Abstract
The sesquiterpene nootkatone (NKT), isolated from Alpiniae oxyphyllae Fructus, was shown to possess protective effects on neurons. In our study, by using an Alzheimer’s disease (AD) model of mice induced by intracerebroventricular (i.c.v.) injection of Aβ1–42 oligomers, we investigated the effects of NKT on memory impairment and further evaluated the pathological changes of mice. AD mice were treated by i.c.v. injection of NKT (at a dose of 0.02 mg/kg and 0.20 mg/kg) or vehicle (PBS) into the lateral ventricle once daily for 5 consecutive days. The behavioral tasks were performed, and levels of some biochemical indicators and histopathological changes of the brain were evaluated to elucidate the mechanism of NKT in the treatment of AD. The results revealed that NKT significantly improved the neurobehavioral performance of the AD mice in the Y-maze and Morris water maze tests. More importantly, NKT treatment decreased the malondialdehyde (MDA), Aβ as well as the acetylcholin esterase (AChE) levels in the mice brain, while increased the glutathione peroxidase (GSH-Px) levels with improved histopathological changes in the hippocampus. These findings provided evidences for the beneficial role of NKT in Aβ1–42-induced mice AD model linking to anti-oxidative and anti-AChE activities with inhibitory effect against Aβ accumulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive and complex neurodegenerative disorder characterized by the occurrence of senile plaques and neurofibrillary tangles (Querfurth and LaFerla 2010; Sierksma et al. 2013). The disease is pathologically characterized by extracellular deposited amyloid β-peptide (Aβ) and intracellular hyper-phosphorylated and tangled tau-protein (Butterfield et al. 2001; Ho et al. 2005; Hynd et al. 2004). Aβ deposition is known to increase oxidative stress, which is arisen from the imbalance between pro-oxidants and antioxidants that leads to excess generation of ROS and free radicals (Rottkamp et al. 2001). Oxidative stress damages the polyunsaturated fatty acids leading to the disruption of cell membrane and its integrity, inactivation of antioxidant enzymes, and neuronal dysfunction and death (Javed et al. 2012). In addition, reduction in cholinergic activity is correlated with the degree of cognitive impairment and is associated with decreased levels of the neurotransmitter acetylcholine (ACh). Acetylcholinesterase (AChE), the enzyme for cholinergic neurons, is involved in the breakdown of ACh and termination of its neurotransmitter action (Ibach and Haen 2004). It was reported that some natural products might slow the progression of AD because they could simultaneously protect neurons from oxidative stress or act as cholinesterases inhibitors (Williams et al. 2011).
Alpiniae oxyphyllae
Fructus (Zingiberaceae) is widely cultivated in South China. The fruits of this plant, ‘Yizhi’ in Chinese, have been used as traditional Chinese medicine for the treatment of intestinal and urethral disorders (Chinese Pharmacopoeia Commission 2010). Previous pharmacological studies have indicated that the extracts of this plant also possess protective effects on neurons and preventative effect on dementia (Koo et al. 2004; Kubo et al. 1995; Shi et al. 2014). However, the components responsible for its protective activity of the nervous system are still unclear.
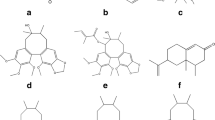
Nootkatone (NKT) (4,4a,5,6,7,8-Hexahydro-6-iso-propenyl-4,4a–dimethyl-2(3H)-naphthalenone) (Fig. 1a), one of the major sesquiterpenoid components in A. oxyphylla, is widely used for flavoring the food and tobacco (Davies and Deroles 2014). Our preliminary work had found the chloroform extract and n-butanol extract from the fruits of Alpiniae oxyphyllae might offer a useful therapeutic choice in either the prevention or the treatment of AD (Shi et al. 2014, 2015). As one of the important bioactive sesquiterpenes of the chloroform extract of Alpiniae oxyphyllae Fructus, we investigated the effects of NKT on memory impairment and neurodegeneration in animal models of AD in the current study.
