Abstract
The complex implantation process is initiated by the recognition and adhesion between the embryo and uterine endometrial epithelium. The expression and interactions between the adhesive molecules from both fetal and maternal sides are crucial for the successful implantation. In this study, we aimed to investigate the expression and adhesive function of sLeX on the trophoblasts and L-selectin on uterine epithelial cells mediated the adhesion at the fetal–maternal interface, and to further explore whether this adhesion system could induce endometrial apoptosis, using in vitro implantation model consisting of the human trophoblast cell line (JAR) and human uterine epithelial cell line (RL95-2). The results showed that sLeX was expressed on JAR cells by indirect immunofluorescence staining. After transfection of JAR cells with fucosyltransferase VII (FUT7) which is the key enzyme for sLeX synthesis, the expression of FUT7 and sLeX synthesis were increased, and the percent adhesion of trophoblast cells to RL95-2 cell monolayer was significantly increased (P < 0.01). L-selectin was strongly expressed but not E- and P-selectin on epithelial RL95-2 cells by RT-PCR, Western blot. Blocking L-selectin with specific antibody or heparin pretreatment in RL95-2 cells inhibited the adhesion of JAR cells to RL95-2 cell monolayer. Furthermore, regulating the expression of sLeX on JAR cells or blocking L-selectin on RL95-2 cells could activate the apoptosis of uterine epithelial cells. These results suggest the sLeX/L-selectin adhesion system at fetal–maternal interface not only mediates the adhesion of embryo to uterine epithelium, but also effectively induces the apoptosis in uterine epithelium. The study supplies a molecular basis for the elucidation of the initial recognition and adhesion during embryo implantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implantation is a complex biological process. It requires the synchrony between the appropriately developed embryo and the receptive uterine endometrium [1, 2]. During this process, many adhesion molecules, such as integrins, cadherins, immunoglobulins, and selectins, are expressed by both the embryo and uterine endometrium [3, 4]. The interactions mediated by these molecules are critical in the recognition and adhesion at fetal–maternal interface, and they are the molecular basis for successful implantation.
Sialyl Lewis X (sLeX) epitopes are stage-specifically expressed in the human endometrium during menstrual cycle, and reach the highest level during the embryo implantation [5, 6]. Therefore, it is regarded as a functional marker of uterine receptivity for embryo. sLeX (NeuAcα2 → 3Galβ1 → 4 [Fucα1–3] GlcNAcβ1 → 3Galβ1 → R) is a mono-fucosylated oligosaccharide, and the fucosylation is the key step for the synthesis. sLeX is catalyzed by the specific α1,3-fucosyltransferase, fucosyltransferases VII (FUT7). Therefore, FUT7 is a critical enzyme that control L-selectin ligand synthesis [7, 8]. We have previously reported that the up- or down-regulation of FUT7 gene expression in RL95-2 cells could promote or inhibit the adhesion potential, respectively [9, 10]. But whether the expression of sLeX on embryo participates the adhesion of embryo to uterine epithelium is unclear.
L-selectin belongs to selectin family. L-selectin and its oligosaccharide ligands, sLeX epitopes, constitute sLeX/L-selectin adhesion system. This adhesion system is correlated with many physiological and pathological processes, such as leukocyte infiltration [11, 12], lymphocyte homing [13], tumor metastasis [14, 15], and embryo implantation [16, 17]. Genbacev et al. [16] found that the strong L-selectin staining was present in the human embryo, whereas the expression of selectin oligosaccharide-based ligands was up-regulated during the implantation window. This carbohydrate–selectin interaction is important for the setup of the initial recognition. However, little is known about the function of L-selectin on uterine epithelium.
Successful implantation needs the embryo adhesion to uterus, and then the embryo must penetrate the endometrial barrier by inducing apoptosis in the uterine epithelium. Recently, evidence suggested that the Fas apoptosis system plays important role in embryo implantation. The adhesion of embryo and uterus induces apoptosis in the endometrium, and the Fas system activated is crucial for the embryo implantation [1, 2, 18].
