Abstract
This study aimed to explore the mechanism of adriamycin resistance in human chronic myelogenous leukemia cells. Proteomic approach was utilized to compare and identify differentially expressed proteins between human chronic myelogenous leukemia K562 cells and their adriamycin-resistant counterparts. The differentially expressed proteins were analyzed by 2-DE (two-dimensional gel electrophoresis), and protein identification were performed on ESI-Q-TOF MS/MS instrument. Out of the 35 differentially expressed proteins between the two cell lines, 29 were identified and grouped into 10 functional classes. Most of identified proteins were related to the categories of metabolism (24%), proteolysis (13%), signal transduction (21%) and calcium ion binding (6%), suggesting that alterations of those biological processes might be involved in adriamycin resistance of K562 cells. We believe this study may provide some clues to a better understanding of the molecular mechanisms underlying adriamycin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myelogenous leukemia accounts for 15% of adult leukemias. In 2009, an estimated 5,050 cases will be diagnosed in the USA, and 470 patients will die from the disease [1]. Although adriamycin has high efficacy against chronic myelogenous leukemia, resistance to adriamycin is a major clinical problem and an important cause for treatment failure. Several mechanisms have been suggested to cause resistance to adriamycin in chronic myelogenous leukemia. They include the upregulation of P-glycoprotein and other plasma membrane multidrug transporters [2, 3]; failure to undergo apoptosis caused by alterations in Bcl-2, Bcl-XL, or Bax expression [4–6]; and changes in drug targets, such as topoisomerase II [7]. But treatments targeting these pathways have been met with little success. Thus, identification of novel diagnostic and therapeutic markers associated with adriamycin-resistance of tumor is very meaningful.
New methods are emerging that facilitate identification of novel diagnostic and therapeutic markers for cancer. In particular, the proteomic approach has resulted in many opportunities and challenges in identifying new tumor markers and therapeutic targets, and in understanding disease pathogenesis [8]. The most consistently successful proteomic method is combination of two-dimensional gel electrophoresis (2-DE) for protein separation and visualization, then mass spectrometric (MS) identification of proteins using peptide mass fingerprints and tandem MS peptide sequencing [9]. Although it has some limitations (for example, labor intensive and the limited dynamic range of proteins), this approach has been used extensively in cancer research [9]. Two dimensional gel electrophoresis-based comparative proteome analysis has been seen as useful for identification of resistance-associated proteins and has been successfully used for screening potential biomarkers for drug resistance in tumor cell lines, serum, and tumor tissues [10–13].
Recently, Zhu et al. compared the global protein profiles between adriamycin-resistant K562 cells and the parental K562 cells, and found eight differentially expressed proteins [14]. However, chemo-resistance acquisition is a very complicated process, and there must be many additional factors that play important roles in the complex processes. And the aim of the present study was to find more proteins involved in adriamycin-resistance using proteomic tools so as to develop more strategies to overcome drug resistance. To accomplish this, the cell line K562 was treated continuously, over 6 months, with stepwise-increasing concentrations of adriamycin to yield the chemo-resistant cell line that was named K562/ADM. The resistance of K562/ADM cells to adriamycin was 239-fold higher than the parental K562 cells, which showed higher resistance than the cells established by Zhu et al. (149-fold increased resistance to ADM) [14]. When subject the two cell lines to proteomic investigation, 29 significantly changed proteins were identified by 2-DE and MS/MS analysis. Altered expression of Annexin A2, Annexin A4, and Phosphoglycerate kinase 1 proteins were further verified by Western blot. The data in the present study may facilitate a better understanding of the molecular mechanisms underlying adriamycin resistance of K562 cells.
