Abstract
Cardiovascular disease is the leading cause of morbidity and mortality in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). More than 44% of these patients present with generalized atherosclerosis at autopsy. It is accepted that endothelial progenitor cells (EPCs) participate in the repair of dysfunctional endothelium and thus protects against atherosclerosis. However, whether COPD affects the repairing capacity of EPCs is unknown. Therefore, the objective of this study was to determine whether and how EPCs are involved in the vascular repair process in patients with COPD. In our study, EPCs from 25 COPD and 16 control patients were isolated by Ficoll density-gradient centrifugation and identified using fluorescence activated cell sorting. Transwell Migratory Assay was performed to determine the number of EPC colony-forming units and the adherent capacity late-EPCs to human umbilical vein endothelial cells. Following arterial damage in NOD/SCID mice, the number of EPCs incorporated at the injured vascular site was determined using a fluorescence microscope. We found that the number of EPC clusters and cell migration, as well as the expression of CXCR4, was significantly decreased in patients with COPD. Additionally, the number of late-EPCs adherent to HUVEC tubules was significantly reduced, and fewer VEGFR2+-staining cells were incorporated into the injured site in COPD patients. Our study demonstrates that EPC capacity of repair was affected in COPD patients, which may contribute to altered vascular endothelium in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is considerable evidence associating the severity of chronic obstructive pulmonary disease (COPD) with the presence and progression of cardiovascular disease. A recent health [1] care database cohort study of more than 10,000 subjects reported patients with COPD were two to four times more likely to die of cardiovascular disease at a 3-year follow-up than were age- and sex-matched control subjects. Additionally, 50% of COPD deaths are attributed to cardiac and other nonrespiratory factors with 44% of patients with COPD presenting with [2] generalized atherosclerosis at autopsy [3]. It has currently been theorized that the presence of co-morbities, COPD and cardiovascular disease (CVD), are the result of altered vascular remodeling as a consequence of endothelial injury from inflammatory cells and cytokines [4–7]. At present, there have been no systematic studies evaluating the factors that contribute to vessel repair, including endothelial progenitor cells (EPCs).
EPCs are a cell subpopulation of large mononuclear cells from circulating bone marrow. They express three cell surface markers: CD133+, CD34+, and vascular endothelial growth factor receptor-2 (VEGFR-2) [8]. Cytokine stimulation and/or ischemia mobilize circulating EPCs from bone marrow which localize at the site of damage where they adhere to the endothelium initiating site-specific neovascularization [9]. There is additional evidence supporting the role of ECs in the developing collateral vessels of ischemic tissues [10, 11], indicating circulating EPCs participate in the formation of new endothelium. More importantly, endothelial dysfunction has been recognized as playing a crucial role in cardiovascular disorders. What has been established is circulating EPCs can restore dysfunctional endothelium and thereby protect against atherosclerotic vascular disease [12].
Accumulating evidence suggests bone marrow–derived circulating progenitor cells contribute to vascular remodeling and repair. This has been shown to be regulated by sympathetic nerve activation and cytokines [13, 14]. Recruitment and incorporation of EPCs need a coordinated sequence of multisteps, adhesive, and signaling events that includes chemoattraction, migration, adhesion, and final differentiation to endothelial cells [9]. An abnormality of any abovementioned functions can alter the repair process. Oren M et al. [15] demonstrated diabetic EPCs had a decreased adherence to human umbilical vein endothelial cells activated by tumor necrosis factor-α (TNF-α), resulting in decreased vascular tubule formation compared with controls. This evidence indicates type II diabetes EPC biology might be altered in processes critical for new blood vessel growth contributing to a population of patients at high risk for morbidity and mortality after occlusive vascular events. Furthermore, Mark Toshner [16] had found late-EPCs from patients with pulmonary artery hypertension (PAH) and bone morphogenetic protein receptor type II (BMPRII) mutations had impaired ability to form vascular networks supporting the role of altered circulating progenitors in the vascular remodeling process responsible for PAH. These data showed functional alterations of circulating EPCs in specific disease states lead to adverse vascular events, confirming EPC dysfunction plays a role in the incidence of CVD.
To date, whether COPD affects function of circulating EPCs to repair injured vasculature is unknown. In this study, we aimed to determine the role of early EPCs in patients with COPD in terms of migration by SDF-1, proliferation, adhesion, and capacities of late-EPCs vascular tube formation which may contribute to the pathogenesis of CVD in patients with COPD.
