Abstract
The most frequently reported symptom of exposure to high altitude is loss of body mass and decreased performance which has been attributed to altered protein metabolism affecting skeletal muscles mass. The present study explores the mechanism of chronic hypobaric hypoxia mediated skeletal muscle wasting by evaluating changes in protein turnover and various proteolytic pathways. Male Sprague–Dawley rats weighing about 200 g were exposed to hypobaric hypoxia (7,620 m) for different durations of exposure. Physical performance of rats was measured by treadmill running experiments. Protein synthesis, protein degradation rates were determined by 14C-Leucine incorporation and tyrosine release, respectively. Chymotrypsin-like enzyme activity of the ubiquitin–proteasome pathway and calpains were studied fluorimetrically as well as using western blots. Declined physical performance by more than 20%, in terms of time taken in exhaustion on treadmill, following chronic hypobaric hypoxia was observed. Compared to 1.5-fold increase in protein synthesis, the increase in protein degradation was much higher (five-folds), which consequently resulted in skeletal muscle mass loss. Myofibrillar protein level declined from 46.79 ± 1.49 mg/g tissue at sea level to 37.36 ± 1.153 (P < 0.05) at high altitude. However, the reduction in sarcoplasmic proteins was less as compared to myofibrillar protein. Upregulation of Ub-proteasome pathway (five-fold over control) and calpains (three-fold) has been found to be important factors for the enhanced protein degradation rate. The study provided strong evidences suggesting that elevated protein turnover rate lead to skeletal muscle atrophy under chronic hypobaric hypoxia via ubiquitin–proteasome pathway and calpains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has now become a well known fact that physical performance of people decreases on ascending to high altitude [1]. People ascending to high altitude expose themselves to a number of challenging environmental conditions such as low air humidity, low temperature, high UV radiations, and most importantly hypoxia. High altitude hypoxia has been reported to be an important factor in skeletal muscle atrophy at moderate altitudes [2]. High altitude mediated hypobaric hypoxia initiates many physiological changes with loss of body mass and protein stores being the most inevitable changes [3]. The decreased physical performance observed at high altitude [4–6] is likely to be a consequence of the dwindled protein stores. Sustained exposure to severe hypoxia has detrimental effects on muscle structure [7]. Some studies showed that muscle cross-sectional area in the thigh decreased by 10% after sojourns went to the Himalayas. Morphologically this loss in muscle mass appeared as a decrease in muscle fiber size mainly due to a loss of myofibrillar proteins [8]. Muscle lipofuscin, a degradation product of lipid peroxidation and an indicator of muscle fiber damage, has been shown to increase by more than two-fold after an expedition to the Himalayas [9]. Human subjects climbing to Peak Lenin (7,134 m) showed a significant decrease in fat-free mass after their return [10]. The study by Bigard et al. [11], clearly demonstrate that hypobaric hypoxia decreases growth rate in rats and increasing the dietary protein intakes in rat had no effect on the depression of muscle growth related to high altitude but had deleterious effects on glycogen deposition in liver and fast muscle.
Decreased protein synthesis is suggested as the underlying mechanism for the loss of skeletal muscle mass [12–14]. Since these studies are acute, presumably, the short duration exposure to hypoxia does not allow sufficient time for relevant hypoxic responses to occur and thus does not determine whether and how the chronic hypoxia affects protein synthesis rate. Only a limited number of chronic studies reporting protein turnover under hypobaric hypoxia have been published. Most of these studies have reported attenuated protein synthesis rate based on indirect measurements such as reduction in mammalian target of rapamycin (mTOR), a marker of protein synthesis after hypobaric hypoxic exposure [15].
Thus, declined protein synthesis has been shown to be detrimental for skeletal muscle mass in numerous studies. However, contrasting results have also been reported. Imoberdorf and co-workers [16] assessed muscle protein synthesis rate after exposure to high altitude and showed that in a group of subjects, investigated acutely after active ascent to high-altitude, muscle protein synthesis rate was higher compared to in a group that was flown to the altitude and compared to the rate at sea level. Similarly, resting skeletal muscle myocontractile protein synthesis rate was shown to be concomitantly elevated by high-altitude induced hypoxia [17].
Increased excretion of proteins in the urine of native high landers [18] provides a clue for enhanced muscle protein catabolism at high altitude. Skeletal muscle atrophy under various catabolic conditions such as cachexia, burn, and sepsis has been linked to upregulation of different proteolytic pathways like ubiquitin–proteasome pathway [19, 20], lysosomal pathways [21], and calpains [22, 23]. However, the role of these pathways is still not clear in the hypobaric hypoxia mediated skeletal muscle loss.
Inconsistency of the earlier results and lack of conclusive evidences for the mechanism of hypobaric hypoxia induced skeletal muscle atrophy led us to design the present study. The purpose of this study was to evaluate the effect of chronic hypobaric hypoxia on skeletal muscle mass and protein turnover and to find out the exact mechanism of loss of skeletal muscle mass under these conditions.
Materials and methods
Ethical approval
The study was approved by the Animal Ethical Committee of our institute in accordance with Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) of the government of India.
Experiments were conducted on Male Sprague–Dawley rats, weighing about 180–200 g. Rats were maintained at 25 ± 2°C in Animal facility, DIPAS, India, and given food and water ad libitum. The animals were housed three rats per cage, and maintained on a 12 h, day–night cycle.