Materials and methods
Animals
Sixty male Kunming mice weighing 30-35 g were provided by the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, China). The mice were maintained on standard laboratory conditions of temperature 25 ± 1 °C and a 12-h light/12-h dark cycle with food and water available ad libitum for the duration of the study. The animal study was carried out in accordance with the Guideline for Animal Experimentation of Shenyang Pharmaceutical University, and the protocol was approved by the Animal Ethics Committee of the institution.
Drugs and chemicals
Aβ1–42 was purchased from Sigma-Aldrich Inc. (St. Louis, MO), and dissolved in PBS (1 mg/ml final concentration) and incubated at 37 °C for 5 days to obtain the oligomers (Maurice et al. 1996). NKT with purity >98% (HPLC) was isolated from the fruits of Alpiniae oxyphyllae Fructus, and suspended in PBS at a stock concentration of 0.2 mg/ml and 2.0 mg/ml. Donepezil with purity >98% (HPLC) was provided by Melone Pharmaceutical Co., Ltd. (Dalian, China), and suspended in PBS at the concentration of 0.2 mg/ml. Commercial kits for detection of malondialdehyde (MDA), glutathione (GSH), glutathione peroxidase (GSH-Px), AChE, β-secretase and Aβ1–42 were purchased from Nanjing Jiancheng Bio engineering Institute (Nanjing, China).
Experimental design
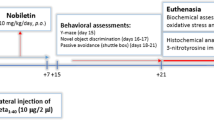
The mice were divided randomly into 6 groups (n = 10 in each group): (i) control group: normal mice without any treatment; (ii) sham group: saline-injection plus intracerebroventricular (i.c.v.) treatment with saline; (iii) model group: Aβ-injection plus i.c.v. treatment with saline; (iv) donepezil (DPZ) group: Aβ-injection plus i.c.v. treatment with donepezil (14 μg/kg/day); (v) NKT(L) group: Aβ injection plus i.c.v. treatment with NKT (0.02 mg/kg/day); (vi) NKT(H) group: Aβ injection plus i.c.v. treatment with NKT (0.20 mg/kg/day). For Aβ1–42 i.c.v. injection, mice were anesthetized by 4% chloral hydrate (10 ml/kg), and Aβ1–42 oligomers or vehicle (PBS) was respectively administrated by stereotaxic injections with a Hamilton micro syringe fitted with a 26-gauge needle. Injection (3 μl for each mouse) was administered over 1 min into the dorsal hippocampus at the coordinates −0.5 mm anterior to posterior (AP) bregma, −1.1 mm mid to lateral (ML), −3.0 mm dorsal to ventral (DV) dura. The needle remained in position for an additional 3 min after injection. After Aβ1–42 injection, animals were implanted with cannula (10 mm, 23 gauge) located 1 mm above the right ventricle (AP: +0.2 mm, ML: +1.0 mm, DV: −3.0 mm). The cannula was fixed to the skull with a screw and dental cement. Following surgery, animals were allowed to recover under a heat lamp (Liu et al. 2010). All mice with surgery received daily intraperitoneal injections of 0.2 mL penicillin sodium (200,000 IU/mL) for consecutive 3 days for prevention of infection (Wu et al. 2017). For i.c.v. administration of drugs, NKT, DPZ or physiological saline was given to animals in each group once daily for 5 consecutive days, mice in sham-operated and model groups received same volume of physiological saline i.c.v. injection. The experimental design was shown in Fig. 1b.
Behavioral analyses
Spontaneous locomotor activity task
Spontaneous locomotor activity was determined in mice that were placed in a transparent chamber (25 cm diameter × 13 cm height) connected to an automatic registration system. The chamber was equipped with nine infrared sensors arranged along the bottom of the wall of the arena. The interruption of beams of two consecutive infrared sensors was collected for 5 min as a reflection of locomotor activity. After each testing session, the enclosures were thoroughly cleaned with 70% ethanol and water (Nagakannan et al. 2012).
