Abstract
Multiple sclerosis (MS) is a neurodegenerative disease with various factors affecting its etiology. Overproduction of nitric oxide and subsequent lesions of biopolymers are some of the possible causes of the disease. This study aimed to measure the most relevant nitrosative and oxidative stress biomarkers and the level of modified DNA bases in patients with MS. Each parameter was assayed in 25 patients with MS and 25 healthy controls. This study involved detecting blood plasma and serum nitric oxide metabolites by chemiluminescence detector Sievers NOA-280i, malondialdehyde (MDA) measurements with thiobarbituric acid reactive substance (TBARS) assay, detection of oxidized purines and pyrimidines with the enzyme-modified comet assay. Statistical analysis of the results was performed by one-way analysis of variance (ANOVA) and unpaired t test for the comparison of less than three data sets. DNA single-strand breaks, levels of modified purines and pyrimidines, as well as nitrite and nitrate levels in plasma and serum samples, were significantly higher in patients with MS than in healthy controls. On the contrary, MDA levels appeared to be lower in patients with MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis is a neurodegenerative disease with chronic and inflammatory characteristics. MS targets axons in the CNS, causing their demyelination, thus disrupting signaling pathways (Stadelmann et al. 2011; Trapp and Nave 2008). A total of 2.8 million people are estimated to live with MS worldwide (Walton et al. 2020). While symptoms can occur at any age, the most common onset age is usually 20 to 40 years (Magyari and Sorensen 2019).
The exact cause of the demyelination is still unknown. This process is supposed to develop as the sequence of various factors such as environmental, genetic, metabolic, viral, or oxidative stress-induced. Together or separately, these factors result in an autoimmune disorder with the consequent immune attack on the CNS (Fiorini et al. 2013; Gonsette 2008; Miller et al. 2012).
Multiple sclerosis is classified into three main subtypes based on the clinical course of the disease. Relapsing-remitting Multiple Sclerosis (RRMS) is the most common type of ailment. It is characterized by relapses of symptoms following the improvement in the patient’s general condition or total disappearance of symptoms. Secondary progressive MS (SPMS) develops as the following stage of the RRMS, and it is characterized by a lack of remissions or gradual deterioration of the patient. Primary progressive MS (PPMS) appears in the form of gradual worsening of symptoms with occasional stability in overall condition. (Ghasemi et al. 2017; Goldenberg 2012; Kalincik 2015; Thompson et al. 2018; Weiner 2008). Alternatively MS phenotypes can be classified according the Expanded Disability Status Scale (EDSS) (Lublin et al. 2014).
Various studies have already confirmed the theory about oxidative stress being a crucial factor in the demyelination process in MS. (Fiorini et al. 2013; Gonsette 2008; Miller et al. 2012). Oxidative stress level can be assessed determining the molecules changed under the influence of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Furthermore, under the effect of oxidative stress, deoxyguanosine is rapidly converted to 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) (Hinokio et al. 1999).
Overproduction of nitric oxide by inducible NO synthase (iNOS) in oligodendrocytes with subsequent lesions of mitochondria is considered crucial in the pathogenesis of MS (Lan et al. 2018). Microglia-mediated neuroinflammation is deemed to be a priming event in the onset and progression of MS. Microglia activation is associated with the release of proinflammatory cytokines and increased production of superoxide and nitric oxide. The two radicals then react to form the neurotoxic peroxynitrite (Kumar et al. 2014). Overproduction of NO in the brain can influence the level of NO metabolites – nitrites and nitrates in the blood (Adamczyk et al. 2017).
ROS-mediated process of lipid peroxidation results in the degradation of polyunsaturated lipids. Reaching biological membranes, this chain of reactions can be induced by various toxic products, including endoperoxides and aldehydes (Rosenblum et al. 1989). MDA is one such secondary product and is commonly used as a biomarker for cell membrane damage evaluation (Esterbauer et al. 1991).