Results
EPCs clonal units from patients with COPD had reduced proliferation rates, were smaller, with a reduced number of clusters compared with control patients. There was significant difference in CD34+ cells isolated and purified by magnetic selection in COPD and control patients 19.99 ± 11.11(×104) versus 32.75 ± 18.25 (×104), respectively) (Table 1). To analyze EPCs ability to proliferate, 106 cells per well were implanted in a 96-well plate and assayed using MTT at 0 h, 24 h, 48 h, 72 h, 96 h, and 120 h. Under normal conditions, cells enter the exponential phase of growth at 24 h to 72 h and rapidly proliferated. Proliferation is typically reduced when cells enter the stationary phase of growth at 96 h. Our data showed impaired proliferation capacity of early EPCs from COPD patients at 48 h to 72 h compared with control patients (P < 0.05), suggesting an impaired ability for cellular repair. The number of EPC clusters was counted in 10 random fields in light-power (×40), the total units were significantly fewer than that of control subjects (22 ± 7 vs. 62 ± 12.9, respectively, P < 0.001) and clone size of patients with COPD was significantly smaller than control. Cell cycle analysis indicated fewer early EPCs in patients with COPD were in S-Phase compared with that of control subjects (0.39 ± 0.10% vs. 1.7 ± 0.59%, respectively, P < 0.05) (Fig. 1a–i).
EPCs proliferation. a early-EPC clusters from COPD and b controls after 48 h (×40 power). c Quantification of cell number in clusters from two groups revealed that COPD EPCs were significantly reduced compared with control (P < 0.05). d A gate was drawn around the high-expressing distinct population of CD34+ cells, and e triple positive events for CD34+, CD133+, and VEGFR2+ were determined. f Growth curves of EPCs (n = 5). g Early EPCs in S-phrase from COPD and h control. i Quantification of early EPCs from COPD patients and control subjects in S-phrase (n = 6). Data are shown as means ± SD (* P < 0.05)
Chemotaxis assays
In the transwell lower chamber, the numbers of early EPCs isolated from patients with COPD were significantly less than that of control subjects at increasing concentrations of SDF-1a (50, 100, 150, 200 ng/ml)(5000/20000 vs. 7200/20000, 6760/20000 vs. 9000/20000,6920/20000 vs. 9800/20000 and 7600/20000 vs. 11200/20000, respectively, P < 0.05). Moreover, the migrating number of early EPCs was not markedly increased with SDF-1a concentration elevated in patients groups. However, the amount of early EPCs from controls was increased in a SDF-1a concentration-dependent manner. These findings indicate the migratory capacity of EPCs from patients with COPD were significantly impaired compared with controls. To investigate whether the impaired migratory activity of EPCs to SDF-1 might be due to a down-regulation of the CXCR4, we analyzed the expression of CXCR4 in isolated EPCs from patients with COPD and controls by flow cytometry, and CXCR4 mRNA was determined by real-time PCR. CXCR4 was significantly reduced in patients with COPD (55.1 ± 9.98% to control 91.53 ± 6.7%, P < 0.05). Quantity of CXCR4 mRNA from controls was 1.5-2 folds greater than that in patients with COPD (Fig. 2a–g).
Chemotaxis assays by SDF-1α gradients and CXCR4 expression and real-time PCR assay of CXCR4 mRNA. a Early EPCs from patients with COPD and b control subjects chemoattracted by SDF-1α (100 ng/ml), c Quantification of early EPCs indicates a reduced number of cells in COPD compared with control subjects. Additionally, in COPD patients groups, there was no correlation with the number of early EPCs and SDF-1a concentration. However, the amount of early EPCs from controls was increased with elevated concentration of SDF-1a. d Expression of CXCR4+ on early EPCs from patients with COPD and e control subjects. f Quantification of CXCR4+ percent on early EPCs from patients with COPD and control subjects. g Real-time PCR assay of CXCR4 mRNA showed controls were 1.5–2 folds more than patients with COPD. Data are shown as means ± SD. (n = 3, * P < 0.05 vs. control at same a concentration, ※ P < 0.05 vs. SDF-1a at multiple concentrations in controls)
Matrigel tubule assay
A Matrigel tubule assay was performed to investigate the ability of EPCs to integrate into vascular structures. It has been shown that early EPCs are not capable of constructing tubular luminal structures in either group (COPD or control) [17]; therefore, we focused on the role of late EPCs. Late EPCs labeled with Dil-acLDL were co-cultured with tubules formed by HUVECs in a 24-well plate coated by Matrigel for 12 h. EPCs labeled with DiI-acLDL were delineated from HUVECs, and analysis under fluorescence revealed that fewer COPD late-EPCs were incorporated into tubules compared with control EPCs (26.4 ± 8.1 vs. 35. ± 7.3, respectively, ×10 power, 5 fields, P < 0.001) (Fig. 3a–d).