In an effort to investigate the role of sLeX on embryo and L-selectin on uterine endometrium, we utilized the in vitro embryo implantation model that mimics the process of embryo implantation. RL95-2 cells and JAR cells were selected because they preserve many properties of invasive embryo and receptive uterine and has been accepted as the models of trophoblast–endometrial interaction [19, 20]. In this study, we found that the recognition of sLeX on JAR cells and L-selectin on RL95-2 cells also mediated the adhesion at the fetal–material interface, and the adhesion could effectively induce the endometrial apoptosis which facilitates the embryo implantation.
Materials and methods
Materials
The human uterine epithelial cell line (RL95-2) and human trophoblast cell line (JAR) were obtained from the American Type Culture Collection (Manassas, VA). Takara RNA PCR Kit (AMV) version 3.0 was from Takara. Trizol, DMEM/F12 (1:1), RPMI 1640, fetal bovine serum (FBS), LipofectamineTM Reagent and PlusTM Reagent and 5-chloromethy carboxyseminapthorhodofluor-1 (SNARF-1) were purchased from Invitrogen. Enhanced chemiluminescence (ECL) assay kit was purchased from Amersham. Antibodies of goat anti-human FUT7, mouse anti-human L-selectin, rabbit anti-human E-selectin, rabbit anti-human P-selectin mouse anti-sLeX IgM, rabbit anti-human Fas, as well as horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies were purchased from Santa Cruz. Antibodies of PE-conjugated mouse anti-goat IgG, PE-conjugated rat anti-mouse IgM, TRITC-conjugated rat anti-mouse IgM, FITC-conjugated goat anti-mouse IgG and DAPI were purchased from Sigma. Coomassie protein assay reagent was purchased from Bio-Rad.
Cell culture
The JAR cells were maintained in RPMI 1640 supplemented with 10% FBS 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C under 5% CO2 in humidified air. RL95-2 cells were grown in DMEM/F12 (1:1) supplemented with 10% FBS, 5 μg/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C under 5% CO2 in humidified air. The growth medium was renewed every 2–3 days. For fluorescent labeling of cells, confluent monolayer of RL95-2 cells were labeled with Cell Tracker Red stain by adding 5 μM SNARF-1 to the cultures, and incubating for 2 h at 37°C under 5% CO2. JAR cells were labeled blue by adding 1% DAPI to the cultures, and incubating for 2 h at 37°C under 5% CO2. Unincorporated stain was removed by washing with culture medium twice. Image was captured using Olympus BX51 microscope (Japan).
Indirect immunofluorescence staining
The cells grown on the coverslips were fixed in 4% paraformaldehyde for 15 min after washing with PBS. After blocking with 1% complete serum for 2 h at 37°C, mouse anti-sLeX antibody (1:100) or mouse anti-L-selectin antibody (1:100) was applied to incubate with the cells overnight at 4°C. Image was captured after incubation of cells with TRITC-conjugated rat anti-mouse IgM (1:100) for sLeX assay, and FITC-conjugated goat anti-mouse IgG (1:100) for L-selectin assay for 30 min using Olympus BX51 fluorescence microscope (Japan).
Transient transfection
The JAR cells (5 × 104/ml) were trypsinized and seeded onto 6-well plate. When cells reached to 90% confluence, FUT7 expression plasmid containing the full coding sequence of FUT7 was transiently transfected into the cells using 400 ng of plasmid in the presence of 2 μl Lipofectamine™ Reagent and Plus™ Reagent per manufacturer’s instructions. The transfection was terminated 6 h later and the cells were harvested after 48 h.