The present study aimed to evaluate the oxidative stress damage in peripheral blood mononuclear cells, measurements of NO metabolite, and lipid peroxidation products in human plasma and serum.
Materials and methods
Patients
Permission of the Central Medical Ethics Committee of the Republic of Latvia No 1/17–10-10 was received to perform this study. Informed consent was obtained from every participant of the study. The study group consisted of 25 unrelated patients with MS randomly chosen among the MS patients attending the Latvian Maritime Medicine Center (detailed information is summarized in Table 1) and the control group of 25 healthy subjects: 4 males and 21 females of 20 to 43 years (average age of the group 34.2 ± 1.7 years). Healthy subjects were volunteers and were defined as those who did not have a history of any chronic and autoimmune diseases. Collection of the MS and control group samples was performed during the same season, samples of both groups were analysed in parallel. Most of the patients were undergoing disease modifying therapies (DMT). Drugs were chosen according to individual preference, availability on the market and effectiveness. Samples of the same patients were used for every method.
Collection and processing of blood samples
Blood was obtained by vein puncture and collected in plastic capillaries with EDTA (BD Vacutainer K2E EDTA 10.8 mg, BD-Plymouth, UK). Peripheral blood mononuclear cells (PBMNC) were isolated using Histopaque-1077 (Sigma-Aldrich, Germany) according to the protocol provided by the manufacturer.
Plasma preparation
Whole blood was collected into EDTA treated tubes and centrifuged for 10 min at 1500 x g in the pre-cooled centrifuge. The supernatant was removed, split into 0.5 mL aliquots, and stored at −20 °C.
Serum preparation
Whole blood was collected into EDTA-free tubes and allowed to clot at room temperature. The clot was removed by centrifugation for 10 min at 1500 x g in the pre-cooled centrifuge. The supernatant was immediately split into 0.5 mL aliquots and stored at −20 °C.
Modified single-cell gel electrophoresis (modified comet assay)
The DNA single-strand damage and oxidized bases were detected in isolated peripheral blood mononuclear cells utilizing single-cell gel electrophoresis in the presence of two DNA repair enzymes: Fpg (Sigma-Aldrich, Germany) and EndoIII (Sigma-Aldrich, Germany). This modification of the comet assay enables detection of the oxidized bases (Collins et al. 1993; Collins et al. 1996). Control and patient samples were analysed under the same conditions. Each of them was analysed in duplicates – two samples per slide. The general procedure for alkaline comet assay was previously described (Borisovs et al. 2019) and performed with a few modifications. 50 μL of isolated PBMNC were mixed with 100 μL of 1% low-melting agarose preheated to 37 °C (Sigma-Aldrich, Germany). 50 μL of the mixture were placed on an agarose-precoated (0.5% type III, Sigma-Aldrich, Germany) microscope slide (two samples per one slide) and covered with 24 × 24 mm cover glass (Corning, USA). The slides were kept on the ice up to the solidification of agarose. 10 mM KBrO3 solution was prepared (0.1 N KBrO3 stock, Merck, Germany), 50 μL of this solution were applied on the prepared sample slides, covered with cover glass, and incubated for 5 min at room temperature. Slides were placed into the lysis solution (2.5 M NaCl, 10 mM Na2EDTA, 10 mM Tris, pH 10 [AppliChem, Germany], 1% Triton-X 100, 5% DMSO [Sigma-Aldrich, Germany]) and left overnight at +4 °C. On the next day and right before use, fresh enzyme reaction (ER) buffer was prepared: 40 mL HEPES (Sigma-Aldrich, Germany), 0.1 M KCl (Sigma-Aldrich), 0.5 mM EDTA (Sigma-Aldrich, Germany), 0.2 mg/mL BSA (Sigma-Aldrich, Germany), pH 8. Fpg protein from Escherichia coli and EndoIII protein from Escherichia coli 1:3000 dilutions in ER buffer were prepared. 50 μL of each enzyme solution were applied to the samples. Samples were then incubated at 37 °C: 30 min for Fpg and 45 min for EndoIII. After the incubation, the standard alkaline comet assay protocol was followed. Cells were visually graded into 5 classes (A0 – A4) (Collins et al. 1993; Collins et al. 1994) from class 0 (undamaged, no discernible tail) to class 4 (almost all DNA in tail, insignificant head). DNA damage index (D) in arbitrary units was calculated as follows: D = A1 + 2 × A2 + 3 × A3 + 4 × A4.