Late-EPC participated in tubulization. HUVECs (105/well) were seeded into 24-well to form tubule structures within Matrigel (4 mg/ml). After 12 h, dil-acLDL labeled late-EPCs (4 × 104, red) were coincubated with HUVECs tubules. Overlay of fluorescent images revealed an increase in late-EPC controls (a) compared with patients with COPD (b). c Late-EPCs simultaneously labeled with dil-acLDL (red) and UEA-1-FITC (green) (×40 power). d Quantification of the number of late-EPCs participated in tubulization revealed that fewer COPD late-EPCs were significantly incorporated into tubules. Data are shown as means ± SD (** P < 0.001). (Color figure online)
Observation of the tube formation
EPC repair capacity is determined by a number of factors, including neovascular tube formation. We measured the development of vascular networks to determine whether they have a role in neovascular tube formation [18]. Angiogenesis assays showed that the formation of vascular networks in a 24-well plate coated with matrigel was markedly impaired in the patients with COPD compared with control subjects (Fig. 4a–c).
Phenotypic analysis of late-EPCs forming tubules. a COPD late-EPCs were markedly deficient in forming intact tubules, whereas b control late-EPCs form intact vascular networks. c Semiquantative analysis of vascular network formation. Experiments were performed in quadruplicate (n = 4). Data are shown as means ± SD (** P < 0.001)
Expression of platelet/endothelial cell adhesion molecule-1 (PECAM-1) on the surface of EPCs
There is evidence in the literature supporting the role of adhesion molecule, PECAM-1, and its contribution to maintaining endothelial integrity and cells migration. Shortening or deletion of the expression of PECAM-1 leads to perturbation of junctional integrity, increased vascular permeability, and impaired transendothelial cells migration [19]. Expression of PECAM-1 in late-EPCs from COPD patients and control subjects were markedly different by FACS. The former were 35.57 ± 8.72%, and the later were 62.98 ± 15.47% (P < 0.05) (Fig. 5a–c).
PECAM-1 expression on late-EPCs analyzed by FACS. Representative images of flow cytometry analyses of late-EPCs for VEGFR2 and PECAM-1 following 14 days in vitro culture incubated with PECAM-1-FITC antibody and VEGFR2-conjuncted-APC from COPD patients (a) and control subjects (b). a PECAM-1 expression percent in late-EPCs from COPD patients and b that of controls. c Quantification of PECAM-1 expression on late-EPCs in two groups (n = 6). Data are shown as means ± SD (* P < 0.05)
EPCs-mediated arterial repair
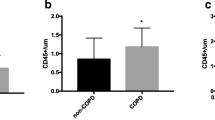
We killed SCID mice 48 h after late-EPCs were injected into the femoral artery to confirm our hypothesis that reduced late-EPCs from patients with COPD altered adherence to injured arterial intima [20]. The injured arterial slices were observed under a fluorescence microscope. The fluorescence density of late-EPCs from patients with COPD at the site of injury site was less than that from control subjects (Fig. 6a–d).
