Abstract
In patients with hepatic encephalopathy (HE) the blood concentration of ammonia is usually highly elevated. Ammonia readily enters brain cells from the blood, and toxic effects of ammonia on brain metabolism and neurotransmission are believed to play a key role in the pathogenesis of HE. It has, however, been a matter of great controversy whether backflux of unmetabolized ammonia (NH3 + NH4 +) from brain cells to the blood occurs in man. In the present analysis of data from a dynamic PET study of brain 13N–ammonia metabolism in healthy subjects and cirrhotic patients with and without HE, we provide the first unambiguous evidence for backflux of ammonia from brain cells to the blood in man. The high temporal and spatial resolution of modern PET technology was employed to distinguish between unidirectional blood-brain transport of ammonia and subsequent metabolism of the ammonia in the brain. In all 16 subjects, clearance of the unidirectional transport of 13N–ammonia from the blood to brain cells (K1) was higher than the metabolic clearance of 13N–ammonia from the blood (Kmet=K1 k3/(k2+k3). This can only be explained by backflux (k2) of ammonia from brain cells to the blood. In conclusion, backflux of ammonia from the brain to the blood does indeed occur in both healthy subjects and cirrhotic patients with and without hepatic encephalopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most patients with cirrhosis and hepatic encephalopathy (HE) have elevated arterial blood concentrations of ammoniaFootnote 1 and the neurotoxic effects of ammonia are numerous (Butterworth 2002). How ammonia enters and leaves the brain is however a matter of controversy (Lockwood and Wack 2006; Sørensen and Keiding 2006; Sørensen and Keiding 2007; Lockwood 2007). Recent positron emission tomography (PET) studies have shown that blood-borne 13N–ammonia readily enters brain tissue (Ahl et al. 2004; Keiding et al. 2006) and the metabolic trapping of 13N–ammonia in brain tissue has been shown to be linearly correlated to the blood concentration of ammonia (Lockwood et al 1979; Keiding et al. 2006). To what extent ammonia diffuses back to the blood has, however, never been settled (Phelps et al. 1977; Raichle and Larson 1981; Ott and Larsen 2004). Some studies have regarded the brain “an infinite sink”, assuming that free ammonia does not leave the brain (Lockwood et al. 1979; Lockwood et al. 1984; Ott and Larsen 2004). Because the magnitude of such a backflux (or washout) of ammonia has been difficult to determine, this model has been convenient from a practical point of view but it is important to emphasize that no studies have actually been able to exclude the existence of a backflux (Ott and Larsen 2004). Washout of unmetabolized ammonia would prevent unrestricted elevation of intracellular concentration of ammonia in cases where it might exceed the metabolic removal capacity of the brain. It would hence be of great pathophysiological importance for the understanding of HE to settle whether a backflux of ammonia exists in man.
In a recent PET study, data were analyzed using a model including possible backflux of ammonia (Keiding et al. 2006). The study included patients with cirrhosis and an episode of HE, patients with cirrhosis and no HE and healthy subjects and the analysis yielded positive rate constants for the backflux in all subjects and all brain regions studied (Keiding et al. 2006). However, the impact of this backflux on the data and whether it was significant compared to the intracellular metabolic trapping was not further elucidated. In a similar study Ahl et al. (2004) also included a rate constant for backflux but did not report the values.
For the present purpose we re-analyzed data from the PET study of brain 13N–ammonia metabolism published by Keiding et al. (2006) in order to explore the significance of a backflux of ammonia from brain tissue to blood. This was done by comparing the unidirectional, non steady-state uptake of 13N–ammonia following a bolus injection with the later quasi steady-state uptake and metabolism.