The rats were randomly allocated to two groups. The groups were control: C and hypoxia treated: H. Hypoxia group was further divided into three batches: hypoxia exposure for 3, 7, and 14 days. These batches (n = 12) were exposed to hypoxia at a simulated altitude of 7,620 m in a hypobaric chamber. Since food intake decreases during hypoxia exposure, one group was pair fed to the intake of hypoxia exposed rats. Control group rats were maintained in the normoxic condition within the same laboratory.
Physical performance measurements: treadmill exercise
Before starting the exposure experiments, all the animals were familiarized for 2 weeks on a motorized treadmill (0% grade) at 25 m/min for 45 min daily. Fatigue time of rats on the treadmill was recorded before and after exposure of rats to hypoxia.
Histology
The animals (n = 12) were anesthesized by injection of sodium pentobarbital (50 mg/kg, i.p.), and perfused intracardially with 0.1 M PBS (pH 7.4) followed by 4% formaldehyde. The muscles were then carefully dissected out and postfixed in the same fixative overnight. Paraffin blocks were then prepared after dehydration, clearing, and wax impregnation. Sections of 5 μm were cut with a rotary microtome, deparaffinized in xylene, and stained with Hematoxylin and Eosin.
Protein synthesis and protein degradation rates
Rats were killed by cervical dislocation and hind limb gastrocnemius muscles were excised and immediately placed in Krebs-Henseleit bicarbonate buffer for incubation as described [24, 25]. The muscles were quickly rinsed and incubated in Krebs-Henseleit buffer consisting of 120 mM NaCl, 4 mM KCl, 25 mM NaHCO3, 2.5 mM CaCl2, 1.2 mM KH2PO4, and 1.2 mM MgSO4 (pH 7.4), supplemented with 5 mM glucose, 5 mM HEPES, 0.1% (w/v) BSA, 0.17 mM leucine, 0.20 mM valine, and 0.10 mM isoleucine.
Protein synthesis rate was measured as previously described [25, 26]. Muscles were first preincubated at 37°C for 30 min. After the preincubation period, fresh Krebs-Henseleit bicarbonate buffer supplemented with 0.10 μCi/ml 14C-leucine were added to the skeletal muscle, and incubated for a further 60 min. At the end of the incubation, muscles were removed from the incubation buffer, washed with cold buffer and homogenized in 10% (w/v) ice-cold TCA. The homogenate was centrifuged at 10,000×g for 10 min at 4°C. The supernatant was decanted and the pellet was suspended in 1 M NaOH and incubated at 37°C for 30 min. Aliquots of this mixture were used to quantify the radioactivity based on liquid scintillation counting of β emission. The rate of protein synthesis was expressed as nmoles of leucine incorporated per hour per milligram of muscle protein.
Protein degradation rate was determined by the release of tyrosine over a period of 2 h as described previously [24, 25]. Because tyrosine is neither synthesized nor degraded by muscles, its release from muscle into the incubation medium reflects the net protein degradation rate. Tyrosine was assayed fluorometrically [27]. The rate of protein degradation was expressed as nmoles of tyrosine released per 2 h per milligram of muscle protein.
Protein degradation pathways
20 S proteasome activity of Ub-proteasome pathways
The ubiquitin proteasome pathway was studied by assaying the chymotrypsin-like enzyme activity of 20 S Proteasome, as described earlier [28]. The fluorogenic peptide succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Suc-LLVY-AMC) served as substrate for the chymotrypsin-like activity. The muscle extracts containing 60 μg protein were incubated for 30 min at 37°C in 50 μl of a buffer containing 100 mM Tris–HCl (pH 8.0), 1 mM DTT, 5 mM MgCl2, 1 mM Suc-LLVY-MCA, 2 mg/ml ovalbumin, and 0.07% SDS. The reaction was terminated by 25 μl of 10% SDS and diluted by 2 ml of 0.1 M Tris–HCl (pH 9.0). Fluorescence of the liberated amidomethylcoumarin was monitored in a Perkin-Elmer fluorometer at excitation 380 nm, emission 460 nm. Chymotrypsin-like enzyme activity was expressed arbitrary units per minute per microgram of muscle protein.
Calpain assay
Calpains, calcium activated proteases, were measured in the homogenate using N-succinyl-Leu-Tyr-7-amido-4-methylcoumarin (SLY-AMC) as a substrate [29]. A stock solution of 50 mM SLY-AMC was prepared in dimethyl sulfoxide and stored at −20°C. Muscles were homogenized in buffer having 50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 10 mM NaH2PO4, 1% nonidet P-40, and 0.4 mM sodium orthovanadate. Homogenate was then centrifuged at 13,000×g for 15 min at 4°C. The following procedure was used for measuring calpain activity in muscle extracts: 30 μl muscle extract was incubated for 60 min at 37°C in a buffer solution (pH 7.4) containing 25 mM HEPES (pH 7.5), 0.1% CHAPS, 10% sucrose, 10 mM DTT, 0.1 mg/ml ovalbumin. After addition of 5 ml of the substrate solution, buffer was added to adjust the volume of the assay to 2 ml. Fluorescence of the liberated AMC was monitored in a Perkin-Elmer fluorimeter (LS-45) at excitation 380 nm, emission 460 nm. Calpain activity, Ca2+ dependent cleavage of SLY-AMC, was expressed as picomole AMC released per microgram of muscle protein.