Nitrite and nitrate measurements
Chemiluminescence detector Sievers NOA-280i was used for the following analyses.
-
Measurements of nitrites. This and the following procedure were performed according to the manual provided by the manufacturer. 5 mL of the reducing agent were prepared by dissolving 50 mg NaI (Sigma-Aldrich, Germany) in 0.5 μL deionized water and mixing with 4.5 mL glacial acetic acid (Sigma-Aldrich, Germany). 10 mL of 100 mM NO2− standard stock solution were prepared by dissolving ~69 mg NaNO2 (Sigma-Aldrich, Germany) in 10 mL deionized water. 10 nM NO2−, 100 nM NO2−, 1 μM NO2− and 10 μM NO2− standard solutions were prepared by diluting the 100 nM standard stock solution. 50 μL of each standard solution were injected in triplicates, and a calibration curve was created before the experiment. 50 μL of each plasma or serum sample were injected into the reducing agent.
-
Measurements of nitrates. 100 mL of the reducing agent were prepared by dissolving 0.8 g VCl3 (Sigma-Aldrich, Germany) in 1 M HCl (Sigma-Aldrich, Germany) and filtered through the filter paper. The gas bubbler was filled with 15 mL of 1 M aqueous NaOH (Sigma-Aldrich, Germany) solution to prevent HCl vapours from entering the NOA. The water bath was connected to the purge vessel, and the temperature was set to 95 °C. 10 mL of 100 mM NO3− standard stock solution were prepared by dissolving ~85 mg NaNO3 (Sigma-Aldrich, Germany) in 10 mL deionized water. 10 nM NO3−, 100 nM NO3−, 1 μM NO3− and 10 μM NO3− standard solutions were prepared by diluting the 100 nM standard stock solution. 50 μL of each standard solution were injected in triplicates, and a calibration curve was created before the experiment. 50 μL of each plasma or serum sample were injected into the reducing agent and NO3−.
Concentrations of NO2− and NO3− were calculated using the provided “NOA Liquid” software based on each calibration curve.
TBARS assay
Measurements were performed by Lambda 25 UV-VIS spectrometer (PerkinElmer, UK) in 1 cm thick single-use cuvettes (Sarstedt, Germany) 4.0 mM TBA standard solution was prepared by dissolving 57.66 mg TBA (Sigma-Aldrich, Germany) in 100 mL 99.5% glacial acetic acid (Sigma-Aldrich, Germany). 1 mM MDA standard stock solution was prepared by dissolving 31.35 mg malondialdehyde tetrabutylammonium salt (Sigma-Aldrich, Germany) in 100 mL 99.5% glacial acetic acid. 0.1, 0.2, 0.4, 0.6, and 0.8 mM standard solutions were prepared by diluting the 4 mM MDA standard stock solution. 500 μL of 4.0 mM standard TBA solution were mixed with the corresponding MDA standard solution, and the mixture was heated for 60 min at 95 °C in the water bath. The absorption of each sample was then measured at 532 nm, and the calibration curve was constructed.
500 μL of plasma or serum were mixed with 500 μL 4.0 mM TBA standard solution. The mixture was then heated for 60 min at 95 °C in the water bath, and the absorption of each sample was measured at 532 nm. MDA concentration in plasma or serum samples was calculated based on the calibration curve.