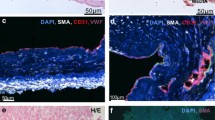
Transplanted late-EPCs from patients with COPD and control subjects into NOS/SCID mice injured femoral artery to determine the capacity of late-EPCs adhesion. Representative images of histological sections stained with mouse-specific endothelial marker CD31 (Green), anti-human VEGFR2+-PE from 48 h post-injury NOS/SCID mouse. a PBS was injected into injured femoral artery of the mice. b Late-EPCs from COPD patients (arrow, red). c Late-EPCs from control subjects (arrowhead, red). d Semiquantitative analysis of late-EPCs incorporated into the injured vascular site. Data are shown as means ± SD (n = 4, * P = 0.045, <0.05). (Color figure online)
Discussion
EPCs have been shown to incorporate into sites of active neovascularization. Based on colonies with different morphology and cell surface markers, EPCs were divided into two different subgroups termed early-EPCs, also known as early outgrowth EPCs, and late-EPCs, also known as late outgrowth EPCs [21]. Early-EPCs exhibit a spindle-like morphology in vitro culture at 4–7 days with increased expression of CD34+, CD133+, and VEGFR2. Late-EPCs demonstrate cobblestone morphology in culture after 2–4 weeks and primarily express CD34+, VEGFR2, and CD31+ [17]. EPCs participate in vascular repair. It was necessary that EPCs had normal function to repair injured endothelium. Therefore, we hypothesized the repair process following vascular injury was altered due to the hypoproliferation of early EPCs and impaired migration resulting in altered tubule formation ability of late-EPCs in patients with COPD [16].
Our data showed a decreased expression of CXCR4 on early EPCs in patients with COPD in addition to impaired migratory ability to SDF-1 compared with controls. In vitro cultured isolated early EPCs from patients with COPD demonstrated a hypo-proliferation compared with control subjects at 48–72 h, and after 72 h. These findings indicate early EPCs from patients with COPD do not adequately proliferate to maintain and repair injured endothelium. Cell cycle analyzed by FACS suggested less early EPCs from patients with COPD were in the proliferative phrase (S-phrase), compared with controls. Furthermore, late-EPCs from patients with COPD had a reduced capacity to adhere to HUVECs tubules and form vascular networks. Expression of PECAM-1 (an adhesion molecule) in late-EPCs from COPD patients and control subjects were markedly different. The former were 35.57 ± 8.72%, the later were 62.98 ± 15.47%. Fewer late-EPCs from in vivo COPD patients were incorporated into injured femoral artery of NOD/SCID mice indicating a reduction in the ability to repair the injured vasculature.
Recruitment and incorporation of EPCs requires a coordinated sequence of multistep adhesive and signaling events that includes chemoattraction, migration, adhesion, and final differentiation into endothelial cells [22]. Dysfunction of any abovementioned roles led to an altered repair process.
EPC production by the bone marrow is initiated by increased sympathetic nerve activity and circulating cytokines in the peripheral blood [13]. Data [23] show chemokine stromal-cell-derived factor-1 (SDF-1), also known as CXCL12, plays a major role in the recruitment and retention of BM stem cells to neo-angiogenic sites supporting revascularization of ischemic tissue. CD34+ cells express CXCR4, which is a receptor of SDF-1 [24] and is expressed in stromal and injured tissue. CD34+ cells attracted by the gradient of SDF-1 are “anchored” to the injured site by close interaction of SDF-1-CXCR4. Although the total number of early EPC was unchanged, we found early EPCs migration was impaired as previous files [25] and the expression of CXCR4 reduced in patients with COPD. This change in expression is believed to contribute to a reduced number of EPCs available to migrate to injured vascular sites [26].
EPCs ability to adhere to injured sites alters the repair process. Oren M [15] found that EPCs of patients with diabetes had a decreased adherence to human umbilical vein endothelial cells activated by tumor necrosis factor-α (TNF-α). This deficiency is believed to contribute to an increased risk of occlusive vascular events. These data supported our findings. We found fewer in vitro culture late-EPCs from patients with COPD that were adherent to HUVEC tubules compared with that of control subjects (P < 0.001). Additionally, we found that the density of late-EPCs were decreased in NOS/SCID mice injected with late EPCs from patients with COPD that had been previously exposed to endothelial femoral artery injury, indicating COPD late-EPCs adhesion capacity was impaired.
Interestingly, it has been shown that late-EPCs expression of platelet/endothelial cell adhesion molecule-1 (PECAM-1) was decreased in COPD subjects. PECAM-1 functions as an adhesive molecule contributing to the maintenance of endothelial integrity. Shortening or deletion of the expression of PECAM-1 leads to perturbation of junctional integrity, increased vascular permeability and impaired transendothelial cell migration [19]. Decreased density of PECAM-1 on late-EPCs may contribute to impaired adhesion of late-EPCs to injured endothelium [27], resulting in fewer late EPCs incorporated into the injured site. This will require further experiments to test these theories.