Lysosomal enzymes assay
Acid phosphatase activity, marker enzyme of lysosomes, was determined using the p-nitrophenyl-phosphate method [30]. An aliquot of 20 μl of the homogenate was incubated at 37°C for 10 min in 2.5 mM sodium acetate buffer, pH 5.0 and 0.5 mM p-nitrophenyl phosphate. The reaction was stopped by adding 0.2 ml NaOH (5 N) and the absorption was read at 405 nm using UV–Vis spectrophotometer, (BioRad, USA). One unit of the enzyme was defined as the amount of enzyme that liberates 1 mol of p-nitrophenol per hour.
Total protein estimation
Total Protein in skeletal muscle homogenate (10% w/v in 150 mM KCl) was assayed using Lowry’s method [31]. Results were expressed as milligram of protein per gram wet tissue weight.
Myofibrillar and sarcoplasmic protein content
Myofibrillar and Sarcoplasmic fractions from rat skeletal muscle were obtained by slight modification in the method described earlier [32]. In brief, muscle samples were homogenized in a 5% ice-cold buffer containing 0.25 m sucrose, 2 mm EDTA, and 10 mm Tris–HCl (pH 7.4). The homogenate was centrifuged at low speed (600×g) and the pellet containing myofibrillar protein was collected. From the supernatant, the sarcoplasmic protein fraction was isolated after centrifugation at 100,000×g for 60 min at 4°C. Protein content in both the fractions was assayed using Lowry’s method [31].
Estimation of hormones
IGF-1 and catecholamines in rat skeletal muscle homogenate and plasma, respectively, were estimated using commercially available kits (Rat/Mouse IGF-1 ELISA, Novozymes, UK and 2-CAT ELISA, LDN, Germany, respectively).
CPK activity
Creatine Phosphokinase (CPK) activity was measured in rat skeletal muscle homogenate using commercially available kit (Randox CK-NAC, UK) as per manufacturer’s instructions. CPK activity was expressed as mIU per mg tissue weight.
Glutaminase enzyme activity
Glutaminase enzyme activity in rat muscle homogenate was measured as described earlier [33]. In brief, GDH (Sigma; 42 units/mg) was incubated in buffer containing l-Glutamine (100 mM), oxoglutarate (50 mM), phosphate buffer (1 M), EDTA (2 mM), and NADH for 5 min at 25°C. Initial absorbance was read at 340 nm. Tissue homogenate was added to it and incubated again for 2 min at 25°C. Final absorbance was again read at 340 nm. Activity was expressed as micromole per minute per milligram muscle protein.
Glutamine synthetase activity
Glutamine synthetase activity in rat muscle homogenate was measured as described earlier [34]. In brief, tissue homogenate was incubated with buffer containing Tris (0.1 M), MgSO4 (20 Mm), sodium glutamate (80 mM), hydroxylamine (6 mM), and ATP (8 Mm) for 5–15 min at 37°C. Reaction was terminated by adding ferric chloride (0.37 M) and absorbance was read at 540 nm using UV–Vis spectrophotometer, (BioRad, USA). Glutamine synthetase activity was expressed as katal units per minute per milligram of muscle protein.
Western blot: ubiquitin, calpain and HIF-1α expressions
The time dependent expression of ubiquitinated proteins, calpain, and HIF-1α on exposure to hypobaric hypoxia was determined by western blot. Primary anti-ubiquitin antibody was obtained from Santa Cruz and anti-μ-calpain antibody and anti-HIF-1α antibody were purchased from Sigma. Muscles were dissected at 4 °C on completion of hypoxia exposure. Ten percent tissue homogenate was prepared in ice cold- lysis buffer (10 mM Tris–HCl, 100 mM NaCl, 0.1 mM dithiothreitol, 1 mM EDTA, 0.1% NaN3, 100 μg/ml PMSF, protease inhibitor cocktail, pH 7.6). The homogenate was centrifuged at 1,000×g for 10 min at 4°C and the supernatant was used for further studies. Total protein content was estimated by Lowry’s method. Fifty μg of sample protein was then resolved by SDS-PAGE and transferred to nitrocellulose membrane pre-soaked in transfer buffer (20% methanol, 0.3% Tris and 1.44% glycine) using a semidry transblot module (BIORAD). The membranes were blocked with 5% non-fat milk, washed with PBST (0.01 M phosphate buffer saline, pH 7.4, 1 ml of 0.01% Tween 20), and probed with primary antibody (1:1000 dilution) for 3 h at room temperature. The membranes were then washed with PBST and incubated for 2 h at room temperature in secondary antibody (Santa Cruz) diluted in 3% non-fat milk. Membranes were then finally washed with PBST and the bands were developed on X-ray film using chemiluminescent substrate (Sigma). The bands thus obtained on the films were quantified by densitometry to determine expression of the protein.
Oxidative stress markers
Free radical generation
The production of free radicals was determined by using 2,7-dichlorofluoroscein diacetate (DCFH-DA) as described earlier [35]. In brief, 150 μl of muscle homogenate was incubated with (10 μl) 100 μM DCFH-DA for 30 min in dark. Fluorescence was read using a fluorimeter (Perkin Elmer, UK) with excitation at 485 nm and emission at 535 nm. Percentage change in free radical generation was expressed as result.
Lipid peroxidation
Malondialdehyde (MDA), a marker for lipid peroxidation, was measured in muscle tissue homogenates as described by Buege and Aust [36]. In brief, 100 mg tissue was homogenized in 15% (w/v) TCA and 0.355% (w/v) TBA and then incubated in boiling water bath for 30 min. It was then centrifuged and absorbance was read at 535 nm using UV–Vis spectrophotometer, (BioRad, USA). Results were expressed as percentage change in lipid peroxidation.