RT-PCR
Total RNA was extracted from untransfected (control), mock vector or FUT7 expression plasmid-transfected cells using Trizol reagent according to the manufacturer’s protocol. The cDNA was synthesized using RNA PCR Kit (AMV) version 3.0. The primers used for FUT7 gene amplification (PCR products 497 bp) were: 5′-cacctccgagcatcttcaactg-3′ (sense) and 5′-cgttggtatcggctc tcattcatg-3′ (antisense). The primers for β-actin gene amplification (PCR products 838 bp) were: 5′-atctggcaccacaccttctacaatgagctgcg-3′ (sense) and 5′-cgtcatactcctgcttgctgatccacatctgc-3′ (antisense). The primers for E-selectin gene amplification (PCR products 244 bp) were: 5′-ggcagtggacacagcaaatc-3′ (sense) and 5′-tggacagcatcgcatctca-3′ (antisense). The primers for P-selectin gene amplification (PCR products 236 bp) were: 5′-caccaatgtgtgaagccatc-3′ (sense) and 5′-acattgcacccctggagtag-3′ (antisense). The primers for L-selectin gene amplification (PCR products 250 bp) were: 5′-aaacccatgaactggcaaag-3′ (sense) and 5′-cgcagtcctccttgttcttc-3′ (antisense). PCR reactions to amplify the DNA fragment were carried as follows: initial denaturation at 94°C for 5 min, 35 cycles of 94°C for 40 s, 57°C for 45 s, 72°C for 45 s, and a final extension for 72°C for 5 min. The amplified products were analyzed by 1% agarose gel electrophoresis, and the bands were then visualized by ethidium bromide (EB) staining, followed by analysis with Labworks 4.6 (UVP).
Flow cytometry assay
Cells were gently trypsinized, and the single cell suspension was collected. After washing with PBS, 0.1% Triton–PBS was added and incubated for 10 min in FUT7 assay. The cells were then incubated with goat anti-FUT7 antibody (1:100), mouse anti-sLeX antibody (1:100), respectively, for 1 h at room temperature. Unbound antibodies were removed by washing with PBS for three times. PE-conjugated mouse anti-goat IgG (1:100) for FUT7 assay, and PE-conjugated rat anti-mouse IgM (1:100) for sLeX assay was then applied to incubate with the cells for 45 min at room temperature. The unbound secondary antibodies were discarded, and the cell mixture was adjusted to 500 μl with PBS for measurement in a FACScan flow cytometer.
Western blot
Total protein from the whole cell lysates was prepared and applied for Western blot. In brief, the cells prepared as indicated were washed with PBS (pH7.4), and incubated with lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 8.0, 0.2 mM Na3VO4, 0.2 mM phenylmethylsulfonyl fluoride, 0.5% Nonidet P-40) for 30 min on ice. Cell lysates were clarified by centrifugation at 9,000×g for 10 min, and the supernatant was collected. Protein concentration was determined with Coomassie protein assay reagent using bovine serum albumin (BSA) as a standard. Total protein (10–60 μg) from the whole cell lysates was separated by 10% SDS-PAGE mini-gel. Proteins separated in the gel were transferred electrophoretically onto nitrocellulose membrane. After blocking with TTBS (50 mM Tris–HCl, pH 7.5, 0.15 M NaCl, 0.1% Tween-20) containing 5% fat-free dry milk for 2 h, the membrane was incubated overnight with specific rabbit anti-Fas antibody (1:100) or anti-L-, anti-E-, or anti-P-selectin antibody at 4°C, respectively. Anti-actin antibody was used to confirm the equal loading. After washing with TTBS three times, 5 min each, the membrane was incubated with HRP-conjugated anti-rabbit or anti-mouse antibody (1:1000) for 40 min at room temperature. The membrane was then developed using an ECL detection system.
Adhesion of JAR cells to RL95-2 cell monolayer
The RL95-2 cells were grown on the coverslips to form a confluent monolayer. Then, the cultured JAR cells were harvested, and counted, followed by gently delivered onto RL95-2 cell monolayer in JAR growth medium. After 1 h, unadhered JAR cells were removed by centrifugation of the coverslips with the cell surface facing down at 12×g for 5 min, and counted under Olympus BX51 microscope after resuspension of the pellet with PBS. The JAR cells which adhered to RL95-2 cell monolayer were also counted for further confirmation. Percent adhesion was calculated as the number of JAR cells attached to RL95-2 cell monolayer compared to the total number of JAR cells plated.