Materials and methods
Cell culture
The human chronic myelogenous leukemia cell K562 cells were maintained in RPMI 1640 medium (Gibco, Grand Island, NY) with 100 U/ml penicillin, 100 g/ml streptomycin, and supplemented with 10% fetal calf serum (Hyclone, Logan, UT) at 37°C in a humidified atmosphere of 5% CO2. A multidrug resistant cell line K562/ADM was established by continuous exposure to stepwise increased dosage of adriamycin (Hisun Pharmaceuticals, China). Briefly cells were exposed to adriamycin starting from 1 × IC50, and the concentrations were escalated at an increasing rate of 50%. Finally, the cells were maintained in the above medium with 1 g/ml adriamycin. For 2-DE analysis, cells (80% confluence) were washed twice with PBS before harvesting. The cells were stored at −80°C until use.
Drug sensitivity assay
Cultured cells were suspended in RPMI 1640 at 6 × 104 cells/ml and 100 μl of cell suspension were seeded into a well of 96-well plate. After an overnight culture, cells were exposed to adriamycin for 48 h. After incubation with MTT (1 mg/ml) for 4 h, medium were removed and 200 μl of dimethylsulfoxide were added to dissolve formazan crystals. Absorbance was measured at 570 nm using an ELISA microplate reader (PerkinElmer, Wellesley, MA, USA). Cell survival rates were calculated according to the formula: survival rate = (mean O.D. of treated wells/mean O.D. of untreated wells) × 100%. IC50 values were determined by drawing dose–effect curves on semi logarithm coordinate paper.
2-DE and image analysis
The 2-DE was performed as described previously [15] with minor modification. Approximately 4 × 107 cells were lysed in 1 ml lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 100 mM DTT, 0.2% pH 3–10 ampholyte, Bio-Rad, USA) containing protease inhibitor cocktail 8340 (Sigma, St. Louis, MO). Samples were then kept on ice and sonicated in six cycles of 15 s, each consisting of 5 s sonication followed by a 10 s break. After centrifugation at 15,000 rpm for 1 h at 4°C, the supernatant was collected and the protein concentrations were determined using the DC protein assay kit (Bio-Rad). Protein samples (2 mg) were applied to IPG strip (17 cm, pH 3–10 NL, Bio-Rad) using a passive rehydration method. After 12–16 h of rehydration, the strips were transferred to an IEF Cell (Bio- Rad). IEF was performed as follows: 250 V for 30 min, linear; 1,000 V for 1 h, rapid; linear ramping to 10 000 V for 5 h and finally 10,000 V for 4 h. The second dimension was performed using 12% SDS-PAGE at 30 mA constant current per gel after equilibration. The gels were stained using CBB R-250 (Merck, Germany) and scanned with a Bio-Rad GS-800 scanner. Three independent runs were made for each cell lines to ensure the accuracy of analyses. The maps were analyzed by PDQuest software Version 6.1 (Bio-Rad). The quantity of each spot in a gel was normalized as a percentage of the total quantity of all spots in that gel and evaluated in terms of OD. For statistical analysis, paired t test was performed to compare data from the three repeated experiments. Only spots that showed consistent and significant differences (±over twofold, P < 0.05) were selected for analysis with MS.
In-gel digestion
In-gel digestion of proteins was carried out using MS-grade Trypsin Gold (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, spots were cut out of the gel (1–2 mm diameter) using a razor blade, and destained twice with 100 mM NH4HCO3/50% ACN at 37°C for 45 min in each treatment. After drying, the gels were preincubated in 10–20 μl trypsin solution for 1 h. Then, 15 μl digestion buffer was added (40 mM NH4HCO3/10% ACN) to cover each gel and incubated overnight at 37°C. Tryptic digests were extracted using MilliQ water initially, followed by two times extraction with 50% ACN/5% TFA for 1 h each time. The combined extracts were dried in a vacuum concentrator at room temperature. The samples were then subjected to MS analysis.
