Abstract
In the current study, the effect of methanolic extract of Mitragyna speciosa leaf (MMS) against the rewarding and reinforcing properties of ethanol using a mouse model of conditioned place preference (CPP) and runway model of drug self-administration was studied. Subsequently, the effect of MMS on dopamine level in the nucleus accumbens (NAc) of the mouse brain was further investigated. From the data obtained, MMS (50 and 75 mg/kg, p.o.) significantly reversed the ethanol-place preference in mice, which is similar to the effect observed in the reference drugs acamprosate (300 mg/kg, p.o.) and clozapine (1 mg/kg, p.o.) treatment groups in CPP test. Likewise, the escalating doses of ethanol-conditioned mice reduced the runtime to reach goal box, infers the positive reinforcing effects of alcohol. Interestingly, MMS (50, 75 and 100 mg/kg, p.o.) significantly prolonged the runtime in ethanol-conditioned mice. Besides, MMS (50 and 75 mg/kg, p.o.) and reference drugs; acamprosate (300 mg/kg, p.o.) and clozapine (1 mg/kg, p.o.) treated mice significantly decreased the alcohol-induced elevated dopamine level in the NAc region of the brain. Overall, this study provides first evidence that MMS inhibits ethanol seeking behaviour in mice. Based on these findings, we suggest that Mitragyna speciosa may well be utilized for novel drug development to combat alcohol dependence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine is a major neurotransmitter in the mediation of the rewarding properties of alcohol and other drugs of abuse. The reinforcing effects of alcohol are partially mediated by activation of the mesolimbic dopaminergic pathway. The mesolimbic pathway (which mainly consists of the nucleus accumbens (NAc), ventral tegmental area (VTA) and prefrontal cortex) is a part of the motivational system in which the effect of various reinforcers such as desire for food and water, self-esteem, sex, sociability and substances abuse are being regulated (Koob and Volkow 2010).
The activation of the mesolimbic pathway through reinforcers, increases the dopaminergic neuronal activity in the VTA and increases dopamine (DA) release into the NAc and other areas, including the prefrontal cortex and amygdala (Brodie et al. 1990; Weiss et al. 1993). The released dopamine then binds to the dopamine receptors involved in reward and positive reinforcement. Repeated activation of the reward system by drugs or alcohol, sensitizes the dopaminergic system, leads to increasing drug salience, increased motivation in the use of addictive substances and the development of craving in response to substance-related stimuli (Nestler 2001).
In view of the theoretical importance of the DA in neurobiology of alcohol dependence, the use of medicines that modify the activity of brain dopamine is of considerable interest, as pharmacotherapy in the treatment of alcohol dependence. In animal models of alcohol dependence, administration of a dopamine receptor antagonist was discovered to be effective in reducing the alcohol consumption (Price and Middaugh 2004; Rassnick et al. 1992; Samson et al. 1993; Thanos et al. 2005), which suggests that dopamine antagonists are ideal for the treatment of addiction to alcohol. Several studies have proven that drugs that target dopaminergic pathways could be an important class of pharmacotherapy in treating alcohol dependence (Ma and Zhu 2014). The use of complementary medicines (CMs) in the treatment of alcohol-use disorder as a part of pharmacotherapy is overwhelming in recent years (Mattioli et al. 2012). CMs are alternative plant-based products used for the treatment of many ailments as they are likely to show effectiveness with minimal side effects (Lee et al. 2003; Sahraei et al. 2006). Besides, a large amount of evidences has been reported on the anti-alcohol effect of plant extract including the root of Pueraria lobata (kudzu), Salvia miltiorrhiza, Salvia przewalskii, Morinda citrifolia and Hypericum perforatum in preclinical as well as clinical study (Khan and Pandy 2016; Lukas et al. 2013; Pandy and Khan 2016b; Rezvani et al. 1999).
Mitragyna speciosa Korth. (M. speciosa) has been used as an herbal medicine to treat many illnesses for several decades. This plant is most abundantly found in Thailand and Malaysia, commonly known as ‘ketum’ in Malaysia and ‘kratom’ in Thailand. Traditionally, M. speciosa has been used to treat diarrhoea, cough, hypertension, muscle ache and fatigue as well as a replacement for morphine in the treatment for addicts (Chee et al. 2008; Hassan et al. 2013; Reanmongkol et al. 2001). Although, M. speciosa is known for its addiction potential and adverse health consequences including hepatotoxicity and mild nephrotoxicity after chronic consumption of this plant, it has also been reported for some neuropharmacological activities like analgesic, antidepressant and attenuation of ethanol withdrawal symptoms in rodents (Hassan et al. 2017; Idayu et al. 2011; Kruegel and Grundmann 2018; Kumarnsit et al. 2007). The pharmacological effect of M. speciosa is mainly contributed by its bioactive constituent, mitragynine and its interaction with dopaminergic, serotonergic and adrenergic receptors (Boyer et al. 2008; Horie et al. 2005; Matsumoto et al. 1996, 1997; Reanmongkol et al. 2001; Shamima et al. 2012; Yamamoto et al. 1999). Moreover, in vitro radioligand-binding assays revealed that mitragynine (the bioactive compound of Mitragyna speciosa) possess inhibitory effect [54.2%] on dopamine (D2) receptors (Boyer et al. 2008). It has also been observed that the long-term consumption of M. speciosa leaf darkened the skin although the user remained indoors (Norakanphadung 1966). The claim for the darker skin of habitual user of M. speciosa leaf is intriguing and suggested that it could be due to combination of psychoactive properties and molecular structure of mitragynine (Jansen and Prast 1988). It has been reported that the activation of the dopamine type 2 (D2) receptors in the rat pituitary gland attenuated the release of α-melanocyte stimulating-like peptides (Kebabian et al. 1984). Therefore, it has been postulated that mitragynine darkened the skin by inhibiting dopamine D2 receptors whereby increasing melanocyte-stimulating substances.