Y-maze task
The Y-maze test involves both cognitive and activities/exploratory behavioral components. The maze is made of black Plexiglas with three identical arms: each arm is 30 cm long, 12 cm high, 5 cm wide at the bottom, and 10 cm wide at the top. Each mouse was placed at the center of the apparatus, and allowed to move freely through the maze during a 6 min period. The number of arm entries was recorded visually. Alternation was defined as successive entry into the three arms, on overlapping triplet sets. Alternation rate (%) was calculated as the number of actual alternations performed divided by the number of possible alternations (defined as the number of arm entries minus two) multiplied by 100 (He et al. 2013).
Morris water maze task
Spatial learning and memory retention were tested in the standard Morris water maze protocol. The maze is a circular pool with 150 cm in diameter made of plastic and painted flat black on the interior walls and floor. The maze is surrounded on all sides by curtains to avoid visual interference during trial. Each wall had unique, large geometric figures mounted on them. The pool was filled to a depth of 80 cm with water made opaque by the addition of a nontoxic paint and temperature was maintained between 23 and 27 °C. The goal quadrant contained a clear, 9 cm diameter submerge platform submerged 1 cm below the water. Mice were trained for 5 days including 2 trials daily with a trial interval of 30 min to find the hidden escape platform. Mice were randomly placed into the pool at one of two starting locations. Mice were guided to the platform when they failed to find the platform within 90 s. After 15 s, they were returned to their cages. The time in which mice reached the platform (escape latency in seconds) was evaluated. For memory retention test on the 6th day, the platform was removed and the animals were allowed to swim for 90 s. The time spent in the target quadrant and the number crossed the platform were recorded in memory retention test.
Biochemical and histopathological tests
Biochemical analysis
Mice were deeply anesthetized after behavioral tests, the cerebral cortex and hippocampus of 8 mice in each group were dissected out and immediately stored at −80 °C. For biochemical analysis, the cerebral cortex and the hippocampus tissue were homogenized in ice-cold saline and centrifuged at 2000 rpm for 10 min, and the supernatant was collected. Levels of SOD, GSH-px, GSH-Px, β-secretase, AChE, Aβ1–42 and MDA were measured using commercial assay kits according to the manufacturers’ protocols.
Histological staining
Two mice in each group were perfused transcardially with saline followed by 4% paraformaldehyde (PFA) in phosphate buffer saline (0.1 mol/l PBS, pH 7.2) after anesthesia. The whole brains of mice were removed and postfixed in 4% PFA at 4 °C until sectioned. Coronal sections of the hemisphere were stained with hematoxylin and eosin (H&E) staining. The sections were deparaffinized and washed with PBS, then stained with hematoxylin for 5 min. After washed with tap water, sections were differentiated in acid alcohol and washed again with tap water. Followed by staining with eosin for 5 min, sections were dehydrated with alcohol and mounted.
Statistics analysis
Data were expressed as the mean ± SEM. The statistical significance in the behavioral and biochemical effects of NKT was determined using SPSS 17.0 (IBM Corp., New York, NY) where statistical significance were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, p < 0.05 was considered statistically significant.
Results
NKT improved short-term memory without affecting spontaneous locomotor activity of mice injected with Aβ1–42 peptide
As shown in Table 1, there was no significant difference in the spontaneous locomotor activity indicated by exploratory behavior among different groups (p > 0.05). The results suggested that the surgeries or i.c.v. injection of Aβ1–42 or NKT had no effect on spontaneous locomotor activity of mice.
Accordantly, there was no significant difference among groups in the number of total entries in the Y-maze task (p > 0.05), which further indicated that surgeries or i.c.v. injection of Aβ1–42 or NKT failed to affect locomotor activity (Fig. 1c). However, the model group showed lower level of the alternation ratio compared with the control group (p < 0.01), indicating the short-term memory was impaired by Aβ1–42. Moreover, in comparison with model group, DPZ, NKT(L) and NKT (H) treatment group showed higher levels of alternation ratio (p < 0.05), which indicated an improvement of Aβ1–42-induced short-term memory impairment in mice by NKT in the Y-maze task. In addition, there was no significant difference between the control group and the sham-operated group (p > 0.05) (Fig. 1d).