Statistical analysis
Results are expressed as means ± standard error of mean (SEM) of three independent experiments. Statistical significance of difference between test groups was assessed by one-way ANOVA followed by Scheffe’s test (post hoc), with P < 0.05 considered to be significant.
Results
Expression of sLeX and up-regulation by FUT7 gene transfection on JAR cells
The expression of sLeX on JAR cells was observed by indirect immunofluorescence staining. The results showed that sLeX on JAR cells was mainly located on cell membrane (Fig. 1A). To further explore the regulation of sLeX expression on JAR cells, FUT7 cDNA was transfected into the cells. The results showed that the gene expression of FUT7 was elevated in the FUT7 cDNA transfected cells compared with the control or mock vector-transfected cells by RT-PCR (Fig. 1B), and expression of FUT7 protein and sLeX was also increased as shown in Fig. 1C. The data indicates that sLeX is expressed on JAR cells and can be regulated by its key synthetic enzyme, FUT7 gene.
Expression and elevated sLeX by FUT7 gene transfection. A Indirect immunofluorescence staining. The cells were incubated with mouse anti-sLeX antibody, followed by incubation with TRITC-conjugated rat anti-mouse IgM for fluorescent staining analysis of sLeX. a sLeX staining on RL95-2 cells; b negative control (cells incubated only with secondary antibody). B Expression of FUT7 gene by RT-PCR in JAR cells after transient transfection with FUT7 cDNA. Control untransfected cells; Mock cells transfected with mock vector; FUT7 cells transfected with FUT7 cDNA; M DNA marker (DL 2000). C Expression of FUT7 and sLeX by flow cytometry assay in JAR cells after transient transfection with FUT7 cDNA. Control untransfected cells; Mock cells transfected with mock vector; FUT7/sLeX cells transfected with FUT7 cDNA; Negative cells were incubated only with secondary antibody
Expression of L-selectin on RL95-2 cells
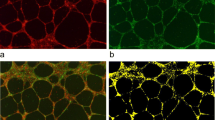
The expression of L- , E- , and P-selectin on RL95-2 cells was detected by RT-PCR, Western blot, and indirect immunofluorescence staining (Fig. 2). The PCR results showed that the expression of L-selectin is the strongest among the three selectins, and the expression of E-selectin was very weak compared with that of L-selectin; whereas P-selectin expression was not detectable although the gene was amplified by 35 cycles of PCR (Fig. 2A). The Western blot results also present similar tendency as those of the gene expression (Fig. 2B). The expression of L-selectin on RL95-2 cells was also further detected by indirect immunofluorescence staining (Fig. 2C). Therefore, the above data suggests that L-selectin is the major type of selectin on RL95-2 cells, and may play an essential role in sLeX/L-selectin-mediated recognition and adhesion at the fetal–maternal interface.
Expression of L-selectin on RL95-2 cells. A Gene expression of L-, E- , and P-selectin was detected by RT-PCR. β-actin was used an internal control. B Expression of L-, E- , and P-selectin was detected by Western blot. Total proteins (30–60 μg) of cell lysates from RL95-2 cells was used for immunoblots with anti-L-, anti-E-, or anti-P-selectin antibody, respectively. Anti-β-actin antibody was used to confirm the equal loading. C Indirect immunofluorescence staining. The cells were incubated with mouse anti-L-selectin antibody, followed by incubation with FITC-conjugated goat anti-mouse IgG for fluorescent staining analysis. a L-selectin staining on RL95-2 cells; b negative control (cells incubated only with secondary antibody)
Elevated sLeX by FUT7 cDNA transfection and anti-sLeX antibody blockage changes adhesion
The effect of sLeX level on the adhesive potential of JAR cells was studied. The percent adhesion of JAR cells to RL95-2 cell monolayer was analyzed (Fig. 3). The results showed that overexpression of FUT7 in JAR cells significantly facilitated the adhesion of JAR cells to RL95-2 cell monomer (84.3 ± 1.4% vs. 68.9 ± 1.9%; P < 0.01). However, the percent adhesion was significantly inhibited when anti-sLeX antibody was incubated with JAR cells, and in a dose dependent manner (Fig. 3A). Figure 3B showed the representative picture of adhesion of JAR cells to RL95-2 cell monolayer in the differently treated groups. JAR cells were labeled with DAPI (blue) and RL95-2 cells labeled with SNARF-1 (red) before JAR cells were delivered to the RL95-2 cell monolayer. These results suggested that the increased sLeX through FUT7 overexpression in JAR cells could enhance embryo adhesion potential.