Recently, we reported the antidopaminergic effect of methanolic extract of Mitragyna speciosa (MMS) leaf using in vivo and ex vivo studies (Vijeepallam et al. 2016). Therefore, we hypothesized that MMS could alleviate the ethanol seeking behaviour mediated through antidopaminergic mechanism. In the present study, we investigate the effect of MMS against ethanol seeking behaviour using conditioned place preference (CPP) paradigm and runway model of drug self-administration in mice. Moreover, the effect of MMS on DA turnover in the NAc of ethanol-dependent mice has been examined.
Material and methods
Drugs and chemicals
Phosphate buffered saline (PBS) (Vivantis Inc., US), clozapine, and acamprosate (Sigma-Aldrich, USA) were used for the present study. The different doses of MMS (50, 75 and 100 mg/kg), acamprosate (300 mg/kg) and clozapine (1 mg/kg) as suspension were prepared using 1% w/v sodium carboxymethyl cellulose (CMC) solution and administered orally (p.o.) at a constant volume (10 ml/kg b.wt). Freshly prepared 10% v/v ethanol was used for animal behavioural studies by diluting of 95% v/v ethanol (Copens Scientific, Malaysia) in sterile water for injection. The escalating doses (0.5, 1, 2, 4 and 4 g/kg, i.p.) of 10% v/v ethanol were used to induce ethanol seeking behaviour in CPP and runway tests as described by (Khan and Pandy 2016; Pandy and Khan 2016a). The different doses of MMS used in the present in vivo study were chosen based on the reported lethal and therapeutic doses of MMS. LD50 of MMS was found at 4.90 g/kg in mice (Reanmongkol et al. 2001) and its CNS activities were reported at 50–1000 mg/kg (Reanmongkol et al. 2001; Sabetghadam et al. 2010; Senik et al. 2012).
Plant collection and identification
The leaf of M. speciosa was collected from a tree of about 12 m tall in Alor Setar Kedah, Malaysia. The leaf was then sent for authentication to Rimba Ilmu, Institute of Biological Science, University of Malaya and a voucher specimen (KUL 47980) was deposited for future reference.
Standardized extract of M. speciosa
Standardized methanolic extract of M. speciosa Korth (MMS) leaf was prepared by using cold extraction with sonication as mentioned in our earlier publication (Vijeepallam et al. 2016). The details of the phytochemical profiling of the extract was reported in our earlier publication and the concentration of mitragynine was found to be 4.4% w/w (Vijeepallam et al. 2016). The dried solvent-free standardized MMS was stored in an amber air tight container at 4 °C for further use.
Animal
Swiss albino male mice weighing about 25-30 g were obtained from the Animal Experimental Unit, University of Malaya 2 wk prior to usage. The mice were randomly segregated into groups of four mice in individually ventilated polycarbonate cages (n = 4 per cage) with ad libitum access to water and standard laboratory food pellet. The experimental animals were maintained under standard laboratory condition; 45–55% relative humidity and temperature of 22 ± 1 °C, 12 h light: 12 h dark cycle (lights off at 19.00 h). Prior to the experimental session, the animals were acclimatized to the laboratory conditions for a week with the utmost care taken to minimize animal suffering. The Institutional Animal Care and Use Committee of Faculty of Medicine, University of Malaya, Kuala Lumpur, approved the experimental protocol (IACUC Ethics No. 2016–190,908/PHAR/R/VP) in accordance with the National Research Council of the National Academies of the USA (Garber et al. 2011) guidelines.
Effect of MMS on ethanol-induced place preference using the CPP paradigm in mice
CPP paradigm
Apparatus
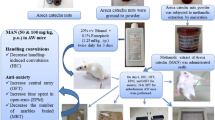
The CPP apparatus used in this study is similar to the one described elsewhere (Khan and Pandy 2016; Pandy and Khan 2016b; Pandy et al. 2018). The apparatus is made out of three compartments: small grey middle compartment, and two large terminal black and white compartments. Each compartment has discrete visual cues that distinguished it from other compartments: the black compartment has a glossy black floor and its walls are covered with horizontal black and white stripes; the white compartment has a matte-finish white floor with a net on top covered with vertical black and white stripes; and the grey compartment has glossy grey walls and grey floor. Each outer compartment measured 15 cm width × 20 cm height × 15 cm length to ensure enough room for the mouse to move. The compartments are separated with two vertical dividers, and the apparatus is covered with a clear acrylic lid. The lid is designed to ensure the formation of a gap between the lid and the top of the apparatus for compartment ventilation. The apparatus was fixed onto the laboratory bench (to minimize the mouse’s visual contact with the experimenter) and remained still in the same place throughout the study. Extra measures were taken to prevent interfering cues from affecting the study, this includes: no direct light on top of the apparatus, the light within the premises was diffused; compartments were cleaned with unscented solution; and the ambient noise was kept under 50db. With the help of a HD camera located above the test box, the animal behaviour during experimentation was recorded. The camera which was connected to a computer captures the animal behaviour and were later analyzed by a trained observer who was blind to the treatment protocol.
Experimental design
Place preference conditioning was performed as described in our previous study (Pandy and Khan 2016b). The CPP test was comprised of four specific phases: habituation, preconditioning, conditioning, and post-conditioning phases (Fig. 1).