Statistical analysis
All the data of DNA damage levels assayed by the enzymatic comet assay were measured in arbitrary units (AU) represented as mean ± standard error of the mean (SEM). Ordinary non-parametric one-way analysis of variance (ANOVA) was performed using the commercially available GraphPad Prism, and the data were considered statistically significant at p < 0.05.
Results
Demographic data
Samples from the same 25 patients with MS (study group is described in Table 1), and 25 healthy subjects were used in each experiment. RRMS was the predominant type of MS in the study group. Table 2 shows the disease-modifying therapy (DMT) synopsis based on administration of the active substance of each drug. Taking into account the total number of the registered MS patients in Latvia (2035), 1.2% of all patients were enrolled in the study.
Modified comet assay
Comet assay results (Figs. 1–1 and –4) show an increased level of DNA single-strand breaks in patients with MS compared with the healthy subjects. This observation supplements previous alkaline comet assay results (Borisovs et al. 2019). To evaluate the number of oxidized bases, DNA damage data was subtracted from the enzyme induced damage data. Apparently, the level of oxidized bases is similar in the MS (Fig. 1–5 and 6) and control groups (Fig. 1–3 and 4). Individual deviations between patient were highly variable, statistically significant differences were not reached (p = 0.919).
Modified comet assay data showing DNA damage levels and levels of oxidized bases (arbitrary units) in PBMNC of healthy controls and patients with MS. 1 – Total DNA damage of healthy subjects; 2 and 3 – levels of oxidized bases in healthy subjects; 4 – total DNA damage of patients with MS; 5 and 6 – levels of oxidized bases in patients with MS. Data are presented as mean ± SEM (arbitrary units). # p < 0.05 vs. the control group; levels of oxidized bases were not statistically significant (p = 0.9190)
TBARS assay in plasma and serum
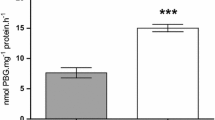
MDA is a widely used biomarker for various disorders, including MS. Results shown in Fig. 2 indicate a decrease in MDA levels in plasma and serum of patients with MS compared to healthy subjects. Plasma and serum control samples have 5.33 ± 0.19 μM MDA (Fig. 3; Control group – P) and 6.07 ± 0.42 μM MDA (Fig. 2; Control group – S) accordingly. As for the plasma and serum from patients with MS – 3.78 ± 0.32 μM MDA (Fig. 2; patients with MS – P) and 4.95 ± 0.23 μM MDA (Fig. 2; Patients with MS – S) accordingly.
NO metabolites in plasma and serum
To our knowledge, there is currently no evidence proving whether it is more accurate to determine metabolite concentrations in blood plasma or serum. In this study, nitrite and nitrate concentrations were determined both in plasma and serum of patients with MS. According to results shown in Fig. 3, nitrite concentration in plasma and serum of healthy subjects are on the same level – 0.64 ± 0.02 μM (Fig. 3; Control group – P) in plasma and 0.65 ± 0.04 μM (Fig. 3; Control group – S) in serum. On the other hand, overall levels of nitrites in plasma and serum of patients with MS are significantly higher than in healthy subjects. However, nitrite concentration in plasma appeared to be higher than in serum – 1.13 ± 0.05 μM (Fig. 3 Patients with MS - P) in plasma and 0.79 ± 0.06 μM (Fig. 3; Patients with MS - S) in serum.
Nitrate concentrations (Fig. 4) follow a similar trend to nitrite concentrations. Namely, both plasma and serum of healthy subjects have the same levels of nitrates – 24.98 ± 1.20 μM (Fig. 4; Control group - P) in plasma and 25.05 ± 3.44 μM (Fig. 4; Control group - S) in serum. Samples taken from patients with MS indicate higher nitrate amounts both in plasma and serum – 33.67 ± 2.97 μM (Fig. 4; Patients with MS - P) in plasma and 36.30 ± 3.81 μM (Fig. 4; Patients with MS - S) in serum.
