colliculus, the raphe nucleus, and the thalamus showed a decreases in BGluM. The inferior colliculis is known to have a role in the processing of auditory information in the environment as well sound-induced flight behaviors (Brandão et al. 1993; Xiong et al. 2015). Similar to inhibition of the superior colliculus, the reduced in BGluM within the inferior colliculus could represent impaired auditory processing, especially as they relate to potential threats. The raphe nucleus supports serotonergic projections to the subthalamic nucleus which is known to compromise motor control during dysfunction (Reznitsky et al. 2016). The thalamus is another critical hub that is responsible for integrating and relaying sensory information (except olfaction). In addition to the other regions involved in sensory processing, impaired functioning of the thalamus through alcohol intoxication may further exasperate this diminished sense of awareness in the environment.
The default mode network (DMN) of the brain has been suggested to support self-referential functions in humans including recollection, conceptual processing, and autobiographical memory (Hsu et al. 2016). Regions in the rat brain model that have been associated with the DMN include the orbital, prelimbic, retrosplenial, cingulate, auditory, visual, post-parietal, frontal association area, and dorsal hippocampus. There are several regions in this study that have been associated with the DMN of the rat brain including the retrosplenial cortex, hippocampus, primary visual cortex, secondary visual cortex, and frontal association area. Decreased BGluM activity in the DMN has been thought to be suppression of activity in regions responsible for processing of introspective thinking and planning (Hsu et al. 2016).
There are several limitations to the current study. First, this study did not include an analysis of BACs. While we include the average dose (g/kg) for each group, this does not always reflect BAC levels, which can vary independent of the dose of ethanol consumed (Dilley et al. 2018). In addition, this study only included male rats. Due to the fact that there are sex differences related to the consumption of alcohol (Wilsnack et al. 2018), future studies should explore this as it relates to BGluM.
In summary, the present study is the first to demonstrate the effects of an acute ethanol binge drinking paradigm on regional BGluM, as measured by PET imaging. Across all three ethanol doses, we see many similarities in regions known to play a role in the processing of sensory and motor information. The results lend insight into the deleterious effects of binge drinking and highlight the functional changes that occur in the brain. Moreover, these results provide clarity into the behavioral manifestations that can occur following binge drinking. Adolescent binge drinking is known to produce a multitude of cognitive impairments, including poor short-term and long-term memory performance, reduced visuospatial function, as well as reduced cognitive flexibility and learning (Lannoy et al. 2019; Lees et al. 2020). The regions of the brain responsible for many of these functions were impacted following binge drinking in our model, thus demonstrating strong translational utility of our design. These changes in functional connectivity help provides anatomical locations for further investigation of molecular and genetic targets involved in binge drinking.
Availability of data and material:
Available from the corresponding author at request.
Code Availability
Not applicable.
References
Bohm C, Peng Y, Geiger JRP, Schmitz D (2018) Routes to, from and within the subiculum. Cell Tissue Res 373:557–563
Borich MR, Brodie SM, Gray WA, Ionta S, Boyd LA (2015) Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia 79:246–255
Brandão ML, Melo LL, Cardoso SH (1993) Mechanisms of defense in the inferior colliculus. Behav Brain Res 58:49–55
Chung T, Creswell KG, Bachrach R, Clark DB, Martin CS (2018) Adolescent Binge Drinking. Alcohol research: current reviews 39:5–15
Crews FT, Braun CJ, Hoplight B, Switzer RC 3rd, Knapp DJ (2000) Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res 24:1712–1723
Crews FT, Vetreno RP, Broadwater MA, Robinson DL (2016) Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol reviews 68:1074–1109
Dean P, Mitchell IJ, Redgrave P (1988) Responses resembling defensive behaviour produced by microinjection of glutamate into superior colliculus of rats. Neuroscience 24:501–510
Dilley JE, Nicholson ER, Fischer SM, Zimmer R, Froehlich JC (2018) Alcohol Drinking and Blood Alcohol Concentration Revisited. Alcoholism, clinical and experimental research. 42, 260–269
Duke AA, Giancola PR, Morris DH, Holt JC, Gunn RL (2011) Alcohol dose and aggression: another reason why drinking more is a bad idea. J Stud Alcohol Drugs 72:34–43
Esser MB, Clayton H, Demissie Z, Kanny D, Brewer RD (2017) Current and Binge Drinking Among High School Students - United States, 1991–2015. MMWR Morb Mortal Wkly Rep 66:474–478
Gispert JD, Figueiras FP, Vengeliene V, Herance JR, Rojas S, Spanagel R (2017) Changes in cerebral [(18)F]-FDG uptake induced by acute alcohol administration in a rat model of alcoholism. Behav Brain Res 327:29–33
Gitelman DR, Parrish TB, Friston KJ, Mesulam MM (2002) Functional anatomy of visual search: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage 15:970–982
Grant BF, Dawson DA (1997) Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 9:103–110
Hermens DF, Lagopoulos J (2018) Binge Drinking and the Young Brain: A Mini Review of the Neurobiological Underpinnings of Alcohol-Induced Blackout. 9
Hingson RW, Edwards EM, Heeren T, Rosenbloom D (2009) Age of drinking onset and injuries, motor vehicle crashes, and physical fights after drinking and when not drinking. Alcohol Clin Exp Res 33:783–790
Hsu LM, Liang X, Gu H, Brynildsen JK, Stark JA, Ash JA, Lin CP, Lu H, Rapp PR, Stein EA, Yang Y (2016) Constituents and functional implications of the rat default mode network. Proc Natl Acad Sci U S A 113:E4541–E4547
Jiang N, Li G, Wei J, Wei B, Zhu FF, Hu Y (2018) Transcranial direct current stimulation of the primary motor cortex on postoperative pain and spontaneous oscillatory electroencephalographic activity following lumbar spine surgery: A pilot study. Restor Neurol Neurosci 36:605–620
Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey BB, Regier DA, Schwab-Stone M (1997) Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol 25, 121–32.