Habituation: The habitation process took place for 3 days to reduce the novelty effect which might affect the measurements taken in the subsequent phases. During each day, the mice groups were individually transported from the animal facility to the laboratory in a specific order that was maintained during the whole experiment, and each mouse was placed in the grey compartment for 5 min, then the compartment dividers were lifted and the mouse was allowed to freely move between the compartments for 10 min. The starting time for the experiment was set to 8:00 am and was maintained during the whole experiment. No measurements were taken during this phase; however, all aggressive mice were removed to avoid behavioural alteration in the study.
Preconditioning: A 1-day phase that was similar to the habituation phase, but with measurements being taken for every mouse. When the compartment dividers were lifted, the times spent of each mouse in each compartment were recorded. Afterwards, conditioning scores were calculated using spreadsheet software. Any mice that showed a strong preference to one of the terminal compartments were excluded from the study, to ensure unbiased post-conditioning measurements.
Conditioning: In this phase, all treatment groups except saline control group were intraperitoneally injected with increasing doses of ethanol (0.5, 1, 2, 4 and 4 g/kg, i.p.) during odd days, and a fixed saline dose (10 ml/kg, i.p.) during even days as shown in Fig. 1 and conditioned in white and black compartments, respectively, for 30 min. As for the saline control group, mice were injected with the aforementioned saline dose during both odd and even days and conditioned for 30 min in the respective compartments. All conditioned animals were given a 1-day break following the 10-day conditioning trials, during which no tests (including any injections) were conducted.
Post-conditioning: A 1-day phase that was carried similar to the pre-conditioning phase in which the control groups (vehicle-saline and vehicle-ethanol) received 1% w/v CMC; the references drugs treated groups received acamprosate (300 mg/kg, p.o.) and clozapine (1 mg/kg, p.o.); the test groups received MMS at different doses (50, 75, and 100 mg/kg, p.o.) 1 h prior to the beginning of the post-conditioning testing. The conditioning score was calculated as mentioned earlier and data were processed in GraphPad Prism 5 software.
Effect of MMS on ethanol seeking behavior in mouse runway model of drug self-administration
Mouse runway model of drug self-administration
Apparatus
The specification and other details about runway apparatus was elaborated in earlier report by our research group (Pandy and Khan 2016a). Briefly, the runway apparatus was designed as z-shaped and made of composite aluminium. The apparatus consists of two square-shaped boxes, namely start box and goal box with a measurement of (150 mm (L) × 150 mm (W) × 200 mm (H)) each. The start and goal boxes are connected together with three straight runway segments in a zig-zag manner (600 mm (L) × 75 mm (W) × 200 mm (H) each segment and two 150 mm curved segments). There were 2 hurdles at a height of 30 mm, each of the segments of the runway, to cut down on the speed with which the animals reached the goal box. The start box and goal box have discrete visual cues to distinguish it from each other: the start box has white horizontal stripes on black walls with black polished floor surface. The start box is separated from the alley by a guillotine door. The goal box, on the other hand, has white wire-mesh floor and white walls with black vertical stripes. The total distance between the start box and the goal box is 1800 mm. The apparatus was fixed onto the laboratory bench (to minimize the mouse’s visual contact with the experimenter) and remained still in the same place throughout the study. Extra measures were taken to prevent interfering cues from affecting the study, this includes: no direct light on top of the apparatus, the light within the premises was diffused; compartments were cleaned with unscented solution; and the ambient noise was kept under 50 db. The animal behaviour during experimentation was recorded through a HD camera located above the test box which was connected to a computer and later analyzed by a trained observer who was blind to the treatment groups.
Procedure
The testing protocol consists of four distinct phases: habituation, preconditioning, conditioning and post-conditioning (Fig. 2).
- i.
Habituation, and Preconditioning
On the habituation day (Day 1–3), each mouse was placed in the start box for 60 s, after which the guillotine door was lifted, thus allowing the animal to explore the apparatus freely (except the goal box) for 10 min. On day 4 (Preconditioning day), each mouse was allowed to run along the runway from the start box to the goal box. The guillotine door of the goal box was immediately closed after entry of the mice to prevent backtracking. The time between the opening of the start box door and the closing of the goal box door was recorded and analyzed as mentioned above. The animals were returned to their home cages immediately after recording the runtimes. This initial runtime on the preconditioning day served as a baseline reading.
- ii.
Conditioning
The conditioning phase was scheduled for the next 5 days for 30-min per day in the goal box (Day 5 to Day 9). Each mouse was allowed to run from the start box along the runway to the goal box during conditioning sessions, with running times being recorded on every trial. Following the animal’s arrival in the goal box, a single injection of the respective dose of ethanol was given and confined in the goal box for 30-min over a period of 5 days for the respective treatment group (saline / ethanol, n = 10/group). One daily injection of saline from Day 5 to Day 9 was given to the saline group and ethanol group had one daily injection of escalating ethanol doses (0.5, 1, 2, 4 and 4 g / kg, i.p.) from Day 5 to Day 9.
- iii.
Post-conditioning
The post-conditioning (test day; Day 10) was scheduled 24 h after the last conditioning session. On Day 10, the effect of acute treatment of vehicle (1% w/v CMC), MMS (50, 75, 100 mg/kg, p.o.), acamprosate (ACAM 300 mg/kg, p.o.), and clozapine (CLZ 1 mg/kg, p.o.) on ethanol self-administration in mice was studied. In addition, the effect of vehicle (1% w/v CMC) and a higher dose of MMS (100 mg/kg, p.o.) on saline self-administration was studied in order to rule out the possible impact of MMS on motor activity. The treatment groups (vehicle-treated and MMS-treated) received an oral gavage of CMC and MMS, 60 min prior to the post-conditioning test. The 60-min after CMC or MMS treatment, the mice were placed in the start box for 60 s and then the guillotine door was lifted to allow the animals to move freely towards the goal box. No ethanol or saline injections were provided upon goal box entry on this “post-conditioning” trial.