Statistical analysis
All the results are presented as mean ± SEM. The experiments were conducted on two different occasions and the data was analyzed using ANOVA followed by Student–Newman–Keuls (SNK) test. Significance level was set at P < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Inc version 15.0). Multi regression analysis was also performed between the physical performance and other biochemical parameters to understand the dependency of these variables.
Results
Effect of chronic hypobaric hypoxia on physical performance of rats
Exposure of rats to chronic hypobaric hypoxia decreased their ability to perform physical activity as shown by decline in their fatigue time on treadmill running. The decline in physical activity was time dependent, showing a significant reduction after 3 days of exposure, continuing to 7 and 14 days. A significant reduction of 24% (P < 0.05) in the fatigue time was observed in rats exposed to 14 days of hypoxia (Table 1). Pair fed group did not show significant difference as compared to the control group (data not shown).
Effect of chronic hypobaric hypoxia on rat gastrocnemius muscle weight/tibial length
With increasing duration of exposure to hypobaric hypoxia, the ratio of rat gastrocnemius muscle weight/tibial length decreased significantly. The ratio decreased by 25% (P < 0.05) following 7 days of exposure and the decrement moved up to 34% (P < 0.05) following 14 days of hypoxia exposure (Table 2).
Histological effects of chronic hypobaric hypoxia on skeletal muscle
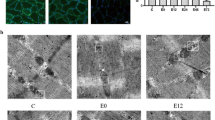
Histological examination of sections from gastrocnemius muscles were used to evaluate the effects of chronic hypobaric hypoxia on their integrity. Muscles were removed from hypobaric hypoxia-treated and control rats and analyzed for the potential presence of histopathological signs. The 40× High power photomicrograph of muscle biopsy showed transverse cut muscle fibers with uniform size and shape revealing the thin, delicate endomysial connective tissue, fibers of uniform size, and the peripherally placed nuclei. After exposure to hypoxia for 3 days the muscle fibers were uniform in size and shape. However, the fibers were smaller in size and had relatively more space between them as compared to control fibers. The photomicrograph of muscle tissue from rats exposed to 7 days of hypoxia exposure showed both atrophic and hypertrophic muscle fibers. Photomicrograph of muscle tissue from rat exposed to 14 days of hypobaric hypoxia, showed further atrophy of muscle fibers with irregularity in fiber size, which had relatively more space between them, compared to 7 days exposed groups. However, no necrotic fiber or cell splitting could be observed in any of the micrographs (Fig. 1).
Hematoxylin and Eosin staining of muscle cells in rat gastrocnemius muscle. Hematoxylin and Eosin staining of muscle cells in rat gastrocnemius muscle: a Control group showing transverse cut muscle fibers having uniform size and shape with peripheral nuclei. The fibers are closely set with little space between them; b 3 days hypoxia exposed group fibers are smaller in size and have relatively more space between them; c 7 days exposed group showing centralized nuclei (arrow), and irregularity of fiber size; d 14 days exposed fibers show further atrophy. Thin endomysium is visible only in the control group (arrow) and 3 days hypoxia group (arrow). A change in shape is observed in all the hypoxia exposed groups, however no cell splitting is visible in any group. Scale bar 10 μm
Effect of chronic hypobaric hypoxia on total protein, myofibrillar and sarcoplasmic protein content
A reduction in protein levels is expected under conditions where skeletal muscle atrophy is observed. Predictably, total protein content in the skeletal muscle homogenates of rats exposed to chronic hypobaric hypoxia of different durations showed a significant decrease. Following 3 days of exposure, 13% (P < 0.05) reduction is obtained in the total protein content of skeletal muscle homogenates of rats followed by 18 and 22% (P < 0.05) reduction over the control rats after 7 and 14 days, respectively (Fig. 2a).
On subfractionation of the total protein, we found a significant decrease of 30% (P < 0.05) in the myofibrillar protein content in the 14 days exposed rats (Fig. 2b). The 7% decrease in sarcoplasmic protein (Fig. 2b), was much less as compared to the loss of myofibrillar protein.
Effect of chronic hypobaric hypoxia on protein turnover
Muscle proteolysis, represented by the release of tyrosine from hind limb muscles, was compared between control and hypoxia exposed groups (Fig. 3a). The hypoxic exposure led to about 2.5-Fold (P < 0.05) and three-fold (P < 0.05) increase in muscle proteolysis following 3 and 7 days exposure, respectively. The proteolysis further increased up to five-fold (P < 0.05) after 14 days of hypoxia exposure. Protein synthesis rates in rat hind limb muscle were determined by measuring 14C-leucine incorporation into newly synthesized proteins. These analyses revealed that synthesis rate also increased in skeletal muscle of rats exposed to chronic hypobaric hypoxia as compared to control animals (Fig. 3b). Though the increment was not significant till 3 days of hypoxia exposure, the augmented rate of protein synthesis (1.2-fold, P < 0.05 following 7 days hypoxia and 1.5-fold, P < 0.05 following 14 days of hypoxia) was obtained on subsequent durations of hypobaric hypoxia exposure.