Discussion
According to different studies subjects with diagnosed Alzheimer’s (AD) and Parkinson’s (PD) diseases have higher levels of single-strand DNA breaks (Milic et al. 2015). Another set of studies described by Kuchařová et al. (2019) support the same idea and indicate that patients with AD and PD also have higher levels of oxidized DNA bases. Interestingly, Souliotis et al. 2019 describe the correlation between the increase of DNA damage and polymorphisms at DNA repair enzymes’ genes of patients with Systematic Sclerosis. DNA damage levels are quite variable for each patient, and so are levels of oxidized bases. However, even though current data is not statistically significant, control group had about the same levels of oxidized bases as the study group. Considering overall DNA damage is higher within patients with MS, bigger sample pool is required to evaluate the potency of oxidized bases as a biomarker for MS.
Adamczyk et al. (2017) conducted research evaluating MDA levels in serum of newly diagnosed (untreated) patients with MS and depending on three different drug therapies. Their results indicate that newly diagnosed patients with RRMS have the highest amount of MDA in serum. Drug-treated patients have lower amounts of MDA in their serum, but values are still higher than control samples. Lower MDA levels contradict previous research performed by Saif Eldeen et al. 2019, which states that patients with MS have increased MDA levels in serum. On the other hand, research by Noroozi et al. (2017) has shown a significant decrease in patients’ serum MDA after a period of IFN-β 1a therapy.
The amount of data regarding NO metabolites, namely, nitrites and nitrates, is currently scarсу. One of the first studies on the given topic shows an increase in nitrite concentration in the human serum of patients with demyelination disorders (Giovannoni et al. 1997).
Examples include acute disseminated encephalomyelitis and neuromyelitis optica. The nitrite and nitrate levels in serum of neuromyelitis optica patients were previously reported to match the NOx levels in patients with MS (Haghikia et al. 2015). Acute disseminated encephalomyelitis is characterized by a profound increase in NOx serum levels than those found in patients with MS (Fominykh et al. 2016).
The primary assumption at this point is that an increase of NO metabolites in blood plasma and serum of the patients might be due to the overproduction of NO by iNOS in oligodendrocytes (Lan et al. 2018).
Overall, the increased levels of NOx in the serum of patients with MS reported in the present study are consistent with previously reported NOx levels for at least several other demyelinating diseases.
Levels of NO metabolites were significantly higher in MS patients. This leads to an assumption that the overproduction of nitric oxide synthases might be the case for MS. No objective evidence to assume the effect of DMTs on the overproduction of NOS.
In the case of MDA, previously described studies indicate that MDA levels should generally be higher within patients of MS, so in this case, DMTs most-likely influence the overall MDA levels. Further investigation with larger study group undergoing DMTs is required.
Conclusions
-
Patients with MS have higher overall DNA damage levels, but oxidized bases appear to be on the same level as in the control group.
-
Compared to healthy subjects, patients with MS have decreased MDA levels both in plasma and serum samples.
-
Nitrite concentrations in plasma and serum of patients with MS are significantly higher compared to healthy subjects.
-
Nitrate concentrations in plasma and serum of patients with MS are on similar levels and significantly higher than in healthy subjects.
Data availability
All data generated or analyzed during the study are summarized into figures and included in the manuscript.