- iv.
Estimation of dopamine level in NAc
Immediately after the runway test, the mice were euthanized by cervical dislocation and the NAc samples were collected as previously described by (Massart et al. 2015; Yoshimoto et al. 2012) with slight modifications. Briefly, the brains were carefully removed from the skull and cooled in ice cold phosphate buffered saline (PBS) for 1 min. Then the brains were sliced using a chilled mouse brain matrix (Ted Pella, Inc. USA) with 1 mm coronal section slice intervals. NAc were then isolated using a 1.5 mm tissue punch (Ueda et al. 1998). The entire procedures were conducted within 5–7 min for each sample. The isolated NAc samples were immediately frozen in dry ice and stored in −80 °C until further use. Furthermore, each sample was weighed and homogenized with 1:50 PBS using a pre-chilled 1 ml Dounce homogenizer. The homogenates were then centrifuged (model # 2-16PK, Sigma, Germany) for 20 min at the speed of 16,000 g rpm at 4 °C and the supernatants were collected in a prechilled polypropylene tubes and stored at −80 °C until analysis. The level of dopamine was measured using mouse dopamine sandwich ELISA kit (Catalogue No: QY-E20493, Qayee Biotechnology Co., Ltd., Shanghai, China) as per the manufacturer’s instructions.
Statistical analysis
The results were analyzed by two-way ANOVA and one-way ANOVA followed by post hoc Tukey’s multiple comparison test. The data are expressed as means ± SEM. Statistical analysis was performed using Graph Pad Prism version 5.0 statistical software (Graphpad Software, Inc., USA). The values of P < 0.05 were considered statistically significant.
Results
Effect of MMS on ethanol-induced place preference using CPP paradigm in mice
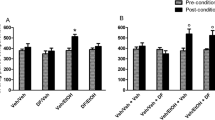
Figure 3 shows the effect of MMS on the ethanol-induced CPP in mice. A significant effect of treatment [F (6, 98) = 2.26; P = 0.04], Trial [F (1, 98) = 4.24; P = 0.04], and Treatment × Trial interaction [F (6, 98) = 4.06; P = 0.001] was found in Two-way ANOVA. A separate one-way ANOVA performed on the preconditioning scores of the different groups (vehicle-saline control, vehicle-ethanol control, acamprosate-treated, clozapine-treated and MMS-treated) revealed no significant differences were observed [F (6, 49) = 0.6532; P = 0.6873]. However, significant differences among the various treatment groups were observed in the postconditioning scores [F (6, 49) = 5.016; P = 0.0004]. The vehicle-ethanol control group that received escalating doses of ethanol (0.5, 1, 2, 4 and 4 g/kg, i.p.) on alternate days, indicated a significant (P < 0.01) CPP on postconditioning day, when compared with the vehicle-saline control group. Interestingly, the acute oral administration of MMS (50 and 75 mg/kg bw), acamprosate (300 mg/kg bw) and clozapine (1 mg/kg bw), an hour before the postconditioning test (on day 16) resulted in a significant reduction in the postconditioning scores (Fig. 3).
The effect of MMS on ethanol-induced CPP in mice. The data represent the differences between the times spent in the compartment associated with ethanol and saline. The negative values represent a preference for the black compartment and vice versa. Each bar represents the mean ± SEM (n = 8). Statistical significance was observed at ## P < 0.001 when compared with the vehicle-saline control group (SAL); *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with the vehicle-ethanol control group (VEH); ns indicates the differences were not statistically significant
Effect of MMS on ethanol seeking behavior in mouse runway model of drug self-administration
Mean runtimes for animals seeking ethanol during each day of the experiment (from pre-conditioning to post-conditioning) is shown in Fig. 4. A significant effect of Treatment [F (7, 148) = 19.25; P < 0.0001], Trial [F (1, 148) = 6.808; P = 0.01], and Treatment × Trial interaction [F (7, 148) = 18.79; P < 0.0001] was revealed by two-way ANOVA analysis. A separate one-way ANOVA computed on preconditioning runtimes of the different treatment groups (saline, ethanol, MMS, ACAM, and CLZ) was not statistically significant [F (7, 74) = 0.3656; P = 0.9194]. However, the difference between the groups (saline, ethanol, MMS, ACAM, and CLZ) on the post-conditioning runtimes were found to be statistically significant [F (7, 74) = 46.80; P < 0.0001]. The vehicle-ethanol control group (CMC + ETOH) that received escalating doses of ethanol (0.5, 1, 2, 4 and 4 g/kg, i.p.) upon goal box entry on each day produced a significant (P < 0.0001) decrease in runtime (faster run) on postconditioning day when compared with the vehicle-saline control (CMC + SAL) group. Interestingly, the acute oral administration of MMS (50, 75 and 100 mg/kg bw), acamprosate (300 mg/kg bw) and clozapine (1 mg/kg bw), 1 h prior to testing on day 10 (postconditioning) resulted in a significant reversal (P < 0.0001) of faster run to goal box to seek ethanol in mice (Fig. 4). However, the runtime recorded for MMS-saline group (MMS 100 + SAL) was not significantly differed from vehicle-saline control (CMC + SAL) group (Fig. 4) which implies the attenuating effect of MMS against ethanol seeking in mice is not mediated by causing the motor deficit.