NKT improved spatial learning and retention memory impairments induced by Aβ1–42 peptide
Acquisition training of the Morris water maze task was carried out for mice to learn to find the hidden platform. Changes of escape latency in the training trials were shown in Fig. 2a. Compared with the control group, model mice exhibited prolonged escape latency during the 5 days training in the place navigation test (p < 0.01). Four days after training, both low and high dose of NKT-treated mice required less time to find the hidden platform (p < 0.05). Similarly, DPZ group exhibited a significant decrease of escape latency compared with model mice on the last day of training (p < 0.05).
Memory retention of the platform location was assessed in a 90 s spatial probe test performed on the day following the place navigation test. Figure 2b showed that model mice spent significantly less time in the target quadrant compared to control mice (p < 0.01), while NKT or DPZ treatment restored the time spent in the target quadrant in mice injected with Aβ1–42. Accordantly, the number of crossing the platform site of mice in model group was lower than those in control group (p < 0.01), while mice treated with NKT or DPZ crossed the platform site significantly more times than the model mice (p < 0.05) (Fig. 2c). In the place navigation test and spatial probe test, there was no significant difference between the control group and the sham-operated group (p > 0.05) (Fig. 2a–c).
NKT treatment improved hippocampal neuronal damage in mice with Aβ1–42 injection
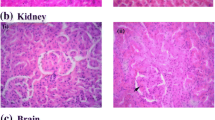
H&E staining was performed to detect the neuronal integrity and orderliness, as well as cell morphology and tissue structure in hippocampal CA1 region. The neurons in control and sham-operated groups appeared to be intact and ordered (Fig. 3a, b), however, eccentrically dispersed nuclei and disordered arrangement of neurons were found in the model group (Fig. 3c). The histopathological changes were significantly ameliorated by DPZ treatment (Fig. 3d). Similarly, NKT (0.02 mg/kg and 0.20 mg/kg) significantly increased the number of neurons, and the degree of the neuronal injury was decreased in mice with Aβ1–42 injection (Fig. 3e, f).
Effects of NKT on the morphology and the number of neurons in an Aβ-induced AD mouse model. Light micrographs of hippocampal neurons from the CA1 region of control group (a), sham-operated group (b), model group (c), donepezil group (d) and NKT at doses of 0.02 mg/kg group (e) and 0.20 mg/kg group (f). The arrows indicate the neurons with degenerated nuclei
NKT decreased MDA level and increased GSH and GSH-Px levels in brain of mice with Aβ1–42 injection
The MDA level in the frontal cortex of model mice was significantly higher than the control mice (p < 0.01). This increase was attenuated by treatment of NKT at doses of 0.02 and 0.20 mg/kg (p < 0.01). However, there was no significant difference in the hippocampus among the groups, although the model group showed the obvious trend of increasing MDA level (p > 0.05) (Table 2).
The level of GSH decreased remarkably both in the frontal cortex and hippocampus of model mice compared with the control mice (p < 0.01). NKT at the both doses could reverse the GSH level in the frontal cortex (p < 0.01), while the treatment failed to up-regulate the GSH level in the hippocampus (p > 0.05) (Table 2).
Table 2 also showed a significant decrease in GSH-Px level in the frontal cortex and hippocampus of model mice as compared with the control group (p < 0.01). DPZ and both doses of NKT significantly increased GSH-Px level in hippocampus of mice with Aβ1–42 injection (p < 0.05).