Theoretical considerations
The kinetic model of brain metabolism of ammonia in Fig. 1 is a two-tissue compartmental model with three compartments: the extra-cellular blood compartment and two intracellular compartments, namely a precursor pool of unmetabolized ammonia and a pool of metabolized ammonia. The kinetic parameters, K1, k2, and k3, can be determined by fitting the model in Fig. 1 to the time courses of tissue concentrations of radioactivity in brain and blood from dynamic PET data. The rate constants for backflux, k2, and intracellular metabolic trapping, k3, (both in terms of per minute, min−1) are in general estimated with some uncertainty, however, mainly because of the influence of metabolites appearing in the blood with time. The determination of the unidirectional clearance from blood to brain, K1 (mL blood/mL tissue/min) is more robust because it is particularly sensitive to the initial time course of PET data and blood samples following a bolus injection of 13N–ammonia (Munk et al. 2001). We have previously shown this for 13N–ammonia by demonstrating that K1 can be estimated without metabolite-correction of the blood input curve because the amount of metabolites in blood builds up over time and is negligible during the first minutes after tracer injection (Sørensen and Keiding 2006).
Two-tissue compartmental model of brain 13N-ammonia transport across the blood-brain-barrier (BBB) and intracellular metabolism. K1 (mL blood/min/mL brain) is the unidirectional clearance of 13N–ammonia from the blood to brain cells, k2 (min−1) is the rate constant for the backflux of 13N–ammonia from brain cells to the blood, and k3 (min−1) is the rate constant for the intracellular quasi steady-state metabolism of 13N–ammonia (primarily to 13N–glutamine). Kmet (mL blood/min/mL brain) is the net metabolic clearance of 13N–ammonia from blood to 13N–metabolites in brain during quasi steady-state metabolism of 13N–ammonia. Kmet is related to the kinetic rate constants as shown in the box
Once inside the brain, 13N–ammonia enters the large pool of ammonia. The model depicted in Fig. 1 assumes intracellular trapping of ammonia by a reaction with glutamate to form glutamine, a reaction catalyzed by the glutamine synthetase enzyme (GS). The anatomical location of this reaction has been shown to be in the peri-capillary end-feet of the astrocytes (Norenberg and Martinez-Hernandez 1979). This is the quantitatively important reaction but the reaction between ammonia and α–ketoglutarate with formation of glutamate also contributes to the trapping (Cooper et al. 1979). The washout of 13N–metabolites is very slow and negligible within the experimental time period of a PET study (Phelps et al. 1981). The intracellular metabolism of 13N–ammonia can hence be considered irreversible. In this case, the net metabolic clearance of 13N–ammonia, Kmet, can be derived from a graphical analysis of the dynamic PET data and blood samples (Gjedde 1982; Patlak et al. 1983). During quasi steady-state metabolism, this representation yields a straight line and the slope of an asymptote fitted to this part equals Kmet. This parameter is related to the other three kinetic parameters as (Gjedde 1982; Patlak et al. 1983):
An important feature of this method is that it is a model-independent analysis in the way that the graphical representation of the data is independent on whether a backflux is present (with the rate constant k2). It relies on the irreversibility of the intracellular metabolic trapping of the tracer during quasi steady-state.
In summary, it is possible to obtain good measurements of both K1 and Kmet of ammonia in brain tissue from dynamic PET data following a bolus injection of 13N–ammonia. As seen from Eq. 1, the Kmet/K1 ratio equals k3/(k2+k3). Accordingly, in cases where the Kmet/K1 ratio is smaller than unity this can only be explained by the presence of a backflux with a rate constant, k2, of a significant size compared to the rate constant for metabolic trapping of 13N–ammonia, k3.
Data and calculations
We calculated the Kmet/K1 ratio from data from a dynamic PET study of brain 13N–ammonia metabolism (Keiding et al. 2006). Six patients with liver cirrhosis and an episode of acute HE, 5 patients with liver cirrhosis and no symptoms of HE, and 5 healthy subjects were included in the study. Median arterial concentrations of ammonia in the three groups were 73 µmol/L (range 29–97 µmol/L), 59 µmol/L (range 28–78 µmol/L), and 16 µmol/L (9–20 µmol/L), respectively (Keiding et al. 2006).