Mean runtimes for animals seeking ethanol during each day of the experiment (from preconditioning to postconditioning) in mouse runway model of drug self-administration. Each point represents the mean ± SEM (n = 10–11). The effect of MMS (50, 75 and 100 mg/kg, p.o.), ACAM (300 mg/kg, p.o.), and CLZ (1 mg/kg, p.o.) on the ethanol seeking behaviour was compared with CMC-ethanol treated group on pre- and post-conditioning days in the mouse runway model of drug self-administration. Statistically significant differences were observed at #### P < 0.0001 and ****P < 0.0001 when compared with CMC-saline and CMC-ethanol groups respectively. When not indicated, the differences were not statistically significant. The effect of a higher dose of MMS (100 mg/kg, p.o.) on saline seeking behaviour was compared with CMC-saline treated group. When not indicated, the differences were not statically significant
Estimation of dopamine level in NAc
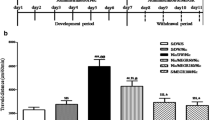
One-way ANOVA results revealed a significant (F (7, 72) = 4.339; P = 0.0005) changes in NAc dopamine level between different treatment groups. Post hoc comparisons revealed that, the dopamine level of vehicle-ethanol control (CMC + ETOH) group was significantly (P < 0.01) increased when compared to vehicle-saline control (CMC + SAL) group. Furthermore, MMS (50, 75, and 100 mg/kg, p.o.), ACAM (300 mg/kg, p.o.) and CLZ (1 mg/kg, p.o.) treated groups significantly attenuated the ethanol-induced increase in the NAc dopamine level (Fig. 5). However, no significant changes in the dopamine levels was observed between MMS-saline group (MMS 100 + SAL) and vehicle-saline control (CMC + SAL) group (Fig. 5) which indicates MMS might not alter the basal dopamine level in the NAc.
Effect of MMS (50, 75, and 100 mg/kg, p.o.), ACAM (300 mg/kg, p.o.) and CLZ (1 mg/kg, p.o.) on the dopamine levels in the NAc of mouse brain. Statistical significance was observed at ###P < 0.001 when compared with the vehicle-saline control group (CMC + SAL); * p < 0.05 and **P < 0.01when compared with the vehicle-ethanol control group (CMC + ETOH); ns indicates the differences were not statistically significant when compared with the vehicle-saline control group (CMC + SAL)
Discussion
In this study, ethanol was administered through the intraperitoneal route. This is because of fast onset of action of intraperitoneal injections that can reduce the conditioning period for at least 30 min. Moreover, the pharmacokinetics of substances administered intraperitoneally share a similarity when given by oral administration even though it is contemplated as a parenteral route of administration because the substance administered will be primarily absorbed by the mesenteric vessels, which later unload into the portal vein and pass through the liver (Turner et al. 2011). Furthermore, there are several reports indicating ethanol administered intraperitoneally produced the reinforcing effect in mice (Cunningham and Henderson 2000; de Licona et al. 2009; Lopez et al. 2014; Pandy and Khan 2016a; Song et al. 2007). Similarly, in this study, the outcome from the CPP test revealed that intraperitoneal injections of ethanol at escalating doses (0.5, 1, 2, 4 and 4 g/kg) in mice produced a significant place preference to the ethanol-paired compartment which infers the administration of escalating doses of ethanol during conditioning successfully produced CPPs in mice. Interestingly, the acute administration of MMS (50 and 75 mg/kg, p.o.), significantly reversed the alcohol-seeking behaviour in mice (Fig. 3). The reference drugs, acamprosate (used in alcohol dependence) and clozapine (dopamine receptor antagonist) also significantly alleviated the craving to ethanol in mice which is consistent with the previous reports (Czachowski et al. 2001; Drake et al. 2000; Kurokawa et al. 2013; Mattioli et al. 2012).
However, the significant downside of the CPP test is evincing false positive results for the candidature drugs acting by impairing learning and memory without influencing drug reward pathways (e.g., NMDA receptor antagonists; Aguilar et al. 2009). Recently, Senik et al. (2012) demonstrated that the methanolic extract of Mitragyna speciosa when given orally facilitated learning and improved the cognitive function in rats. Therefore, the present attenuation of ethanol-induced CPP by the MMS is unlikely due to drug-induced memory impairment. In general, the drugs affecting motor activity can influence the results of any animal behavioural studies and this could be avoided by evaluating the compound’s effect on spontaneous locomotor activity. In a previous study, the methanolic extract of M. speciosa (50–200 mg/kg, p.o.) and its alkaloidal fraction (5-20 mg/kg, p.o.) per se did not affect the spontaneous locomotor activity in mice (Reanmongkol et al. 2001), which suggests that MMS at the tested doses might not alter the motor activity, thereby, influencing CPP results in mice.
Nevertheless, to further confirm these findings from ethanol-CPP results, another set of experiment was conducted using a mouse runway model of drug self-administration. A significant decrease in the time of trained (conditioned) mice to reach the goal box confirmed the subjects’ motivation to seek ethanol on day 10 (expression) as shown in Fig. 4. Interestingly, MMS at (50, 75 and 100 mg/kg, p.o.) significantly prolonged the runtime of trained (conditioned) mice to reach the goal box as demonstrated for the reference drugs, acamprosate and clozapine in mice (Fig. 4). These results also confirmed the anticraving properties of MMS against ethanol seeking behaviour in mice.