NKT showed inhibitory effect on accumulation of Aβ in the hippocampus without affecting the level of β-secretase in the cortex
As shown in Fig. 4a, an increased accumulation of extracellular Aβ in the hippocampus of Aβ1–42-injected mice was observed compared with the control mice (p < 0.05). However, NKT prevented the accumulation of Aβ in the mice received Aβ1–42 injection (p < 0.05). The level of β-secretase in the cortex of Aβ-injected mice brain was further analyzed (Fig. 4b). In comparison with the control mice, the β-secretase level of the model mice was significantly higher (p < 0.05), while it was not furthered affected by NKT treatment (p > 0.05) (Fig. 4b).
Effects of NKT on Aβ accumulation in the hippocampus (a), β-secretaselevel in the frontal cortex (b), and AChE activity in the cortex (c) and hippocampus (d) of Aβ1–42-injected mice. Data are shown as mean ± S.E.M (n = 8 animals per group). # p < 0.05 v.s. control group; *p < 0.05 and **p < 0.01 v.s. model group
NKT inhibited AChE activity in brain of mice with Aβ1–42 injection
The activity of AChE within the cortex and hippocampus were increased in model mice with Aβ1–42 injection (p < 0.01) (Fig. 4c, d). The administration of DPZ or both high and low doses of NKT significantly down-regulated the activity of AChE in cortex (p < 0.01) (Fig. 4c). The increased activity of AChE was also significantly inhibited by the treatment with NKT in the hippocampus (p < 0.01) (Fig. 4d).
Discussion
Alzheimer’s disease (AD) is a neurodegenerative disorder of advanced age characterized by loss of memory and the accumulation of Aβ deposits and decreased levels of the neurotransmitter acetylcholine (Tesseur et al. 2013). DPZ, a selective inhibitor of AChE that had been approved for treatment of AD (Wilkinson 1991), was used as a positive control drug to evaluate the effectiveness of NKT in AD treatment. Because hippocampal dysfunction caused by Aβ is one of the pathologic mechanisms of AD (Butterfield et al. 2001), we chose the human Aβ1–42 i.c.v. injection model to test the efficacy of NKT on recovery of memory deficit. Blood-brain barrier (BBB) is a main obstacle to the effective delivery of drugs to the brain (Mao et al. 2015). It is reported that after volatile oil of Alpiniae oxyphyllae Fructus was intragastric administrated to rats, nootkatone could be detected in the cerebrospinal fluid (Tan et al. 2004). The results indicated NKT might cross BBB freely. In the current study, NKT and DPZ were given to mice by i.c.v. injection through an alloy-steel tube to investigate the effectiveness against Aβ1–42 induced AD. Our results revealed that Aβ1–42 induced marked deficiencies in both short- and long-term memory of mice, and increased deposition and dissemination of Aβ in the hippocampus of mice, which were consistent with the biochemical and histological observations.
Y-maze task was conducted to investigate short term stressless memory processes, while Morris water maze test was performed to evaluate long term stressfull learning of mice. These intensive behavioral evaluations allow a detailed description of the non-cognitive behavioral abnormalities and cognitive impairments in this AD model and provide useful information for studies investigating potential treatments or interventions (Mao et al. 2015; Wu et al. 2017). In our research, i.c.v. injection of Aβ1–42 impaired both short term and long term memory of mice, while DPZ or NKT treatment could effectively reverse Aβ1–42-induced impairment (Figs. 1 and 2).
All multifactorial pathological conditions of AD are evidenced to link with oxidative stress, which results from imbalance between production and removal of reactive oxygen species (ROS) and leads to neuronal injury (Markesbery 1997). MDA, a by-product of lipid peroxidation, is a major biomarker of ROS accumulation induced membrane lipid peroxidation (Ziech et al. 2010). In the present study, MDA level in the frontal cortex was increased by Aβ1–42 induction, which was effectively inhibited by NKT treatment. The level of GSH, an endogenous antioxidant against free radicals (Kharrazi et al. 2008), was decreased in the frontal cortex of model mice, which was restored by NKT i.c.v. injection. Moreover, GSH-Px, functions to reduce lipid hydroperoxides (Kharrazi et al. 2008), was also up-regulated by NKT in mice with Aβ1–42 injection (Table 2). Frontal cortex is a cortical region known to be involved in AD, increases in oxidative markers and declines in antioxidants in frontal cortex were AD-dependent (Ansari and Scheff 2010). Our findings indicated an anti-oxidative activity of NKT against Aβ1–42 might be involved in the neuroprotective effect. However, DPZ only showed a weak anti-oxidative effect against Aβ1–42 (Table 2).