Effect of chronic hypobaric hypoxia on a PD protein degradation rate, b PS protein synthesis rates, and c fold increase over control in protein synthesis and degradation rate n rat gastrocnemius muscle (“a” indicates P < 0.05 versus control group, “b” indicates P < 0.05 versus 3 days, “c” indicates P < 0.05 versus 7 days)
However, increase in protein degradation rate was significantly higher in comparison to synthesis rate (Fig. 3c), leading to overall enhanced protein depletion.
Effect of chronic hypobaric hypoxia on Ub-proteasome pathway, calpain activity, and lysosomal pathway
In accordance with the proteolytic rate, the chymotrypsin-like activity of Ub-proteasome pathway (Fig. 4a) was increased in the hypoxia exposed group compared with the control group. The increment was approximately five-fold (P < 0.05) over control in the 14 days hypoxia exposed group. Similarly, calpain activity also showed a three-fold increase (P < 0.05) over control (Fig. 4b). Acid phosphatase activity was measured as a marker for lysosomal pathway. No significant change was observed in the acid phosphatase activity after 3 and 7 days of exposure, but the activity increased by about 20% after 14 days of hypoxia exposure (Fig. 4c).
Effects of chronic hypobaric hypoxia on IGF-1 and catecholamine
IGF-1 is an anabolic hormone while catecholamine inhibits skeletal muscle proteolysis. Chronic exposure to hypobaric hypoxia does not result in any significant change in the IGF-1 level and epinephrine (Table 2). However, an increase in nor-epinephrine levels was observed after 3 and 7 days of hypoxia exposure but the increase was not significant (Table 3).
Effects of chronic hypobaric hypoxia on enzymes CPK, glutaminase, and glutamine synthetase
CPK levels were measured as an index of muscle damage under hypobaric hypoxia. With increase in duration of hypoxia exposure, the CPK content of muscle homogenates decreased significantly. There was two-times decrease in the CPK activity (P < 0.05) in the 14 days hypoxia exposed group as compared with the control group (Fig. 5a). Glutamine is known to play a very important role in protein metabolism and muscle mass accounts for about 90% of all glutamine synthesized in the body. Glutaminase and glutamine synthetase are therefore measured for conclusive study of protein turnover in skeletal muscle. A time dependent increase in the glutaminase activity supports the observed increment in skeletal muscle proteolysis. Following 3 days of exposure, significant change was observed in the Glutaminase activity. A significant increase of 3.5-fold (P < 0.05) and four-fold (P < 0.05) was obtained after 7 and 14 days of hypoxia exposure, respectively, Fig. 5b. Similar trend was observed in glutamine synthetase enzyme activity. It showed a significant increase (Fig. 5c) of 1.5 times over control following 14 days of hypoxia exposure (P < 0.05).
Effects of chronic hypobaric hypoxia on expressions of ubiquitinated proteins and calpain
Expressions of ubiquitin, calpain were studied using western blot (Fig. 6a). The results showed that there was an increased expression of ubiquitinated proteins after hypoxic exposure (Fig. 6b). Exposure to 3 days lead to approximately three-fold increase over control (P < 0.05) which gradually increased to 3.5-fold (P < 0.05) after 7 days exposure and up to five-fold increase (P < 0.05) over control in 14 days exposed rats. Similarly, μ-calpain expression also increased in hypobaric hypoxia exposed animals with about 2.7-fold increase (P < 0.05) over control after 14 days of hypoxia exposure (Fig. 6c).
Effect of chronic hypobaric hypoxia on a ubiquitinated proteins, μ-calpain and HIF-1α as determined by western blotting. Blots showing increasing band intensities at different hypoxia durations represent upregulated ub-proteasome pathway, calpains and HIF-1α, b densitometry of ubiquitinated proteins, c densitometry of calpain and d Densitometry of HIF-1α (“a” indicates P < 0.05 versus control group, “b” indicates P < 0.05 versus 3 days, “c” indicates P < 0.05 versus 7 days)
Effects of chronic hypobaric hypoxia on HIF-1α and oxidative stress markers
HIF-1α is an important transcription factor for the expression of hypoxia responsive genes. Hence to assess the magnitude of hypobaric hypoxic stress, expression of HIF-1α was studied using western blot. The results showed a time dependent increase in expression of HIF-1α in rat skeletal muscle homogenate with maximum increase of five-fold over control observed in the 14 days exposed rats (Fig. 6d).
Free radical generation and lipid peroxidation were measured as markers of oxidative stress. A significant increase of 1.5-fold over control was observed in free radical generation after 3 days of hypoxia exposure (P < 0.05). Free radical generation increased further with 2.4-fold increase (P < 0.05) over control after 7 days of hypobaric hypoxia and up to 3.5-fold increase (P < 0.05) over control after 14 days of hypobaric hypoxia exposure (Fig. 7a). Similar results were obtained in lipid peroxidation with a maximum increase of 80% over control (P < 0.05) in 14 days hypoxia exposed rats (Fig. 7b).
Discussion
Although the atrophic phenomenon at altitude has been studied for decades, the underlying mechanisms remain unresolved and only few data on protein turnover in response to chronic hypobaric hypoxia exist. High altitude affects the human body because of oxygen deprivation. A consistent consequence of severe altitude exposure where hypoxia is inevitable, such as during an expedition to the Himalayas, is a loss of body mass and a similar loss of muscle volume [7, 8]. The main findings in the present study are: (i) chronic hypobaric hypoxia lead to elevated skeletal muscle protein synthesis rate. It proves that synthesis rate is not a factor responsible for loss of skeletal muscle mass under chronic exposure. (ii) The fold increase in protein degradation rate is much higher than fold increase in protein synthesis rate with chronic hypobaric hypoxic exposure leading to overall decreased protein turnover. (iii) upregulation of ubiquitin–proteasome pathway and calpain activity are responsible for the enhanced protein degradation following chronic hypobaric hypoxia.