In vitro, we found late-EPCs from patients with COPD failed to form intact capillary tube networks just as previously described [16], indicating a role of late-EPCs in the repair process of injured vessels. It has been shown that late-EPCs from patients with COPD could not protect the functional integrity of the endothelium. Damaged endothelium impairs the release of nitric oxide (NO), leading to an increased risk of arteriosclerosis, thrombosis, and hypertension [28], which accelerate the development of atherosclerosis [29, 30]. The endothelium plays a crucial role in the regulation of vascular homeostasis. Lack of endothelial cells contributes to reduced vascular tone altering the vessel’s ability to adapt to increased flow and modulates hypoxic vasoconstriction. These alterations in endothelial dysfunction initiate the early vascular changes in the development of arteriosclerosis [31]. Furthermore, due to lack of endothelium protection, dysfunction of late-EPCs in patients with COPD alter intimal repair and result in hyperplastic medial changes accelerating vascular remodeling and increasing the incidence of atherosclerosis in patients with COPD.
Additionally, the MTT analysis of cell proliferation showed that EPCs from patients with COPD had a markedly hypoproliferative capacity at 24 h to 72 h in contrast to that of controls cells cultured (P < 0.05) and that the number of COPD EPCs clusters were significantly decreased. These findings further support our hypothesis that patients with COPD are at risk for CVD due to a reduced ability to proliferation. Cell Cycle analysis indicated fewer early EPCs in patients with COPD were in S-Phase compared with that of control subjects. These results can be explained by the fact that early EPCs became senescent more rapidly than those from control. Reduced proliferation has been demonstrated to be related to endothelial dysfunction in the systemic circulation [32].
In general, our data showed that hypoproliferation and the decreased expression of CXCR4 on early EPCs from COPD patients leads to reduced migration by SDF-1 gradients. This suggests early EPCs are senescent and in CD34+ are reduced in patients with COPD compared with controls. Fewer late-EPCs incorporated into HUVEC tubules in vitro and at the injured NOS/SCID femoral artery in vivo showed that late-EPCs repairing capacity was impaired in patients with COPD.
The major limitation of the present study was the limited number of matched controls and the lack of gender diversity, thereby not representing the entire clinical spectrum of patients with COPD. Due to the limited access of vessel tissue from patients with COPD, we were not able to determine changes in SDF-1 levels following vascular injury. Changes in SDF-1 may contribute to decreased recruitment of early EPCs in patients with COPD. Nevertheless, we concluded that early EPCs hypoproliferation, impaired capacity of migration and late-EPCs impaired capacity to repair injured vasculature in patients with COPD may contribute to an increase in the incidence of CVD in patients with COPD.
Materials and methods
Twenty-five patients with COPD were enrolled with a previous history of smoking and 16 age-matched healthy, nonsmoking volunteers (controls) after signing informed consent for all procedures (Table 1). Inclusion criteria for patients with COPD were (1) moderate-to-severe COPD (Global Initiative for Chronic Obstructive Lung Disease stage II, III) (2) clinically stable (no change in pulmonary function tests or exacerbation in the 4 weeks preceding the study); No resting hypoxemia (arterial oxygen tension: PaO2 > 60 mmHg) and no patient treated with systemic steroids 2 weeks prior to the study. (3) age <70 years. Exclusion criteria: Patients with primary cardiovascular, (hypertension, cardiomyopathy, acute myocardial infarction), cerebrovascular, neuromuscular, or rheumatological (SLE) diseases as well as any history of tumors and/or metabolic disorders such as diabetes mellitus, were excluded from the study.
Inclusion criteria for controls were the following: (1) age <70 years; (2) normal spirometry and arterial blood gases; (3) and no evident diseases. These protocols were used in accordance with procedures approved by the Human Experimentation and Ethics Committees of the Sun Yat-sen University.
All subjects received a complete clinical assessment including pulmonary function tests and a 12-lead ECG. Blood samples were drawn from the radial artery at rest while breathing room air for arterial blood gas determination. 20 ml venous blood was obtained from the antecubital vein for CD34+ isolation. Plasma was assayed for the level of cytokines by ELISA.