Cholinergic dysfunction and oxidative stresses have been linked to the pathological development of AD (Ho et al. 2003). It has been reported that a reduction in the acetylcholine concentration in the cholinergic synaptic cleft was observed in the AD brain (Craig et al. 2011). As the enzyme responsible for terminating cholinergic neurotransmission, AChE is able to promote Aβ aggregation in neurons, and Aβ could also in turn increase the activity of AChE (Hu et al. 2004). Previous study reported that NKT and some sesquiterpenoids isolated from the ethanolic extract of Alpiniae oxyphyllae Fructus showed weak to moderate AChE inhibitory activities (Chen and Xiang 2013). Accordantly, our study revealed that the activity of AChE within the cortex and hippocampus were increased in model mice with Aβ1–42 injection, while NKT and DPZ significantly down-regulated the activity of AChE in brain tissues. Unexpectively, AChE was slightly, although insignificantly, activated in sham group compared with control group, which might be caused by the slight inflammation induced by surgery (Fig. 4). Our study indicated that NKT may mediate neuroprotective effect against Aβ-induced cognitive impairment partly through the inhibition of AChE.
Moreover, the accumulation of the Aβ in the brain has been thought to be a key factor in the pathogenesis of AD, and the production of Aβ is due to sequential cleavages of amyloid precursor protein (APP) by β- and γ-secretases (Chami et al. 2012). Levels of Aβ and β-secretase in brain of mice were increased significantly in mice with Aβ1–42 injection, although a slightly increase of Aβ and β-secretase was also shown in mice with sham surgery, the difference was not significant. Moreover, our results showed an inhibitory effect of NKT rather than DPZ on the augmentation of Aβ accumulation in hippocampus of brain, while the level of β-secretase was not affected by NKT (Fig. 4). Exogenous Aβ was injected for induction of AD animal model in our study, and results indicated that NKT may directly inhibit accumulation of exogenous Aβ in brain without affecting the activity of β-secretase that promotes endogenous Aβ production. In addition, accordant with the results from biochemical analysis, H&E staining showed that the number of neurons in the NKT-treated mice was significantly increased, the degree of neuronal injury was decreased compared with the model mice (Fig. 3).
In summary, NKT from Alpinia oxyphylla Miq. significantly protected mice from Aβ1–42-induced memory impairment in behavioral tests including Y-maze and MWM tasks. The memory ameliorating effects of NKT were mediated, in part, by the anti-oxidative and anti-AChE activities. Additionally, NKT effectively inhibited Aβ accumulation in hippocampus of mice after Aβ1–42 injection. Unlike the commercial drugs such as DPZ with single target against AChE, our results suggest that NKT may represent a potential therapeutic to improve cognitive function in patients with AD through the regulation of multi-targets.