We studied various aspects of skeletal muscle alterations under the conditions of hypobaric hypoxia by assessing physical, biochemical, and histological parameters in rats after exposing them to different durations of hypobaric hypoxia. As shown in our results, chronic hypobaric hypoxia decreases physical performance which is indicated by a significant decrease in the fatigue time in treadmill running. Earlier studies from our lab have demonstrated that exposure to intermittent hypobaric hypoxia for 7 days results in decreased muscle performance [37]. They used electrical stimulation to induce six tetanic muscular contractions in the gastrocnemius muscle after completion of exposure. Percentage mean performed work (PW), time of decay to 50% peak force of contraction (T50), and peak force of contraction (F peak) were measured during titanic contractions. Fpeak was reduced in the hypoxia exposed group at the second, third, fourth, fifth, and sixth titanic contractions as compared with respective forces in the unexposed control group. Similarly, T50 was also reduced in the fifth and sixth titanic contractions as compared with respective forces in the unexposed control group. They also observed a reduction in PW in the third, fourth, and sixth contractions when compared with respective values of the unexposed group. Fluctuations in body weight as occur with aging make body weight an unreliable reference for normalizing muscle weight. We measured the effect of hypobaric hypoxia on skeletal muscle mass by measuring the ratio of gastocnemius muscle weight to tibial length. Predictably, chronic hypobaric hypoxia resulted in significant reduction in gastrocnemius muscle/tibial length ratio. A decline in muscle to tibial length ratio indicates muscle atrophy under such conditions which might serve as an important factor responsible for hampered physical activities of people ascending to high altitude. Histological results also showed a time dependent skeletal muscle atrophy in the hypoxia exposed rats. Pair fed groups did not show any significant difference in any parameter when compared to the control groups and therefore its data is not shown. But this confirms that the changes observed are restricted to hypobaric hypoxia exposure.
Our current results indicate that prolonged periods of hypobaric hypoxia has severely adverse effects on skeletal muscle protein status. Total protein content of skeletal muscle decreased significantly after hypoxia exposure. The decrease is mainly because of depletion of myofibrillar proteins. This decline in myofibrillar protein may be due to a marked increase in the rate of skeletal muscle protein degradation. Multiple regression analysis (using SPSS 15) was also carried out between the physical performance (PP) and muscle mass (MM), myofibrillar proteins (MP), and degradation rate (DR) to understand the dependency of these parameters. The multiple regression equation between these parameters is PP = 55.05 − 0.673 DR + 0.09 MW + 0.810 MP. The equation is significant at P < 0.001 with r 2 = 0.95. Since maximum changes in all the parameters were observed after 14 days of hypoxia exposure, the above equation depicts the dependency of the parameters in that group. This further signifies the role of these biochemical parameters in decreasing physical performance under chronic hypobaric hypoxia.
The most interesting and unpredictable outcome in our study is that chronic hypobaric hypoxia also lead to an increase in the protein synthesis rate which could possibly be explained as an adaptive response under these conditions. However, the fold increase in hypoxia exposed muscle over control, for protein degradation rate is much higher than fold increase in protein synthesis resulting in an overall decreased skeletal muscle mass.
As protein degradation emerged to be the cause of skeletal muscle loss under chronic hypobaric hypoxia, we further studied various proteolytic pathways. Our results indicate that Upregulation of the ubiquitin and calpains results in amplified activities of these two pathways which eventually lead to increased degradation of skeletal muscle proteins. The Ub-proteasome pathway has also been shown to account for the majority of skeletal muscle degradation in cancer cachexia where hypoxia is encountered [38]. We have found in this study that both the calpains and ubiquitin–proteasome pathways are implicated simultaneously leading to muscle atrophy during hypobaric hypoxia. Recent evidences also point toward interactive involvement of these systems in proteolysis under different catabolic conditions. Since oxidization of proteins lead to their degradation, hypobaric hypoxia induced skeletal muscle protein oxidation might play a role in upregulation of these proteolytic pathways.
It is also believed that the observed protein turnover is not driven by changes in hormones as we and others [16, 39] have not been able to detect any significant changes in the hormonal levels.
In this study, we also studied enzymatic activities which could affect protein metabolism under chronic hypobaric hypoxia. An increase in glutaminase enzyme activity supports the observed increase in protein degradation rate as glutaminase catalyzes the breakdown of glutamine residue resulted from proteolysis of skeletal muscle proteins. Similarly, increased glutamine synthetase enzyme activity may be a factor responsible for the enhanced protein synthesis. These results are in accordance with the results of previous study carried by Vats et al. [40].
CPK is the key energy reservoir in skeletal muscles. Lower levels of CPK in skeletal muscle of rats exposed to chronic hypobaric hypoxia are also a contributing factor to the hampered physical activity under these conditions. Hypobaric hypoxia leads to increased muscle permeability which results in leakage of the CPK from muscle to the bloodstream. Thus, the decreased creatinine phosphokinase activity in skeletal muscle homogenates, as shown in our results, is an indicator of the muscle permeability under chronic hypobaric hypoxia.