ESI-Q-TOF analysis and protein identification
Mass spectra were acquired using a Q-TOF mass spectrometer (Micromass, Manchester, UK) fitted with an ESI source (Waters). Tryptic digests were dissolved in 18 μl 50% ACN. MS/MS was performed in a data-dependent mode in which the top 10 most abundant ions for each MS scan were selected for MS/MS analysis. Trypsin autolysis products and keratin derived precursor ions were automatically excluded. The MS/MS data were acquired and processed using MassLynx software (Micromass) and MASCOT (http://www.matrixscience.com) was used to search the database. Database searches were carried out using the following parameters: Database, Swiss-Prot; taxonomy, homo sapiens; enzyme, trypsin; mass tolerance, ±0.1 Da; MS/MS tolerance, ±0.05 Da; and an allowance of one missed cleavage. Fixed modifications of cysteine carboamidomethylation, and variable modifications of methionine oxidation were allowed. The data format was selected as Micromass PKL and the instrument was selected as ESI-Q-TOF. Proteins with probability based MOWSE scores, derived from ions scores as a non-probabilistic basis for ranking protein hits when using Mascot searching engine, exceeding their threshold (P < 0.05) were considered to be positively identified. To eliminate the redundancy of proteins appearing in the database under different names or accession numbers, the one-protein member with the highest MASCOT score was further selected from the relevant multiple-member protein family.
Western blot
K562 and K562/ADM cell proteins were extracted in RIPA buffer (50 mM Tris-base, 1.0 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% Sodium deoxycholate, 1 mM PMSF) and quantified by the DC protein assay kit (Bio-Rad). Samples were separated by 12% SDS-PAGE and transferred to PVDF membranes (Amersham Biosciences). The membranes were blocked overnight with PBS containing 0.1% Tween 20 in 5% skimmed milk at 4°C, and subsequently probed by the primary antibodies: rabbit-anti-Annexin A2 (diluted 1:500 Santa Cruz Biotechnology, USA), rabbit-anti-Annexin A4 (diluted 1:1,000 Santa Cruz Biotechnology, USA), and rabbit-anti-Phosphoglycerate kinase 1 (diluted 1:500 Santa Cruz Biotechnology, USA). Blots were incubated with the respective primary antibodies for 2 h at room temperature and washed three times in TBST. After that, the blots were incubated with secondary antibody (diluted 1:5,000) conjugated to HRP for 2 h at room temperature. Target proteins were detected by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, USA). GAPDH was used as an internal control.
Results
K562/ADM cell line acquirement and 2-DE analysis
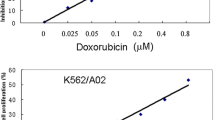
Adriamycin-resistant cell line K562/ADM was established by treating continuously, over 6 months, with stepwise-increasing concentration of adriamycin. The IC50 values for adriamycin treatment in K562 and K562/ADM cells were 0.06 ± 0.04 and 14.36 ± 1.08 μM, respectively. The resistance of K562/ADM cells to adriamycin was 239-fold higher than that of the parental K562 cells, which meant adriamycin-resistant cell line was successfully established. The protein expression profile of K562/ADM and K562 cells were examined by 2-DE. The experiments were repeated three times. A pair of representative 2-DE maps was shown in Fig. 1. The spots with remarkable expression changes were selected as examples and shown in Fig. 2 in an enlarged form.
Enlargement of selected regions as examples showing spots with consistent expression variation in 2-DE gels. a Spots # 1, # 2, and # 4 (corresponding to ANXA4, PSMD8, and PDAP1, respectively) were significantly up-regulated in K562/ADM cells compared with K562 cells. b The quantity of each spot was determined by PDQuest software. # 2 spot was up-regulated 3.6-fold ± 1.3 (P < 0.05) in K562/ADM cells compared with control K562 cells; # 1 and # 4 were also significantly up-regulated (P < 0.01). Data were shown as mean values ± SD
Identification of differentially expressed proteins
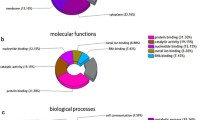
Out of the 35 differentially expressed proteins in pair wise comparison between the two cell lines, 29 proteins were successfully identified using ESI-Q-TOF MS/MS analysis (Table 1). These proteins can be grouped into 10 main functional classes including metabolism (24%), proteolysis (13%), signal transduction (21%) and calcium ion binding (6%), and so on (Fig. 3). Representative MS maps of spot #8 were shown in Fig. 4. The MOWSE score and the number of unique peptides identified by MS/MS sequencing were 685 and 33, respectively (Table 1).