CD34+ isolation and purification in samples from 25 COPD patients and 16 subject controls, flow cytometric analysis
Twenty milliliter venous blood was diluted 1:1 with Hanks’ (Invitrogen, USA) and mononuclear cells were isolated by density gradient centrifugation over Ficoll-Hypaque 1.077 (Haoyang, Tianjin, China) in 15-ml tubes as previously described [33]. The mononuclear cells were washed three times in Hanks’ and cells were incubated with CD34+ Positive Selection Cocktail (EasySep®, Stemcell, Canada) (100 μl/ml) in polystyrene round-bottom tubes at room temperature for 15 min according to the manufacturer’s operating instruction. EasySep® Magnetic Nanoparticles were added and incubated at room temperature for 10 min, cell suspension was brought to a total volume of 2.5 ml by adding RoboSep® medium (Buffer, Catalog #20104) and the tube was placed into the magnet. After 5 min, the magnet was picked up, and in one continuous motion, the magnet and tube were inverted and the supernatant fraction was poured off. The tube was removed from the magnet and 2.5 ml of medium were added to the tube. The cell suspension was gently mixed by pipetting up and down 2–3 times, and the tube was placed back in the magnet and set aside for 5 min and the supernatant fraction was poured off again. The last two steps were repeated three times. CD34+ cells were obtained and counted under a light microscope. One-fourth of the purified CD34+ cells were assessed by FACS. Purification of CD34+ cells were 93% in these cells and seeded onto 6-well plates coated with 3% human gelatin (Sigma, USA) (2 × 106 cells/well) and cultivated in EBM-2 containing 10% heat-inactivated fetal calf serum, 10% horse serum, SCGF (10 ng/ml; TEBU, Frankfurt, Germany), l-glutamine (2 mM) and VEGF (5 ng/ml; TEBU, Sigma) at 37°C 5% CO2. After 48 h, nonadherent cells were removed, adherent cells were resuspended, and 25% the cells were incubated for 30 min at room temperature with anti-human CD34+ conjugated-FITC (Miltenyi Biotec), anti-human CD133+ labeled phycoerythrin (PE) antibody (Miltenyi Biotec), mouse anti-human vascular endothelial growth factor receptor-2 (VEGFR-2) first antibody and rabbit anti-mouse-APC second antibody (Santa, Biotec), followed by flow cytometry on a FACS Calibur (Becton–Dickinson) with Cell Quest software. Cells suspensions were prepared in PBS containing 1% (vol/vol) FCS. 2% BSA was used to block nonspecific antigen on cells.
Colony forming unit assay
CD34+ progenitor cells were seeded onto a 6-well plate (Corning, USA) coated with 3% human gelatin (Sigma, USA) (2 × 106 cells/well) and cultivated in EBM-2 (Lonza, USA) containing 10% heat-inactivated fetal calf serum, 10% horse serum, SCGF (10 ng/ml; TEBU, Frankfurt, Germany), l-glutamine (2 mM) and VEGF (5 ng/ml;TEBU,Sigma) at 37°C, 5% CO2 [33]. After 48 h, nonadherent cells were removed and colony-forming units were counted in 10 random fields under a light microscope.
MTT assay of EPCs proliferation
Early-EPCs (20000/well) were seeded in 96 well-plates (Corning, USA) containing 100 μl EBM-2 medium (Lonza, USA) per well in a humidified atmosphere (37°C, 5% CO2). 20 μl of MTT (0.5 mg/ml) were added into each well after 0, 24, 48, 72, 96,120 h. All plates were allowed to incubate under the same conditions for 4 h, (37°C, 5% CO2) and then 150 μl of DMSO (sigma, USA) were added to each well. Plates were oscillated gently for 10 min and then measured using a microtiter plate (ELISA) reader, wavelength 490 nm.
Chemotaxis assays and flow cytometry analysis expression of CXCR4 and real-time PCR assay of CXCR4 mRNA
Chemotaxis was studied by transwell assay (6.5 mm diameter and 5 mm pore, Corning). Cultured early EPCs (2 × 104) were resuspended in 200 μl 0.1% FCS medium and plated in the transwell upper chamber. The bottom compartment was filled with 500 μl of the same medium supplemented with SDF-1α gradient (50,100,150,200 ng/ml, R&D systems, USA). After incubation at 37°C for 2 h, migrating cells were harvested from the bottom compartments and the number of migrating cells was counted under a light microscope.