Krieger H, Young CM, Anthenien AM, Neighbors C (2018) The Epidemiology of Binge Drinking Among College-Age Individuals in the United States. Alcohol Res 39:23–30
Kuntsche E, Kuntsche S, Thrul J, Gmel G (2017) Binge drinking: Health impact, prevalence, correlates and interventions. Psychol Health 32:976–1017
Lannoy S, Billieux J, Dormal V, Maurage P (2019) Behavioral and Cerebral Impairments Associated with Binge Drinking in Youth: A Critical Review. Physiol Belgica 59:116–155
Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM (2020) Effect of alcohol use on the adolescent brain and behavior. Pharmacology, biochemistry, and behavior. 192, 172906–172906
Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF (1998) Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Res 82:135–146
McDowell MA, Fryar CD, Hirsch R, Ogden CL (2005) Anthropometric reference data for children and adults: U.S. population, 1999–2002. Adv Data. 1–5
Miller JW, Naimi TS, Brewer RD, Jones SE (2007) Binge drinking and associated health risk behaviors among high school students. Pediatrics 119:76–85
Mitchell AS, Czajkowski R, Zhang N, Jeffery K, Nelson AJD (2018) Retrosplenial cortex and its role in spatial cognition. Brain Neurosci Adv 2:2398212818757098
Nakayama D, Baraki Z, Onoue K, Ikegaya Y, Matsuki N, Nomura H (2015) Frontal association cortex is engaged in stimulus integration during associative learning. Curr Biol 25:117–123
Patrick ME, Schulenberg JE (2013) Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Res 35:193–200
Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH (1998) Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcohol Clin Exp Res 22:176–182
Reznitsky M, Plenge P, Hay-Schmidt A (2016) Serotonergic projections from the raphe nuclei to the subthalamic nucleus; a retrograde- and anterograde neuronal tracing study. Neurosci Lett 612:172–177
Saab CY, Willis WD (2003) The cerebellum: organization, functions and its role in nociception. Brain Res Brain Res Rev 42:85–95
SAMSHA (2015) Substance Use Disorders. Vol., ed.^eds. Substance Abuse and Mental Health Services Administration, SAMSHA
Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL (2003) A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods 129:105–113
Stoodley CJ, Valera EM, Schmahmann JD (2012) Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59:1560–1570
Strother WN, Lumeng L, McBride WJ (2008) Acute ethanol effects on local cerebral glucose utilization in select central nervous system regions of adolescent alcohol-preferring (P) and alcohol-nonpreferring (NP) rats. Alcohol Clin Exp Res 32:1875–1883
Thanos PK, Wang GJ, Volkow ND (2008) Positron emission tomography as a tool for studying alcohol abuse. Alcohol Res Health 31:233–237
Tootell RBH, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM (1998) Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci U S A 95:811–817
Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S et al (1990) Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res 35:39–48
Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Li K, T (2006) Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage 29:295–301
Volkow ND, Kim SW, Wang GJ, Alexoff D, Logan J, Muench L, Shea C, Telang F, Fowler JS, Wong C, Benveniste H, Tomasi D (2013) Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage 64:277–283
WHO, W.H.O (2018) Global Status Report on Alcohol and Health 2018. World Health Organization
Williams-Hemby L, Porrino LJ (1994) Low and moderate doses of ethanol produce distinct patterns of cerebral metabolic changes in rats. Alcohol Clin Exp Res 18:982–988
Williams-Hemby L, Porrino LJ (1997) I. Functional consequences of intragastrically administered ethanol in rats as measured by the 2-[14 C]deoxyglucose method. Alcohol Clin Exp Res 21:1573–1580
Wilsnack RW, Wilsnack SC, Gmel G, Kantor LW (2018) Gender Differences in Binge Drinking. Alcohol research: current reviews 39:57–76
Woo C-W, Krishnan A, Wager TD (2014) Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage 91:412–419
Xiong XR, Liang F, Zingg B, Ji X-y, Ibrahim LA, Tao HW, Zhang LI (2015) Auditory cortex controls sound-driven innate defense behaviour through corticofugal projections to inferior colliculus. Nat Commun 6:7224
Funding
This work was supported by the Research Foundation for the State University of New York (#RIAQ0094).
Author information
Authors and Affiliations
Contributions
CR: Investigation, Data curation, Writing, Formal analysis, Visualization. JH: Writing, Visualization, Formal analysis. KR: Supervision, Writing. MS: Resources. RY: Resources. PT: Conceptualization, Resources, Writing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
None.
Ethical approval
All experiments were conducted in conformity with the National Academy of Science Guide for Care and Use Laboratory Animals and approved by the University Institutional Animal Care and Use Committee.
Consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rapp, C., Hamilton, J., Richer, K. et al. Alcohol binge drinking decreases brain glucose metabolism and functional connectivity in adolescent rats. Metab Brain Dis 37, 1901–1908 (2022). https://doi.org/10.1007/s11011-022-00977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-00977-8