Protein validation by Western blot
The alteration of Annexin A2, Annexin A4 and Phosphoglycerate kinase 1 proteins were further validated by Western blot. Consistent with the observations in 2-DE analysis, Annexin A2, Annexin A4 and Phosphoglycerate kinase 1 were found up-regulated in the K562/ADM compared with the parental K562 cells (Fig. 5).
Western blot confirmation of the alterations of Annexin A2, Annexin A4, and Phosphoglycerate kinase 1 proteins. The images were representative results of at least three independent experiments. Annexin A2, Annexin A4, and Phosphoglycerate kinase 1 proteins were significantly up-regulated (P < 0.01) in K562/ADM cells compared with K562 cells. GADPH was used as a loading control
Discussion
Acquired resistance to multiple anticancer drugs is a major problem in chemotherapy, and some mechanisms of resistance have been investigated previously [2–7]. However, the exact mechanisms that lead to chemo-resistance are largely unknown. In the present study, a comparative proteome study using 2-DE and MS based analysis was conducted and totally 29 proteins were positively identified to be differentially expressed between K562/ADM and the parental K562 cells.
Compared with the previous study [14], we identified more differentially expressed proteins potentially involved in adriamycin-resistance. Most of identified proteins were related to the categories of metabolism, proteolysis, signal transduction and calcium ion binding, suggesting that alterations of those biological processes might be involved in adriamycin resistance of K562 cells. Among them, some of the proteins have been reported to be associated with the development of tumor drug resistance, such as Annexin A2, while some might be new drug-resistance-associated proteins, such as PGK1, and there are also some proteins that have not been previously linked to drug resistance. The functional roles of the changed proteins associated with drug-resistance of tumor cells were briefly discussed below.
Hypoxia was often observed in solid tumors [16]. The oxygen-deficient hypoxic tumor cells have been shown to influence prognosis in terms of both a negative effect on response to chemotherapy/radiotherapy and a positive effect on malignant progression [17–19]. The mechanisms by which hypoxia contributes to chemoresistance are largely unknown. Tumor hypoxia may develop in tumors with a high growth rate relative to the capacity of the microvasculature to supply oxygen and nutrients, causing cell cycle disruption or arrest, DNA over-replication, and induction of stress proteins [20]. Indeed, our proteomic analysis demonstrated over-expression of glycolytic enzymes such as phosphoglycerate kinase 1, transaldolase, fructose-bisphosphate aldolase A, and l-lactate dehydrogenase B chain, indicating the presence of hypoxia in the chemoresistant tumor cells. Thus, it may be an effective method to deal with the drug resistance by reversing the hypoxia.
The proteasome, a multicatalytic proteinase complex, is responsible for the majority of intracellular protein degradation [21]. Pharmacologic inhibitors of the proteasome possess in vitro and in vivo anti-tumor activity [22, 23]. PS-341 is a dipeptidyl boronic acid derivative that specifically inhibits the function of the 26S proteasome [24]. The 26S proteasome is a large multisubunit ATP-dependent threonine protease complex found in the cytoplasm and nucleus of all eukaryotic cells; its principle function is to degrade proteins via the ubiquitin pathway [25]. Preclinical and clinical studies demonstrated that the PS-341 potentiates the sensitivity of conventional chemotherapeutic agents, in part by down-regulating chemo-resistance pathways [26]. In this study, we found that some subunits of proteasome including 26S proteasome non-ATPase regulatory subunit 8, 26S proteasome non-ATPase regulatory subunit 11, 26S proteasome non-ATPase regulatory subunit 13 and proteasome subunit beta type-4 were obviously up-regulated in K562/ADM cells compared with K562 cells. These data showed inhibition of proteasome activity may be an effective strategy to reverse chemoresistance.