In earlier studies, it was demonstrated that dopamine antagonists attenuated the craving to alcohol reinforcement in patient receiving controlled dose of alcohol in a laboratory setting (Swift 2010). Besides, many other preclinical and clinical studies demonstrated that the dopamine antagonists such as quetiapine, clozapine, olanzapine and tiapride were found to reduce alcohol craving (Drake et al. 2000; Hutchison et al. 2006; Hutchison et al. 2001; Shaw et al. 1994). Moreover, in a retrospective study, haloperidol (dopamine D2 antagonist) was found to be effective in decreasing the craving for alcohol in alcohol dependent patients when compared to the placebo group (Modell et al. 1993).
The rewarding effect and behavioural stimulation induced by ethanol in humans and rodents are known to be mediated by activation of the mesolimbic dopaminergic pathway (Ahlenius et al. 1973; Carlsson et al. 1974; Engel and Lilijequist, 1983). Moreover, intracerebral (20–120 mM) or systemic (0.5–1 g/kg) administration of ethanol to Sprague-Dawley and wild type Wistar rats exalted the dopamine release in the nucleus accumbens (Blanchard et al. 1993; Di Chiara and Imperato 1988; Imperato and Di Chiara 1986; Lof et al. 2007; Yim and Gonzales 2000; Yim et al. 1998). The present study results are corroborated with the earlier reports in which the dopamine level in the NAc of ethanol treated group was significantly elevated. The test groups treated with different doses of MMS (50–100 mg/ kg, p.o.) and the reference groups treated with ACAM (300 mg/kg, p.o.) and CLZ (1 mg/kg, p.o.) significantly decreased the ethanol-induced elevated dopamine level in mice. The present results do not clarify the exact neuronal mechanism involved in the de-addictive like effect of MMS. However, in our previous study, we found that MMS at lower doses exhibit D2 receptor antagonistic effect (Vijeepallam et al. 2016). Hence, we postulate that MMS attenuate the ethanol seeking behavior by blocking dopamine D2 receptors in mice.
Overall, this study highlights the attenuating effect of MMS against ethanol seeking at lower doses (50–100 mg/ kg, p.o.) which could be utilized in the novel drug discovery for the treatment of alcohol dependence. Further receptor-ligand binding assays are warranted to confirm the actual mechanism of action of MMS as an anticraving agent to treat alcohol dependence.
References
Aguilar MA, Manzanedo C, Do Couto BR, Rodriguez-Arias M, Minarro J (2009) Memantine blocks sensitization to the rewarding effects of morphine. Brain Res 1288:95–104. https://doi.org/10.1016/j.brainres.2009.06.100
Ahlenius S, Carlsson A, Engel J, Svensson T, Sodersten P (1973) Antagonism by alpha methyltyrosine of the ethanol-induced stimulation and euphoria in man. Clin Pharmacol Ther 14:586–591. https://doi.org/10.1002/cpt1973144part1586
Blanchard BA, Steindorf S, Wang S, Glick SD (1993) Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res 17:968–973. https://doi.org/10.1111/j.1530-0277.1993.tb05650.x
Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH (2008) Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 103:1048–1050. https://doi.org/10.1111/j.1360-0443.2008.02209.x
Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508:65–69. https://doi.org/10.1016/0006-8993(90)91118-Z
Carlsson A, Engel J, Strombom U, Svensson TH, Waldeck B (1974) Suppression by dopamine-agonists of the ethanol-induced stimulation of locomotor activity and brain dopamine synthesis. Naunyn Schmiedebergs Arch Pharmacol 283:117–128. https://doi.org/10.1007/bf00501138
Chee JW, Amirul AA, Majid MI, Mansor SM (2008) Factors influencing the release of Mitragyna speciosa crude extracts from biodegradable P(3HB-co-4HB). Int J Pharm 361:1–6. https://doi.org/10.1016/j.ijpharm.2008.05.007
Cunningham CL, Henderson CM (2000) Ethanol-induced conditioned place aversion in mice. Behav Pharmacol 11:591–602. https://doi.org/10.1097/00008877-200011000-00006
Czachowski CL, Legg BH, Samson HH (2001) Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res 25:344–350. https://doi.org/10.1111/j.1530-0277.2001.tb02220.x
de Licona HK, Karacay B, Mahoney J, McDonald E, Luang T, Bonthius DJ (2009) A single exposure to alcohol during brain development induces microencephaly and neuronal losses in genetically susceptible mice, but not in wild type mice. Neurotoxicology 30:459–470. https://doi.org/10.1016/j.neuro.2009.01.010
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278. https://doi.org/10.1073/pnas.85.14.5274
Drake RE, Xie H, McHugo GJ, Green AI (2000) The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 26:441–449. https://doi.org/10.1093/oxfordjournals.schbul.a033464
Engel J, Lilijequist S (1983) The involvement of different central neurotransmitters in mediating stimulatory and sedative effects of ethanol. In: Pohorecky L, Brick JJ (eds) Stress and alcohol Use. Elsevier, New York, pp 153–169. https://doi.org/10.1007/s11011-019-00477-2
Garber JC et al (2011) Guide for the care and use of laboratory animals. The National Academies Collection, Washington, DC. https://doi.org/10.17226/5140
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NHM, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NIW, Jayabalan N, Hazim AI, Mansor SM, Müller CP (2013) From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 37:138–151. https://doi.org/10.1016/j.neubiorev.2012.11.012