Q-PCR was performed on the ABI 9700 Q-PCR System (ABI, USA) and the data were analyzed using the accompanying software package. CXCR4 mRNA was isolated from early EPCs and detected by RT-PCR as previously described [34], using 5′-GCCCTTAGCCCACTACTTCA-3′ and 5′-TCACTTCCAATTCAGCAAGC-3′ as oligonucleotides.
Late-EPCs adhesion to matrigel tubule
Twenty-four-well culture plates (Corning, USA) were coated with 250 μl of cold-matrigel (Becton–Dickinson, Bedford, MA), diluted to 4 mg/ml with complete culture medium (to reduce viscosity). Cells were incubated for 12 h at 37°C and 5% CO2 to solidify. HUVECs (1 × 105) were pretreated for 12 h with TNF-α (Peprotech, Asia) (1 ng/ml), and seeded on top of the gel then incubated for 12 h at 37°C in a humidified atmosphere with 5% CO2. 2 × 104/well late-EPCs with diI-acLDL (Molecular Probe, USA) were then added for a 6-h coincubation to determine adhesion to the matrigel tubule. Adherent EPCs were counted in 10 random fields under an invert-fluorescence microscope (Leica, German, ×10 power).
Late-EPCs form tubule
The spontaneous formation of capillary-like structures by late-EPCs on a basement membrane matrix preparation, Matrigel (Becton–Dickinson, Bedford, MA), was used to assay angiogenic potential. Twenty-four-well plates (Corning, USA) were coated with Matrigel (3 mg/ml). Late-EPCs (1 × 105 cells/well) were seeded on Matrigel-coated plates and incubated at 37°C. After 72 h, endothelial networks were photographed under phase-contrast microscopy (Leica, German, ×10 power).
Assay expression of PECAM-1
Isolated CD34+ cells were cultured with EBM-2 (12% FCS, VEGF 5 ng/ml; TEBU) in a collagen-coated 24-well plate. After 24 h, nonadherent cells were discarded, and the media was replaced daily for the next 2–4 weeks [35, 36]. Cells expressing CD34+ and VEGFR-2 [37] were mainly late EPCs. Late EPCs (1 × 105) from COPD and controls were blocked by 2% BSA for 30 min, then incubated with mouse anti human-VEGFR-2 for 40 min, followed with rabbit anti-mouse-APC second antibody, and mAb-CD31-FITC (BD, Canada) for 30 min at room temperature, and washed with 500 μl PBS 3 times in 5 min, resuspended with 300ul PBS. Cells were analyzed on a FACS Calibur flow cytometer (Becton–Dickinson) with Cell Quest software, which analyzes 10,000 cells for the presentation of the specific antigen.
NOD/SCID mice femoral artery wire injury model
Eight-week-old NOD/SCID mice, weighing between 20 and 25 g, were acquired from Sun Yat-Sen University Laboratory. To assess the ability of late-EPCs to participate in in vivo arterial repair, the intima of the femoral artery of NOD/SCID mice were injured by insertion of an acupuncture needle as previously described [20, 38]. Following arterial incision, the needle was introduced into the lumen, advanced toward the head, and passed five times in order to denude the endothelium and mechanically stretch the vessel [20]. Subsequently, a plastic capillary tube was inserted and 5 × 105 late-EPCs from controls and COPD were injected into femoral artery. All animals had full use of the limb after 24 h post-surgery. 10% chloral hydrate (0.3 ml/100 g) was used to kill the animals 48 h post surgery to assess re-endothelialization. At death, the femoral artery was carefully excised and placed on ice for frozen slices. All procedures involving experimental animals were approved by Sun Yat-sen University Clinic Institutional Animal Care and Use Committee.
Immunofluorescence staining of frozen tissue
Frozen sections (0.2 μm thick) for fluorescence immunostaining were permeabilized in acetone at 4°C for 10 min, washed, blocked with 2% BSA, incubated at 4°C overnight in anti-Mouse CD31-eFluor (eBioscience, Inc), first rabbit-anti-human VEGFR2+ (Santa) (diluted 1:100 in 2% BSA) and second goat-anti-rabbit-PE (1:200 in 2% BSA). All procedures were protected from light. Slides were viewed with a fluorescence microscope (ZEISS, AX10, German) (×20 power).