The Rho GDP dissociation inhibitor (RhoGDI), which is a member of a family of GDIs that include RhoGDI (also known as RhoGDIa or RhoGDI-1), D4-GDI, and RhoGDI-3, is a cellular regulatory protein that works primarily by controlling the cellular distribution and activity of Rho GTPases [27–29]. It is ubiquitously expressed in cells and tissues and binds to most of the Rho GTPases thus far examined, including Rac1, RhoA, and Cdc42 [30]. The mechanism by which RhoGDI can negatively regulate Rho proteins is as follows: (a) by blocking their interaction with guanine nucleotide exchange factors (GEF), thereby inhibiting GTPase activation; (b) by shielding the membrane-anchoring domain of the GTPases, thereby restricting them to a cytosolic (non-active) localization; and (c) by blocking binding to downstream target molecules (e.g., various kinases regulated by small GTPases). RhoGDI is overexpressed in multiple types of human cancers. Previous studies showed that RhoGDI is highly expressed in stable chemoresistant cancer cell lines, such as melanoma and ovarian cells, raising the possibility that RhoGDI might play a role in the development of drug resistance of cancer cells [31–33]. Moreover, the recent data displayed that overexpression of RhoGDI increased resistance of human breast cancer cell MDA-MB-231 and lymphoma cell JLP-119 to the induction of apoptosis by two chemotherapeutic agents: etoposide and doxorubicin. Conversely, silencing of RhoGDI expression by small interfering RNA sensitized these cells to drug-induced apoptosis. The mechanism is RhoGDI has the ability to inhibit caspase-mediated cleavage of Rac1 GTPase, which is required for maximal apoptosis to occur in response to cytotoxic drugs [34]. Our data displayed that both Rho GDP-dissociation inhibitor 1 and Rho GDP-dissociation inhibitor 2 were upregulated in the adriamycin-resistant cells, which means RhoGDI maybe an ideal target for overcoming the adriamycin-resistance.
Ion binding proteins Annexin A2 and A4 correlate with increasing drug resistance. Annexin A2 was identified as highly expressed in adriamycin-resistant human breast carcinoma cells, but not in adriamycin-sensitive human breast carcinoma cells. Small interference RNA-mediated gene suppression demonstrated that Annexin A2 was required for enhanced cell proliferation and invasion of the adriamycin-resistant human breast carcinoma cells. Down-regulation of Annexin A2 alone was not sufficient to reverse the cell sensitivity to adriamycin, suggesting that Anxa2 was not required for MDR phenotype [35]. Previous study has also demonstrated elevated levels of Annexin A4 protein is associated with enhanced chemoresistance to carboplatin in human epithelial ovarian cancer cells in part by enhancing drug efflux [36]. In this study, these two proteins were positively identified with high confidence and further validated by Western blot. Taken together, ion binding proteins Annexin A2 and A4 may play important roles in the development of chemo-resistance.
Molecular chaperones are responsive to a variety of stresses such as cytotoxic agents, and therefore comprise a set of proteins with the potential to be used as biomarkers in response to cytotoxic agents. Each member of molecular chaperones appears to have a distinct set of functions within the cells. Some of these functions are well documented, such as the modulatory effects of HSPs on apoptosis; others need further elucidation, for example the role of special HSPs in thermotolerance [37, 38]. Previous study reported that HSP70A8 and HSP60, CCT3, and HSPC124 were increased in the taxotere-resistant squamous lung carcinoma cell line. Conversely, in the taxol-resistant squamous lung carcinoma cell line, HSP70A8, HSP27, and HSP70 9B were all down regulated [39]. In our study, some molecular chaperones like CCT7, HSPB1, TCP1, and CCT2 were changed significantly (2.6, 3.3, 3.5, and 3.3 folds, respectively) in the adriamycin-resistant cells compared with parental cells, some of which may be involved in the resistance to adriamycin.