Details of the experimental procedures are described elsewhere (Keiding et al. 2006). In short, 13N–ammonia was injected intravenously and the time course of the brain tissue concentration of radioactivity recorded by a PET camera. Arterial blood concentrations over time of 13N–ammonia, 13N–urea, and 13N–glutamine were determined from blood samples analysed using a well-counter and a three-column solid phase extraction method validated by radio-HPLC (Rosenspire et al. 1990; Nieves et al. 1986). The unidirectional clearance, K1, was calculated by fitting the kinetic model in Fig. 1 to brain and blood concentrations of 13N–ammonia and 13N–metabolites by non-linear regression analysis. Kmet was determined from graphical analysis of data also using metabolite corrected input, as outlined in “Theoretical considerations”.
Results and discussion
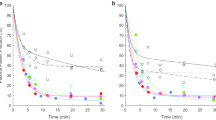
As shown in Fig. 2, Kmet was smaller than K1 in all three brain regions studied and in all subjects. Intravenously injected (exogenous) 13N–ammonia is hence subject to a backflux with a washout rate constant (k2) of a quantitatively significant size when compared to the rate constant for intracellular metabolic trapping, k3. This means that not all blood-borne ammonia entering the brain is metabolized but that a quantitative amount diffuses back to the circulation without being metabolized.
Backflux of ammonia from brain cells to the blood expressed in terms of the ratio of the metabolic clearance of 13N–ammonia Kmet to the initial unidirectional clearance K1 as determined by dynamic PET studies of 13N–ammonia metabolism. If K met/K1 = 1, then there is no significant backflux compared to the intracellular metabolic trapping of 13N–ammonia. If K met/K1 < 1, then there is a significant backflux of 13N–ammonia (see Fig. 1). Results are shown for the three brain regions studied. Cirr+HE is the group of patients with cirrhosis and hepatic encephalopathy; Cirr-HE is the group of patients with cirrhosis without hepatic encephalopathy; Healthy is the group of healthy subjects
The analysis reported here was performed qualitatively without individual estimates of the rate constant for the backflux, k2, but it illustrates that k2 is significant compared to the rate constant for the metabolic trapping of ammonia, k3, as seen from Eq. 1. The possibility of ammonia leaving the brain tissue without being metabolized also opens up the possibility of ammonia produced within the brain (endogenous ammonia) may leave the brain.
Not only does the presence of a backflux affect the estimation of the clearance (Kmet vs. K1) but calculations of other kinetic parameters will inevitable be biased if a wrong clearance is used. For example, in our study (Keiding et al. 2006) we calculated the permeability-surface area product, PS, for ammonia across the blood-brain-barrier from the Kety-Renkin-Crone relation (Kety 1951; Renkin 1959; Crone 1964):
where CBF is the cerebral blood flow. The relation shows that at low CBF, which is often observed in patients with HE (Iversen et al. 2008) the estimates of the PS-product are particularly sensitive to errors in the estimation of K1. It is hence crucial that K1 is calculated from data measured immediately following the bolus injection 13N–ammonia. Another example is the calculation of extraction fractions:
where K is either K1 which yields the unidirectional extraction fraction, E0, or Kmet which then yields the net metabolic extraction fraction, Emet. The Emet/E0 ratio accordingly equals the Kmet/K1 ratio.
In conclusion, the PET data after injection of 13N–ammonia in cirrhotic patients with or without HE and healthy subjects are incompatible with a kinetic model that does not allow for backflux of unmetablolized ammonia. Thus, ammonia entering brain tissue from the blood can re-enter the blood circulation without being metabolized. Because of the existence of a backflux it is hence of utmost importance to discriminate between the unidirectional clearance, K1, and the net metabolic clearance, Kmet, which are determined from data obtained during the initial non steady-state phase of distribution and the subsequent quasi steady-state phase of metabolic trapping, respectively.
Notes
In this paper, the term ammonia refers to the sum of the unionized NH3 and the ionized NH4 +.