References
Ansari MA, Scheff SW (2010) Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 69(2):155–167. https://doi.org/10.1097/NEN.0b013e3181cb5af4
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med 7(12):548–554. https://doi.org/10.1016/S1471-4914(01)02173-6
Chami L, Buggiaprévot V, Duplan E, Delprete D, Chami M, Peyron JF, Checler F (2012) Nuclear factor-κB regulates β APP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J Bio Chem 287(29):24573–24584. https://doi.org/10.1074/jbc.M111.333054
Chen P, Xiang L (2013) Acetylcholinesterase inhibitory activities of 48 traditional Chinese medicinal herbs. J Chin Pharm Sci 22:106–109
Chinese Pharmacopoeia Commission (2010) Pharmacopoeia of the People's Republic of China, vol 1. China Medical Science and Technology Press, Beijing
Craig LA, Hong NS, McDonald RJ (2011) Revisiting the cholinergic hypothesis in the development of Alzheimer's disease. Neurosci Biobehav Rev 35(6):1397–1409. https://doi.org/10.1016/j.neubiorev.2011.03.001
Davies KM, Deroles SC (2014) Prospects for the use of plant cell cultures in food biotechnology. Curr Opin Biotechnol 26:133–140. https://doi.org/10.1016/j.copbio.2013.12.010
He P, Ouyang X, Zhou S, Yin W, Tang C, Laudon M, Tian S (2013) A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’disease. Horm Behav 64(1):1–7. https://doi.org/10.1016/j.yhbeh.2013.04.009
Ho SC, Liu JH, RY W (2003) Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 4(1):15–18. https://doi.org/10.1023/A:1022417102206
Ho GJ, Drego R, Hakimian E, Masliah E (2005) Mechanisms of cell signaling and inflammation in Alzheimer's disease. Curr Drug Targets Inflamm Allergy 4(2):247–256. https://doi.org/10.2174/1568010053586237
Hu W, Gray NW, Brimijoin S (2004) Amyloid-beta increases acetylcholinesterase expression in neuroblastoma cells by reducing enzyme degradation. J Neurochem 86(2):470–478. https://doi.org/10.1046/j.1471-4159.2003.01855.x
Hynd MR, Scott HL, Dodd PR (2004) Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int 45(5):583–585. https://doi.org/10.1016/j.neuint.2004.03.007
Ibach B, Haen E (2004) Acetylcholinesterase inhibition in Alzheimer's disease. Curr Pharm Design 10(3):231–251. https://doi.org/10.2174/1381612043386509
Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A, Ashafaq M, Islam F, Siddiqui MS, Safhi MM, Islam F (2012) Rutin prevents cognitive impairments by ameliorating oxidative stress and neuro in ammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 210:340–345. https://doi.org/10.1016/j.neuroscience.2012.02.046
Kharrazi H, Vaisi-Raygani A, Rahimi Z, Tavilani H, Aminian M, Pourmotabbed T (2008) Association between enzymatic and non-enzymatic antioxidant defense mechanism with apolipoprotein E genotypes in Alzheimer disease. Clin Biochem 41(12):932–936. https://doi.org/10.1016/j.clinbiochem.2008.05.001
Koo BS, Lee WC, Chang YC, Kim CH (2004) Protective effects of alpinae oxyphyllae fructus (Alpinia oxyphylla Miq.) water-extracts on neurons from ischemic damage and neuronal cell toxicity. Phytother Res 18(2):142–148. https://doi.org/10.1002/ptr.1382
Kubo M, Matsuda H, Suo T, Yamanaka J, Sakanaka M, Yoshimura M (1995) Study on Alpiniae Fructus. I. Pharmacological evidence of efficacy of Alpiniae Fructus on ancient herbal literature. Yakugaku Zasshi 115(10):852–862. https://doi.org/10.1248/yakushi1947.115.10_852
Liu RT, Zou LB, JY F, QJ L (2010) Effects of liquiritigenin treatment on the learning and memory deficits induced by amyloid β-peptide (25-35) in rats. Behav Brain Res 210(1):24–31. https://doi.org/10.1016/j.bbr.2010.01.041