Previously Zhu et al. compared the global protein profiles between adriamycin-resistant K562 cells and the parental K562 cells, and found eight differentially expressed proteins [14]. However, it was surprised that the eight proteins found by Zhu et al. did not appeared in the 29 differentially expressed proteins identified in the present study. The following reasons may partly explain why two similar studies lead to different results. Firstly, as we know, chemo-resistance acquisition is a very complicated process, and chemo-resistant cells are very heterogeneous, and maybe different cells gained chemo-resistance through different mechanisms during long time screening strength. Secondly, the type of IPG strip and concentration of SDS-PAGE used in the present study were non-linear and 12%, respectively, which were different from the study performed by Zhu et al. Thirdly, the resistance to adriamycin of K562/ADM cells established in this study was 239-fold higher than their parental K562 cells, and are different from that used by Zhu et al. (149-fold).
Accumulating data suggests that multiple complex mechanisms may be involved, simultaneously or complementarily, in the emergence and development of drug resistance in cancers. In the present study, using proteomic approach, we identified 29 protein spots that have altered expression in the adriamycin-resistant human chronic myelogenous leukemia cell line K562/ADM compared with the parental sensitive cell line K562, and the alteration of three representative proteins were further confirmed by Western blot. We believe the data in the present study may be useful for further study of resistance mechanisms and screening of resistant biomarkers.
Abbreviations
- 2-DE:
-
Two-dimensional gel electrophoresis
- ACN:
-
Acetonitrile
- CBB:
-
Coomassie brilliant blue
- DMEM:
-
Dulbecco-modified Eagle medium
- IEF:
-
Isoelectric focusing
- IPG:
-
Immobilized pH gradient
- MS:
-
Mass spectrometry
- PBS:
-
Phosphate-buffered saline
- PVDF:
-
Polyvinylidene fluoride
- Q-TOF:
-
Quadrupole time-of-flight
- TBST:
-
Tris-buffered saline Tween-20
References
Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Faneyte IF, Kristel PM, Maliepaard M et al (2002) Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res 8:1068–1074
Walker J, Martin C, Callaghan R (2004) Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. Eur J Cancer 40:594–605
Ruiz de Almodovar C, Ruiz-Ruiz C, Munoz-Pinedo C et al (2001) The differential sensitivity of Bcl-2-overexpressing human breast tumor cells to TRAIL or doxorubicin-induced apoptosis is dependent on Bcl-2 protein levels. Oncogene 20:7128–7133
Gariboldi MB, Ravizza R, Riganti L et al (2003) Molecular determinants of intrinsic resistance to doxorubicin in human cancer cell lines. Int J Oncol 22:1057–1064
Pommier Y, Sordet O, Antony S et al (2004) Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 23:2934–2949
Prost S (1995) Mechanisms of resistance to topoisomerases poisons. Gen Pharmacol 26:1673–1684
Vimalachandran D, Costello E (2004) Proteomic technologies and their application to pancreatic cancer. Expert Rev Proteomics 1:493–501
Chen R, Pan S, Brentnall TA, Aebersold R (2005) Proteomic profiling of pancreatic cancer for biomarker discovery. Mol Cell Proteomics 4:523–533
Yang YX, Sun XF, Cheng AL et al (2009) Increased expression of HSP27 linked to vincristine resistance in human gastric cancer cell line. J Cancer Res Clin Oncol 135:181–189
Shi YQ, Hu WH, Yin F et al (2004) Regulation of drug sensitivity of gastric cancer cells by human calcyclin-binding protein (CacyBP). Gastric Cancer 7:160–166
Chen R, Yi EC, Donohoe S et al (2005) Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology 129:1187–1197
Shekouh AR, Thompson CC, Prime W et al (2003) Application of laser capture microdissection combined with two-dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics 3:1988–2001
Zhu F, Wang Y, Zeng S, Fu X, Wang L, Cao J (2009) Involvement of Annexin A1 in multidrug resistance of K562/ADR cells identified by the proteomic study. OMICS 13:467–476