To assess the magnitude of hypoxic stress, expression of HIF-1α was studied. Our results show that with increase in hypoxia duration HIF-1α expression also increased. Chronic hypoxia also induced oxidative stress which was indicated by a significant increase in the free radicals and malondialdehyde levels. This increased oxidative damage may be a trigger for the increased protein degradation under chronic hypoxia. Several studies show that oxidative stress upregulate ubiquitin proteasome pathway in different conditions such as in lens epithelial cells exposed to H2O2 [41], retinal endothelial cells exposed to H2O2 [42], coronary atherosclerosis [43], and in C2C12 myotubes exposed to FeSO4 and H2O2 [44]. The reactive oxygen species is also known to activate NF-Kb [45] which may have a role in upregulation of the proteasome pathway. Inhibition of ubiquitin–proteasome activity has been shown to down-regulate NF-kB-mediated inflammatory pathways [46] and vice versa inhibition of NF-kB resulted in decreased Ub-proteasome activity [37].
Detailed mechanism of chronic hypobaric hypoxia induced skeletal muscle atrophy has been explained diagrammatically in Fig. 8.
Conclusion
Excessive protein degradation during exposure to chronic hypobaric hypoxia resulted in skeletal muscle atrophy which could be detrimental for performing any physical task under these conditions. The majorly affected protein is myofibrillar protein and the pathways responsible for this loss of skeletal muscle mass are Ub-proteasome pathway and calpains. Attenuation of these pathways could be significant for preventing or impeding the observed skeletal muscle atrophy at high altitude and under various catabolic conditions mimicking the symptoms of high-altitude maladies such as COPD. Our results also concluded that increased oxidative stress induced at high altitude may also be important factor responsible for the skeletal muscle loss.
References
Fulco CS, Friedlander AL, Muza SR, Rock PB, Robinson S, Lammi E, Baker R, Fulco CJ, Lewis SF, Cymerman A (2002) Energy intake deficit and physical performance at altitude. Aviat Space Environ Med 73:758–765
Bharadwaj H, Prasad J, Pramanik SN, Kishnani S, Zachariah T, Chaudhary KL, Sridharan K, Srivastava KK (2000) Effect of prolonged exposure to high altitude on skeletal muscles of Indian soldiers. Def Sci J 50:167–176
Macdonald JH, Oliver SJ, Hillyer K, Sanders S, Smith Z, Williams C, Yates D, Ginnever H, Scanlon E, Roberts E, Murphy D, Lawley J, Chichester E (2009) Body composition at high altitude: a randomized placebo-controlled trial of dietary carbohydrate supplementation. Am J Clin Nutr 90:1193–1202
Sridharan K, Mukherjee AK, Grover SK, Kumaria MML, Arora BS, Rai RM (1987) Assessment of nutritional status and physical work capacity of road construction workers at altitude of 2150–2750 m on two different ration scales. Nutr Rep Int 35:1269–1277
Brouns F (1992) Nutritional aspects of health and performance at lowland and altitude. Int J Sports Med 13:S100–S106
Schols AM (2002) Pulmonary cachexia. Int J Cardiol 85:101–110
Hoppeler H, Vogt M (2001) Muscle tissue adaptations to hypoxia. J Exp Biol 204:3133–3139
Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P (1990) Muscular exercise at high altitude. II. Morphological adaptation of skeletal muscle to chronic hypoxia. Int J Sports Med 11:S3–S9
Martinelli M, Winterhalder R, Cerretelli P, Howald H, Hoppeler H (1990) Muscle lipofuscin content and satellite cell volume is increased after high altitude exposure in humans. Experientia 46:672–676
Kung-tung C, Yu-yawn C, Huey-june W, Chen-kang C, Wen-tsung L, Yen-yuan L, Chieh-chung L, Rong-sen Y, Jung-charng L (2008) Decreased anaerobic performance and hormone adaptation after expedition to Peak Lenin. Chin Med J 121:2229–2233
Bigard AX, Douce P, Merino D, Lienhard F, Guezennec CY (1996) Changes in dietary protein intake fail to prevent decrease in muscle growth induced by severe hypoxia in rats. J Appl Physiol 80:208–215
Preedy VR, Smith DM, Sugden PH (1985) The effects of 6 hr hypoxia on protein synthesis in rat tissue in vivo & in vitro. Biochem J 228:179–185
Preedy VR, Sugden PH (1989) The effects of fasting or hypoxia on rates of protein synthesis in vivo in subcellular fractions of rat heart and gastrocnemius muscle. Biochem J 257:519–527
Iioka TK, Sugito K, Moriya T, Kuriyama T (2002) Effects of insulin like growth factor on nitrogen balance during hypoxic exposure. Eur Respir J 20:293–299
Vigano A, Ripamonti M, Palma SD, Capitanio D, Vasso M, Wait R, Lundby C, Cerretelli P, Gelfi C (2008) Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics 8:4668–4679
Imoberdorf R, Garlick PJ, McNurlan MA, Casella GA, Marini JC, Turgay M, Bartsch P, Ballmer PE (2006) Skeletal muscle protein synthesis after active or passive ascent to high altitude. Med Sci Sports Exerc 38:1082–1087
Holm L, Haslund ML, Robach P, van Hall G, Calbet JA, Saltin B, Lundby C (2010) Skeletal muscle myofibrillar and sarcoplasmic protein synthesis rates are affected differently by altitude-induced hypoxia in native lowlanders. PLoS ONE 20:e15606
Rennie MJ (1985) Muscle protein turnover and the wasting due to injury and disease. Br Med Bull 41:257–264