Hassan Z, Bosch OG, Singh D, Narayanan S, Kasinather BV, Seifritz E, Kornhuber J, Quednow BB, Müller CP (2017) Novel psychoactive substances-recent progress on neuropharmacological mechanisms of action for selected drugs. Front Psychiatry 8:152. https://doi.org/10.3389/fpsyt.2017.00152
Horie S, Koyama F, Takayama H, Ishikawa H, Aimi N, Ponglux D, Matsumoto K, Murayama T (2005) Indole alkaloids of a Thai medicinal herb, Mitragyna speciosa, that has opioid agonistic effect in Guinea-pig ileum. Planta Med 71:231–236. https://doi.org/10.1055/s-2005-837822
Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A (2001) Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology 155:27–34. https://doi.org/10.1007/s002130000629
Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R (2006) The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology 31:1310–1317. https://doi.org/10.1038/sj.npp.1300917
Idayu NF, Hidayat MT, Moklas MA, Sharida F, Raudzah AR, Shamima AR, Apryani E (2011) Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 18:402–407. https://doi.org/10.1016/j.phymed.2010.08.011
Imperato A, Di Chiara G (1986) Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther 239:219–228
Jansen KL, Prast CJ (1988) Psychoactive properties of mitragynine (kratom). J Psychoactive Drugs 20:455–457. https://doi.org/10.1080/02791072.1988.10472519
Kebabian JW, Beaulieu M, Itoh Y (1984) Pharmacological and biochemical evidence for the existence of two categories of dopamine receptor. Can J Neurol Sci 11:114–117. https://doi.org/10.1017/S0317167100046254
Khan Y, Pandy V (2016) Methanolic extract of Morinda citrifolia L. (noni) unripe fruit attenuates ethanol-induced conditioned place preferences in mice. Front Pharmacol 7:352. https://doi.org/10.3389/fphar.2016.00352
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. https://doi.org/10.1038/npp.2009.110
Kruegel AC, Grundmann O (2018) The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 134:108–120. https://doi.org/10.1016/j.neuropharm.2017.08.026
Kumarnsit E, Keawpradub N, Nuankaew W (2007) Effect of Mitragyna speciosa aqueous extract on ethanol withdrawal symptoms in mice. Fitoterapia 78:182–185. https://doi.org/10.1016/j.fitote.2006.11.012
Kurokawa K, Mizuno K, Shibasaki M, Higashioka M, Oka M, Hirouchi M, Ohkuma S (2013) Acamprosate suppresses ethanol-induced place preference in mice with ethanol physical dependence. J Pharmacol Sci 122:289–298. https://doi.org/10.1254/jphs.13056FP
Lee SY, Song DK, Jang CG (2003) Effects of Coptis japonica on morphine-induced conditioned place preference in mice. Arch Pharm Res 26:540–544. https://doi.org/10.1007/BF02976878
Lof E, Ericson M, Stomberg R, Soderpalm B (2007) Characterization of ethanol-induced dopamine elevation in the rat nucleus accumbens. Eur J Pharmacol 555:148–155. https://doi.org/10.1016/j.ejphar.2006.10.055
Lopez MF, Becker HC, Chandler LJ (2014) Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol 48:639–645. https://doi.org/10.1016/j.alcohol.2014.09.002
Lukas SE, Penetar D, Su Z, Geaghan T, Maywalt M, Tracy M, Rodolico J, Palmer C, Ma Z, Lee DYW (2013) A standardized kudzu extract (NPI-031) reduces alcohol consumption in nontreatment-seeking male heavy drinkers. Psychopharmacology 226:65–73. https://doi.org/10.1007/s00213-012-2884-9
Ma H, Zhu G (2014) The dopamine system and alcohol dependence. Shanghai Arch Psychiatry 26:61–68. https://doi.org/10.3969/j.issn.1002-0829.2014.02.002
Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, Kennedy P, Nestler EJ, Szyf M, Yadid G (2015) Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci 35:8042–8058. https://doi.org/10.1523/jneurosci.3053-14.2015
Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai SI, Aimi N, Watanabe H (1996) Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol 317:75–81. https://doi.org/10.1016/S0014-2999(96)00714-5
Matsumoto K, Mizowaki M, Takayama H, Sakai S, Aimi N, Watanabe H (1997) Suppressive effect of mitragynine on the 5-methoxy-N,N-dimethyltryptamine-induced head-twitch response in mice. Pharmacol Biochem Behav 57:319–323. https://doi.org/10.1016/S0091-3057(96)00314-0
Mattioli L, Titomanlio F, Perfumi M (2012) Effects of a Rhodiola rosea L. extract on the acquisition, expression, extinction, and reinstatement of morphine-induced conditioned place preference in mice. Psychopharmacology 221:183–193. https://doi.org/10.1007/s00213-012-2686-0
Modell JG, Mountz JM, Glaser FB, Lee JY (1993) Effect of haloperidol on measures of craving and impaired control in alcoholic subjects. Alcohol Clin Exp Res 17:234–240. https://doi.org/10.1111/j.1530-0277.1993.tb00755.x
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128. https://doi.org/10.1038/35053570
Norakanphadung P (1966) Pramuan Khuamru Ruang Yaseptit Hai Thot. Thanyarak Hospital, Bangkok
Pandy V, Khan Y (2016a) Design and development of a modified runway model of mouse drug self-administration. Sci Rep 6:21944. https://doi.org/10.1038/srep21944
Pandy V, Khan Y (2016b) Noni (Morinda citrifolia Linn.) fruit juice attenuates the rewarding effect of ethanol in conditioned place preference in mice. Exp Anim 65:437–445. https://doi.org/10.1538/expanim.16-0018