References
Ahl B, Weissenborn K, van den Hoff J, Fischer-Wasels D, Köstler H, Hecker H, Burchert W (2004) Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology 40:73–79
Butterworth RF (2002) Pathophysiology of hepatic encephalopathy: A new look at ammonia. Metab Brain Dis 17:221–227
Cooper AJL, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE (1979) The metabolic rate of 13N labelled ammonia in rat brain. J Biol Chem 254:4982–4992
Crone C (1964) Permeability of capillaries in various organs as determined by use of the indicator diffusion method. Acta Physiol Scand 58:292–305
Gjedde A (1982) Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a re-examination. Brain Res 257:237–274
Iversen P, Sørensen M, Bak LK, Waagepetersen HS, Vafae MS, Borghammer P, Mouridsen K, Jensen SB, Vilstrup H, Schousboe A, Ott P, Gjedde A, Keiding S (2008) Low cerebral oxygen consumption and blood flow in patients with cirrhosis and an acute episode of hepatic encephalopathy. Gastroenterology (in press)
Keiding S, Sørensen M, Bender D, Munk OL, Ott P, Vilstrup H (2006) Brain metabolism of 13N–ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology 43:42–50. Correction in Hepatology 44:1056
Kety SS (1951) The theory and application of the exchange of inert gas at lung and tissues. Pharmacol Rev 3:1–41
Lockwood AH, McDonald JM, Reiman RE, Gelbard AS, Laughlin JS, Duffy TE, Plum F (1979) The dynamics of ammonia metabolism in man. J Clin Invest 63:449–460
Lockwood AH, Bolomey L, Napoleon F (1984) Blood-brain barrier to ammonia in humans. J Cerebral Blood Flow Metab 4:516–522
Lockwood AH, Wack DS (2006) To the editor: The brain permeability-surface product for ammonia. Hepatology 44:1052–1053
Lockwood AH (2007) Controversies in ammonia metabolism: implications for hepatic encephalopathy. Metab Brain Dis 22:285–289
Munk OL, Bass L, Roelsgaard K, Bender D, Hansen SB, Keiding S (2001) Liver Kinetics of Glucose analogs measured in Pigs by PET: Importance of dual-input blood sampling. J Nucl Med 42:795–801
Nieves E, Rosenspire KC, Filc-DeRicco S, Gelbard AS, Cooper AJ (1986) High-performance liquid chromatographic on-line flow-through radioactivity detector system for analyzing amino acids and metabolites labeled with nitrogen-13. J Chromatogr 383:325–337
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 151:303–310
Ott P, Larsen FS (2004) Blood-brain barrier permeability to ammonia in liver failure: a critical reappraisal. Neurochem Int 44:185–198
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3:1–7
Phelps ME, Hoffman EJ, Raybaud C (1977) Factors which affect cerebral uptake and retention of 13NH3. Stroke 8:694–702
Phelps ME, Huang SC, Hoffman EJ, Selis C, Kuhl DE (1981) Cerebral extraction of N-13 ammonia: its dependence on cerebral blood flow and capillary permeability-surface area product. Stroke 12:607–619
Raichle ME, Larson KB (1981) The significance of the NH3-NH4 + equilibrium on the passage of 13N-ammonia from blood to brain. A new regional residue detection model. Circ Res 48:913–937
Renkin EM (1959) Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol 197:1205–1210
Rosenspire KC, Schwaiger M, Mangner TJ, Hutchins GD, Sutorik A, Kuhl DE (1990) Metabolic fate of [13N]ammonia in human and canine blood. J Nucl Med 31:163–167
Sørensen M, Keiding S (2006) Ammonia metabolism in cirrhosis. In D Häussinger, G Kircheis, F Schliess (eds.), Hepatic Encephalopathy and Nitrogen Metabolism, Springer pp. 406–419
Sørensen M, Keiding S (2007) New findings on cerebral ammonia uptake in HE using functional 13N–ammonia. Metab Brain Dis 22:277–284
Acknowledgements
The study was supported by grants from the Danish Medical Research Council (22-02-0337) and the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sørensen, M., Munk, O.L. & Keiding, S. Backflux of ammonia from brain to blood in human subjects with and without hepatic encephalopathy. Metab Brain Dis 24, 237–242 (2009). https://doi.org/10.1007/s11011-008-9126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-008-9126-1