Mao X, Liao ZZ, Guo L, Xu X, Wu B, MJ X, Zhao X, Bi KS, Jia Y (2015) Schisandrin C ameliorates learning and memory deficits by Abeta1-42-induced oxidative stress and neurotoxicity in mice. Phytother Res 29(9):1373–1380. https://doi.org/10.1002/ptr.5390
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer's disease. Free Radical Bio Med 23(1):134–147. https://doi.org/10.1016/S0891-5849(96)00629-6
Maurice T, Lockhart BP, Privat A (1996) Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res 706(2):181–193. https://doi.org/10.1016/0006-8993(95)01032-7
Nagakannan P, Shivasharan BD, Thippeswamy BS, Veerapur VP, Bansal P (2012) Protective effect of hydroalcoholic extract of Mimusops elengi Linn. flowers against middle cerebral artery occlusion induced brain injury in rats. J Ethnopharmacol 140(2):247–254. https://doi.org/10.1016/j.jep.2012.01.012
Querfurth HW, LaFerla FM (2010) Mechanisms of disease. New Engl J Med 362(4):329–344. https://doi.org/10.1056/NEJMra0909142
Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA (2001) Redox-active iron mediates amyloid-β toxicity. Free Radical Bio Med 30(4):447–450. https://doi.org/10.1016/S0891-5849(00)00494-9
Shi SH, Zhao X, Liu B, Li H, Liu AJ, Wu B, Bi KS, Jia Y (2014) The effects of Sesquiterpenes-rich extract of Alpinia oxyphylla Miq. on amyloid-β-induced cognitive impairment and neuronal abnormalities in the cortex and hippocampus of mice. Oxidative Med Cell Longev 1:451802
Shi SH, Zhao X, Liu AJ, Liu B, Li H, Wu B, Bi KS, Jia Y (2015) Protective effect of n-butanol extract from Alpinia oxyphylla on learning and memory impairments. Physio Behav 139:13–20. https://doi.org/10.1016/j.physbeh.2014.11.016
Sierksma AS, Rutten K, Sydlik S, Rostamian S, Steinbusch HW, Dl VDH, Prickaerts J (2013) Chronic phosphodiesterase type 2 inhibition improvesmemory in the APPswe/PS1dE9 mouse model of Alzheimer's disease. Neuropharmacology 64:124–136. https://doi.org/10.1016/j.neuropharm.2012.06.048
Tan R, Chen SL, Yang DJ (2004) Alpinia oxyphylla oil constituents of blood brain barrier permeability GC-MS analysis. Chin Tradit Herbal Drugs 35:624–625
Tesseur I, Pimenova AA, Lo AC, Ciesielska M, Lichtenthaler SF, De Maeyer JH, Schuurkes JAJ, D’Hooge R, De Strooper B (2013) Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol Aging 34(7):1779–1789. https://doi.org/10.1016/j.neurobiolaging.2013.01.020
Wilkinson DG (1991) The parmacology of donepezil: a new treatment of Alzheimer's disease. Exp Opin Pharmacother 1(1):121–135
Williams P, Williams P, Sorribas A, Howes MJ (2011) Natural products as a source of Alzheimer's drug leads. Nat Prod Rep 28(1):48–77. https://doi.org/10.1039/C0NP00027B
Wu LD, Tong T, Wan ST, Yan TX, Ren FY, Bi K, Jia Y (2017) Protective effects of puerarin against Abeta1-42-induced learning and memory impairments in mice. Planta Med 83(3–4):224–231
Ziech D, Franco R, Georgakilas AG, Georgakila S, Malamou-Mitsi V, Schoneveld O, Pappa A, Panayiotidis MI (2010) The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem Biol Interact 188(2):334–339. https://doi.org/10.1016/j.cbi.2010.07.010
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 81573580, No. 81503229), Foundation of Liaoning Education Committee (51120427), Doctoral Starting up Foundation of Liaoning Science and Technology Department (51120390), Key Laboratory of Polysaccharide Bioactivity Evaluation of TCM of Liaoning Province and Key Laboratory of Quality Control of TCM of Liaoning Province (17-137-1-00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
He, B., Xu, F., Xiao, F. et al. Neuroprotective effects of nootkatone from Alpiniae oxyphyllae Fructus against amyloid-β-induced cognitive impairment. Metab Brain Dis 33, 251–259 (2018). https://doi.org/10.1007/s11011-017-0154-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0154-6