References
Adamczyk B, Wawrzyniak S, Kasperczyk S, Adamczyk-Sowa M (2017) The evaluation of oxidative stress parameters in serum patients with relapsing-remitting multiple sclerosis treated with II-line immunomodulatory therapy. Oxidative Med Cell Longev 2017:1–12. https://doi.org/10.1155/2017/9625806
Borisovs V, Ļeonova E, Baumane L, Kalniņa J, Mjagkova N, Sjakste N (2019) Blood levels of nitric oxide and DNA breaks assayed in whole blood and isolated peripheral blood mononucleated cells in patients with multiple sclerosis. Mutat. Res. Toxicol. Environ. Mutagen. The Comet Assay – Human Biomonitoring 843:90–94. https://doi.org/10.1016/j.mrgentox.2018.11.008
Collins AR, Duthie SJ, Dobson VL (1993) Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis 14:1733–1735. https://doi.org/10.1093/carcin/14.9.1733
Collins AR, Fleming IM, Gedik CM (1994) In vitro repair of oxidative and ultraviolet-induced DNA damage in supercoiled nucleoid DNA by human cell extract. Biochim Biophys Acta BBA Gene Struct Expr 1219:724–727. https://doi.org/10.1016/0167-4781(94)90236-4
Collins AR, Dusinská M, Gedik CM, Stĕtina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104:465–469
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128. https://doi.org/10.1016/0891-5849(91)90192-6
Fiorini A, Koudriavtseva T, Bucaj E, Coccia R, Foppoli C, Giorgi A, Schininà ME, Domenico FD, Marco FD, Perluigi M (2013) Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: the spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS One 8:e65184. https://doi.org/10.1371/journal.pone.0065184
Fominykh V, Onufriev MV, Vorobyeva A, Brylev L, Yakovlev AA, Zakharova MN, Gulyaeva NV (2016) Increased S-nitrosothiols are associated with spinal cord injury in multiple sclerosis. J Clin Neurosci 28:38–42. https://doi.org/10.1016/j.jocn.2015.09.017
Ghasemi N, Razavi S, Nikzad E (2017) Multiple sclerosis: pathogenesis, symptoms, Diagnoses and Cell-Based Therapy. Cell J Yakhteh 19:1–10
Giovannoni G, Heales SJ, Silver NC, O’Riordan J, Miller RF, Land JM, Clark JB, Thompson EJ (1997) Raised serum nitrate and nitrite levels in patients with multiple sclerosis. J Neurol Sci 145:77–81. https://doi.org/10.1016/s0022-510x(96)00246-8
Goldenberg MM (2012) Multiple sclerosis review. Pharm Ther 37:175–184
Gonsette RE (2008) Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci 274:48–53. https://doi.org/10.1016/j.jns.2008.06.029
Haghikia A, Kayacelebi AA, Beckmann B, Hanff E, Gold R, Haghikia A, Tsikas D (2015) Serum and cerebrospinal fluid concentrations of homoarginine, arginine, asymmetric and symmetric dimethylarginine, nitrite and nitrate in patients with multiple sclerosis and neuromyelitis optica. Amino Acids 47:1837–1845. https://doi.org/10.1007/s00726-015-2015-0
Hinokio Y, Suzuki S, Hirai M, Chiba M, Hirai A, Toyota T (1999) Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia 42:995–998. https://doi.org/10.1007/s001250051258
Kalincik T (2015) Multiple sclerosis relapses: epidemiology, outcomes and management a systematic review. Neuroepidemiology 44:199–214. https://doi.org/10.1159/000382130
Kuchařová M, Hronek M, Rybáková K, Zadák Z, Štětina R, Josková V, Patková A (2019) Comet assay and its use for evaluating oxidative DNA damage in some pathological states. Physiol Res 68:1–15. https://doi.org/10.33549/physiolres.933901
Kumar A, Chen S-H, Kadiiska MB, Hong J-S, Zielonka J, Kalyanaraman B, Mason RP (2014) Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells. Free Radic Biol Med 73:51–59. https://doi.org/10.1016/j.freeradbiomed.2014.04.014
Lan M, Tang X, Zhang J, Yao Z (2018) Insights in pathogenesis of multiple sclerosis: nitric oxide may induce mitochondrial dysfunction of oligodendrocytes. Rev Neurosci 29:39–53. https://doi.org/10.1515/revneuro-2017-0033
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L et al (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286. https://doi.org/10.1212/WNL.0000000000000560