Tong A, Zhang H, Li Z et al (2007) Proteomic analysis of liver cancer cells treated with suberonylanilide hydroxamic acid. Cancer Chemother Pharmacol 61:791–802
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276
Brown JM (1999) The hypoxic cell: a target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 59:5863–5870
Evans SM, Koch CJ (2003) Prognostic significance of tumor oxygenation in humans. Cancer Lett 195:1–16
Bos R, Groep PVD, Greijer AE et al (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97:1573–1581
Teicher BA (1994) Hypoxia and drug resistance. Cancer Metastasis Rev 13:139–168
Voorhees PM, Dees EC, O’Neil B, Orlowski RZ (2003) The proteasome as a target for cancer therapy. Clin Cancer Res 9:6316–6325
Ruggeri B, Miknyoczki S, Dorsey B et al (2009) The development and pharmacology of proteasome inhibitors for the management and treatment of cancer. Adv Pharmacol 57:91–135
Milano A, Iaffaioli RV, Caponigro F (2007) The proteasome: a worthwhile target for the treatment of solid tumours? Eur J Cancer 43:1125–1133
Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4:349–360
Maniatis T (1999) A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev 13:505–510
Ma MH, Yang HH, Parker K et al (2003) The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res 9:1136–1144
Olofsson B (1999) Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal 11:545–554
Sasaki T, Takai Y (1998) The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun 245:641–645
Golovanov AP, Chuang TH, DerMardirossian C et al (2001) Structure–activity relationships in flexible protein domains: regulation of rho GTPases by RhoGDI and D4 GDI. J Mol Biol 305:121–135
Fukumoto Y, Kaibuchi K, Hori Y et al (1990) Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 5:1321–1328
Poland J, Schadendorf D, Lage H et al (2002) Study of therapy resistance in cancer cells with functional proteome analysis. Clin Chem Lab Med 40:221–234
Sinha P, Kohl S, Fischer J et al (2000) Identification of novel proteins associated with the development of chemoresistance in malignant melanoma using two-dimensional electrophoresis. Electrophoresis 21:3048–3057
Takano M, Goto T, Sakamoto M et al (2003) Identification of paclitaxel-resistance related genes by differential display using cDNA microarray in ovarian cancer cell lines. Proc Am Soc Clin Oncol 22:462
Zhang BL, Zhang YQ, Dagher MC et al (2005) Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res 65:6054–6062
Zhang F, Zhang L, Zhang B et al (2009) Anxa2 plays a critical role in enhanced invasiveness of multidrug resistant human breast cancer cells. J Proteome Res 8:5041–5047
Kim A, Enomoto T, Serada S et al (2009) Enhanced expression of Annexin A4 in clear cell carcinoma of the ovary and its association with chemoresistance to carboplatin. Int J Cancer 125:2316–2322
Hütter G, Sinha P (2001) Proteomics for studying cancer cells and the development of chemoresistance. Proteomics 1:1233–1248
Sinha P, Poland J, Kohl S et al (2003) Study of the development of chemoresistance in melanoma cell lines using proteome analysis. Electrophoresis 24:2386–2404
Murphy L, Henry M, Meleady P, Clynes M, Keenan J (2008) Proteomic investigation of taxol and taxotere resistance and invasiveness in a squamous lung carcinoma cell line. Biochim Biophys Acta 1784:1184–1191
Acknowledgments
This study was supported by the grants from the National Natural Sciences Foundation of China (30801294), Fok Ying Tung Education Foundation (201080) and the special major science and technology project for “creation of major new drugs” of China (2009ZX09103-132).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xingchen Peng and Fengming Gong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Peng, X., Gong, F., Xie, G. et al. A proteomic investigation into adriamycin chemo-resistance of human leukemia K562 cells. Mol Cell Biochem 351, 233–241 (2011). https://doi.org/10.1007/s11010-011-0730-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0730-8