Cai D, Lee KK, Li M, Tang MK, Chan KM (2004) Ubiquitin expression is upregulated in human and rat skeletal muscles during aging. Arch Biochem Biophys 425:42–50
Reid MB (2005) Response of the ubiquitin–proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol 288:R1423–R1431
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Piccolo PD, Burden SJ, Lisi RD, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6:458–471
Attaix D, Mosoni L, Dardevet D, Combaret L, Mirand PP, Grizard J (2005) Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol 37:2098–2114
Enns DL, Raastad T, Ugelstad I, Belcastro AN (2007) Calpain/calpastatin activities and substrate depletion patterns during hindlimb unweighting and reweighting in skeletal muscle. Eur J Appl Physiol 100:445–455
Dardevet D, Sornet C, Vary T, Grizard J (1996) Phosphotidylinositol 3-kinase and p70 S6 kinase participate in the regulation of protein turnover in skeletal muscle by insulin and insulin-like growth factor I. Endocrinology 137:4089–4094
Vary TC, Dardevet D, Grizard J, Voisin L, Buffiere C, Denis P, Breuille D, Obled C (1998) Differential regulation of skeletal muscle protein turnover by insulin and IGF-1 after bacteremia. Am J Physiol Endocrinol Metab 275:E584–E593
Ventrucci G, Mello MAR, Marcondes G (2004) Proteasome activity is altered in skeletal muscle tissue of tumour-bearing rats fed a leucine-rich diet. Endocr Relat Cancer 11:887–895
Waalkes TP, Udenfriend S (1957) A fluorimetric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med 50:733–736
Hepple RT, Qin M, Nakamoto H, Goto S (2008) Caloric restriction optimizes the proteasome activity. Am J Physiol Regul Integr Comp Physiol 295:R1231–R1237
Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P (2008) Muscle wasting in diabetic and in tumor bearing rats: role of oxidative stress. Free Radic Biol Med 44:584–593
Oron U (1990) Proteolytic enzyme activity in rat hind limb muscle in fetus and during post natal development. Int J Dev Biol 34:457–460
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Koopman R, Gehrig SM, Leger B, Walrand S, Murphy KT, Lynch GS (2010) Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice. J Physiol 588:4811–4823
Kvamme E, Torgner IA, Svenneby G (1985) Glutaminase from mammalian tissue. Methods Enzymol 113:241–244
Elliott WH (1955) Glutamine synthesis. In: Colowick SP, Kaplan NO (eds) Methods enzymol II. pp 337–339
Cathcart R, Schwiers E, Ames BN (1983) Detection of pico mole levels of hyderoperoxides using fluorescent dichlorofluoroscein assay. Anal Biochem 134:111–116
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Dutta A, Ray K, Singh VK, Vats P, Singh SN, Singh SB (2008) L-carnitine supplementation attenuates intermittent hypoxia-induced oxidative stress and delays muscle fatigue in rats. Exp Physiol 93:1139–1146
Tisdale MJ (2005) The Ub-proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol 3:209–217
Howald H, Pette D, Simoneau JA, Uber A, Hoppeler H, Cerretelli P (1990) Effect of chronic hypoxia on muscle enzyme activities. Int J Sports Med S10–S14
Vats P, Mukherjee AK, Kumria MM, Singh SN, Patil SK, Rangnathan S, Sridharan K (1999) Changes in activity levels of glutamine synthetase, glutaminase and glycogen synthetase in rats subjected to hypoxic stress. Int J Biometeorol 42:205–209
Shang F, Gong Taylor A (1997) Activity of ubiquitin-dependent pathway in response to oxidative stress. Ubiquitin-activating enzyme is transiently up-regulated. J Biol Chem 272:23086–23093
Fernandes R, Ramalho J, Pereira P (2006) Oxidative stress upregulates ubiquitin proteasome pathway in retinal endothelial cells. Mol Vis 12:1526–1535
Hermann J, Gulati R, Napoli C, Woodrum LLO, Porcel MR, Sica V, Simari RD, Ciechanover A, Lerman A (2003) Oxidative stress-related increase in ubiquitination in early coronary atherogenesis. FASEB 17:1730–1732
Gomes M, Maria CC, Tisdale MJ (2002) Induction of protein catabolism and the ubiquitin proteasome pathway by mild oxidative stress. Cancer Lett 180:69–74
Sagi SKS, Patir H, Mishra C, Pradhan G, Mastoori SR, Ilavazhagan G (2008) Role of oxidative stress and NFkB in hypoxia induced pulmonary edema. Exp Biol Med 233:1088–1098
Marfella R, Amico MD, Filippo CD, Baldi A, Siniscalchi M, Sasso FC, Portoghese M, Carbonara O, Crescenzi B, Sangiuolo P, Nicoletti GF, Rossiello R, Ferraraccio F, Cacciapuoti F, Verza M, Coppola L, Rossi F, Paolisso G (2006) Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: effects of rosiglitazone treatment. JACC 47:2444–2455
Acknowledgments
This study was supported and funded by the Defence Research and Development organization, Ministry of Defence, Government of India. The authors are grateful to the Director, Defence Institute of Physiology and Allied Sciences, Delhi for providing facilities to carry out these investigations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhary, P., Suryakumar, G., Prasad, R. et al. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin–proteasome pathway and calpains. Mol Cell Biochem 364, 101–113 (2012). https://doi.org/10.1007/s11010-011-1210-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1210-x