Statistical analysis
All statistical analyses were performed with SPSS version 16 for Windows (SPSS, Inc). Data are presented as mean ± SD. For pair comparisons, we applied a 2-tailed paired t test. The number of CD34+ cells between the two groups was compared by analysis of variance. P < 0.05 was considered statistical significance. The authors had full access to the data and take full responsibility for its integrity.
References
Finkelstein J, Cha E, Scharf SM (2009) Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis 4:337–349
Mannino DM, Brown C, Giovino GA (1997) Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 156(3 Pt 1):814–818
Zvezdin B et al (2009) A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest 136(2):376–380
Macnee W, Maclay J, McAllister D (2008) Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc Am Thorac Soc 5(8):824–833
Eickhoff P et al (2008) Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178(12):1211–1218
Barbera JA, Peinado VI, Santos S (2003) Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J 21(5):892–905
Chung KF, Adcock IM (2008) Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 31(6):1334–1356
Peichev M et al (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95(3):952–958
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95(4):343–353
Zampetaki A, Kirton J, Xu Q (2008) Vascular repair by endothelial progenitor cells. Cardiovasc Res 78(3):413–421
Peinado VI et al (2006) Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 34(3):257–263
Giannotti G, Landmesser U (2007) Endothelial dysfunction as an early sign of atherosclerosis. Herz 32(7):568–572
Aguila HL (2006) Regulation of hematopoietic niches by sympathetic innervation. Bioessays 28(7):687–691
Katayama Y et al (2006) Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124(2):407–421
Tepper OM et al (2002) Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106(22):2781–2786
Toshner M et al (2009) Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 180(8):780–787
Mukai N et al (2008) A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res 314(3):430–440
Tressel SL et al (2007) Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler Thromb Vasc Biol 27(10):2150–2156
Zocchi MR, Poggi A (2004) PECAM-1, apoptosis and CD34+ precursors. Leuk Lymphoma 45(11):2205–2213
Hibbert B et al (2009) Inhibition of endothelial progenitor cell glycogen synthase kinase-3beta results in attenuated neointima formation and enhanced re-endothelialization after arterial injury. Cardiovasc Res 83(1):16–23
Sermsathanasawadi N et al (2009) Enhanced adhesion of early endothelial progenitor cells to radiation-induced senescence-like vascular endothelial cells in vitro. J Radiat Res (Tokyo) 50(5):469–475
Heida NM et al (2010) Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J Am Coll Cardiol 55(4):357–367
Carbajal KS et al (2010) Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci USA 107(24):11068–11073
Petit I, Jin D, Rafii S (2007) The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 28(7):299–307
Takahashi T et al (2011) Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology 16(4):680–687
Stellos K et al (2008) Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 117(2):206–215
Newman PJ, Newman DK (2003) Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 23(6):953–964
Hirata Y et al (2010) Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J 51(1):1–6
Pan S (2009) Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal 11(7):1669–1682
Hamed S, Roguin A (2006) Endothelial progenitor cells and atherosclerosis. Harefuah 145(5):358–361, 397
Sitia S et al (2010) From endothelial dysfunction to atherosclerosis. Autoimmun Rev 9(12):830–834
Hill JM et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348(7):593–600
Gehling UM et al (2000) In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 95(10):3106–3112
Sandstedt J et al (2010) C-kit+ CD45− cells found in the adult human heart represent a population of endothelial progenitor cells. Basic Res Cardiol 105(4):545–556
Zhang Y et al (2009) Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol 296(5):H1675–H1682
Thill M et al (2008) Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 49(6):2696–2708
Timmermans F et al (2007) Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol 27(7):1572–1579
Sata M et al (2000) A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol 32(11):2097–2104
Acknowledgments
We thank Dr. Lihua Liu for technical assistance, and Dr. Weiping Tan, Dr. Shuxiang Zhang and the staff of nurses in respiratory department of the First Affiliated Hospital of Sun Yat-sen University for collecting clinical cases, and Prof Zhengyu Dong for the preparation of the manuscript and cover letter.
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaoran Liu and Canmao Xie authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, X., Xie, C. Human endothelial progenitor cells isolated from COPD patients are dysfunctional. Mol Cell Biochem 363, 53–63 (2012). https://doi.org/10.1007/s11010-011-1157-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1157-y