Magyari M, Sorensen PS (2019) The changing course of multiple sclerosis: rising incidence, change in geographic distribution, disease course, and prognosis. Curr Opin Neurol 32:320–326. https://doi.org/10.1097/WCO.0000000000000695
Milic M, Frustaci A, Del Bufalo A, Sánchez-Alarcón J, Valencia-Quintana R, Russo P, Bonassi S (2015) DNA damage in non-communicable diseases: a clinical and epidemiological perspective. Mutat Res 776:118–127. https://doi.org/10.1016/j.mrfmmm.2014.11.009
Miller E, Walczak A, Saluk J, Ponczek MB, Majsterek I (2012) Oxidative modification of patient’s plasma proteins and its role in pathogenesis of multiple sclerosis. Clin Biochem 45:26–30. https://doi.org/10.1016/j.clinbiochem.2011.09.021
Noroozi S, Arababadi MK, Meimand HAE, Asadikaram G (2017) The effect of IFN-beta 1a on biochemical factors in multiple sclerosis patients. Iran Red Crescent Med J 19:8
Rosenblum ER, Gavaler JS, Van Thiel DH (1989) Lipid peroxidation: a mechanism for alcohol-induced testicular injury. Free Radic Biol Med 7:569–577. https://doi.org/10.1016/0891-5849(89)90034-8
Saif Eldeen EMS, El Sharkawy R, Abd El Azim G, Mohamed N, Abd Elmajed M (2019) Role of nitric oxide and malondialdehyde biomarkers in relapsing-remitting multiple sclerosis. Sci J Al-Azhar Med Fac Girls 3:544. https://doi.org/10.4103/sjamf.sjamf_59_19
Souliotis VL, Vlachogiannis NI, Pappa M, Argyriou A, Ntouros PA, Sfikakis PP (2019) DNA damage response and oxidative stress in systemic autoimmunity. Int J Mol Sci 21. https://doi.org/10.3390/ijms21010055
Stadelmann C, Wegner C, Brück W (2011) Inflammation, demyelination, and degeneration — recent insights from MS pathology. Biochim. Biophys. Acta BBA - Mol. Basis dis., Molecular Basis of Multiple Sclerosis 1812:275–282. https://doi.org/10.1016/j.bbadis.2010.07.007
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Trapp BD, Nave K-A (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269. https://doi.org/10.1146/annurev.neuro.30.051606.094313
Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P (2020) Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler Houndmills Basingstoke Engl 26:1816–1821. https://doi.org/10.1177/1352458520970841
Weiner HL (2008) A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol 255(Suppl 1):3–11. https://doi.org/10.1007/s00415-008-1002-8
Acknowledgments
We express our deepest gratitude to the Centre for Marine Medicine staff in Riga, especially N. Mjagkova, for obtaining and preparation of human samples. The help of the participants of the COST action CA15132, “Comet assay as human biomonitoring tool (hCOMET)” in the mastering of the modified comet assay and interpretation of the results is highly acknowledged.
Code availability
Not applicable.
Funding
The study was funded from the European Regional Development Fund Project No. 1.1.1.1/16/A/016 “Identification of proteasome related genetic, epigenetic, and clinical markers for multiple sclerosis.”
Author information
Authors and Affiliations
Contributions
V. Borisovs designed the study as a part of his Ph.D. thesis. V. Borisovs and J. Bodrenko performed experiments. Samples were received from patients with MS under the supervision of J. Kalnina. V. Borisovs wrote the manuscript, and N. Sjakste revised the final version. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were declared regarding the study, authorship, and publication of this article.
Ethics approval
Permission of the Central Medical Ethics Committee of the Republic of Latvia No 1/17–10-10 was received to perform this study. Informed consent was obtained from every participant of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borisovs, V., Bodrenko, J., Kalnina, J. et al. Nitrosative stress parameters and the level of oxidized DNA bases in patients with multiple sclerosis. Metab Brain Dis 36, 1935–1941 (2021). https://doi.org/10.1007/s11011-021-00786-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00786-5