Pandy V, Wai YC, Amira Roslan NF, Sajat A, Abdulla Jallb AH, Vijeepallam K (2018) Methanolic extract of Morinda citrifolia Linn. Unripe fruit attenuates methamphetamine-induced conditioned place preferences in mice. Biomed Pharmacother 107:368–373. https://doi.org/10.1016/j.biopha.2018.08.008
Price KL, Middaugh LD (2004) The dopamine D1 antagonist reduces ethanol reward for C57BL/6 mice. Alcohol Clin Exp Res 28:1666–1675. https://doi.org/10.1097/01.ALC.0000145783.39748.E6
Rassnick S, Pulvirenti L, Koob GF (1992) Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology 109:92–98. https://doi.org/10.1007/BF02245485
Reanmongkol W, Keawpradub N, Sawangjaroen K (2001) Effects of the extracts from Mitragyna speciosa Korth. Leaves on analgesic and behavioral activities in experimental animals. Songklanakarin J Sci Technol 29:39–48
Rezvani AH, Overstreet DH, Yang Y, Clark E, Jr. (1999) Attenuation of alcohol intake by extract of Hypericum perforatum (St. John's wort) in two different strains of alcohol-preferring rats. Alcohol alcohol (Oxford, Oxfordshire) 34:699-705. https://doi.org/10.1093/alcalc/34.5.699
Sabetghadam A, Ramanathan S, Mansor SM (2010) The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharm Res 2:181–185. https://doi.org/10.4103/0974-8490.65514
Samson HH, Hodge CW, Tolliver GA, Haraguchi M (1993) Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull 30:133–141. https://doi.org/10.1016/0361-9230(93)90049-H
Sahraei H, Fatemi SM, Pashaei-Rad S, Faghih-Monzavi Z, Salimi SH, Kamalinegad M (2006) Effects of Papaver rhoeas extract on the acquisition and expression of morphine-induced conditioned place preference in mice. J Ethnopharmacol 103:420–424
Senik MH, Mansor SM, JTK J, Abdullah JMB (2012) Effect of acute administration of Mitragyna speciosa Korth. Standardized methanol extract in animal model of learning and memory. J Med Plant Res 6:1007–1014. https://doi.org/10.5897/JMPR11.601
Shamima AR, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MA, Arulselvan P (2012) Antinociceptive action of isolated mitragynine from Mitragyna Speciosa through activation of opioid receptor system. Int J Mol Sci 13:11427–11442. https://doi.org/10.3390/ijms130911427
Shaw GK, Waller S, Majumdar SK, Alberts JL, Latham CJ, Dunn G (1994) Tiapride in the prevention of relapse in recently detoxified alcoholics. Br J Psychiatry 165:515–523. https://doi.org/10.1192/bjp.165.4.515
Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L (2007) Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res 31:2001–2005. https://doi.org/10.1111/j.1530-0277.2007.00522.x
Swift R (2010) Medications acting on the dopaminergic system in the treatment of alcoholic patients. Curr Pharm Des 16:2136–2140. https://doi.org/10.2174/138161210791516323
Thanos PK, Katana JM, Ashby CR Jr, Michaelides M, Gardner EL, Heidbreder CA, Volkow ND (2005) The selective dopamine D3 receptor antagonist SB-277011-a attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav 81:190–197. https://doi.org/10.1016/j.pbb.2005.03.013
Turner PV, Brabb T, Pekow C, Vasbinder MA (2011) Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613
Ueda S, Aikawa M, Ishizuya-Oka A, Koibuchi N, Yamaoka S, Yoshimoto K (1998) Age-related degeneration of the serotoninergic fibers in the zitter rat brain. Synapse 30:62–70. https://doi.org/10.1002/(sici)1098-2396(199809)30:1<62::aid-syn8>3.0.co;2-i
Vijeepallam K, Pandy V, Kunasegaran T, Murugan DD, Naidu M (2016) Mitragyna speciosa leaf extract exhibits antipsychotic-like effect with the potential to alleviate positive and negative symptoms of psychosis in mice. Front Pharmacol 7:464. https://doi.org/10.3389/fphar.2016.00464
Weiss F, Lorang MT, Bloom FE, Koob GF (1993) Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267:250–258
Yamamoto LT, Horie S, Takayama H, Aimi N, Sakai SI, Yano S, Shan J, Pang PKT, Ponglux D, Watanabe K (1999) Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa. Gen Pharmacol 33:73–81. https://doi.org/10.1016/S0306-3623(98)00265-1
Yim HJ, Gonzales RA (2000) Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 22:107–115. https://doi.org/10.1016/S0741-8329(00)00121-X
Yim HJ, Schallert T, Randall PK, Gonzales RA (1998) Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res 22:367–374. https://doi.org/10.1111/j.1530-0277.1998.tb03662.x
Yoshimoto K, Watanabe Y, Tanaka M, Kimura M (2012) Serotonin2C receptors in the nucleus accumbens are involved in enhanced alcohol-drinking behavior. Eur J Neurosci 35:1368–1380. https://doi.org/10.1111/j.1460-9568.2012.08037.x
Acknowledgements
We are indebted to the University of Malaya for providing financial assistance [BKS051-2017] to carry out this research. The funding source is not involved in study design. Besides, the authors would like to thank Ms.Bitna Joo from Korea Brain Research Institute, Daegu for her helpful advice and guidance in dissection of brain regions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vijeepallam, K., Pandy, V., Murugan, D.D. et al. Methanolic extract of Mitragyna speciosa Korth leaf inhibits ethanol seeking behaviour in mice: involvement of antidopaminergic mechanism. Metab Brain Dis 34, 1713–1722 (2019). https://doi.org/10.1007/s11011-019-00477-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00477-2