Elevated sLeX by FUT7 cDNA transfection and anti-sLeX antibody blockage on JAR cells changes adhesion. A Percent adhesion of JAR cells to RL95-2 cell monolayer was analyzed. Control untreated cells; FUT7 JAR cells were transfected with FUT7 cDNA; sLeX-Ab JAR cells were pre-incubated with anti-sLeX antibody for 3 h. **P < 0.01. B Fluorescent staining of JAR cells by DAPI (blue) and RL95-2 cells by SNARF-1 (red). a Untreated cells; b JAR cells were transfected with FUT7 cDNA; c JAR cells were pre-incubated with anti-sLeX antibody. (Color figure online)
Blocking L-selectin on RL95-2 cells inhibits adhesion at the fetal–maternal interface
To check if the highly expressed L-selectin on RL95-2 cells plays an important function in the adhesion of embryonic cells to uterine epithelial cells, the adhesion inhibition assay by specific antibody against L-, E-, or P-selectin blockage on RL95-2 cells before JAR cell delivery, as well as heparin pre-incubation was undergone (Fig. 4). Statistical analysis showed that the percent adhesion was significantly decreased when RL95-2 cells was pre-incubated with anti-L-selectin antibody, and the lowest percent adhesion was 42.6 ± 2.4% when the concentration of L-selectin is 30 μg/ml. However, there was no significant change with E- or P-selectin antibody blockage in in vitro implantation model (Fig. 4A). Heparin is an inhibitor of glycan ligands/selectin binding [21, 22], and heparin pretreatment (5 mg/ml) of RL95-2 cells also inhibited the percent adhesion (67.8 ± 2.8% vs. 48.6 ± 3.7%) (Fig. 4B). The results that the adhesion of embryonic cells to uterine epithelial monolayer was significantly inhibited with anti-L-selectin antibody blockage, but not with anti-E- or anti-P-selectin antibody blockage, indicating that sLeX/L-selectin mediated adhesion in in vitro implantation model.
sLeX/L-Selectin-mediated adhesion induces apoptosis in RL95-2 cells
To analyze whether sLeX/L-selectin adhesion system could induce the apoptosis of uterine epithelial cells, which is an important marker of successful embryo implantation, the expression level of Fas was detected in RL95-2 cells after the differential pretreatment of JAR cells or RL95-2 cells. As shown in Fig. 5A, Fas was not detectable in the untreated JAR cells, and used as an expression control [23], increased sLeX level by FUT7 gene transfection or blockage of functional sLeX with anti-sLeX antibody in JAR cells greatly inhibited Fas expression in RL95-2 cells, indicating that sLeX on embryonic cells participates in inducing the apoptosis of uterine epithelial cells. Meanwhile, Fas expression decreased in RL95-2 cells pre-incubated with anti-L-selectin antibody or heparin compared with the untreated RL95-2 cells (Fig. 5B). The results suggest that sLeX/L-selectin adhesion system can induce the apoptosis of uterine epithelial cells.
sLeX/L-Selectin-mediated adhesion triggers the apoptosis in RL95-2 cells. A Fas was detected by Western blot in RL95-2 cells after JAR cells were differently treated before delivery. Total protein used for immunoblots was from the cell lysates of JAR cells plated directly on the well (expression control, lane 1), JAR cells of untreated (control, lane 2), FUT7 transfected (lane 3), and sLeX antibody blocked (lane 4). B Fas was detected by Western blot in RL95-2 cells after RL95-2 cells were differently treated before untreated JAR cells delivery. RL95-2: untreated RL95-2 cells (lane 1). The untreated JAR cells delivered onto untreated RL95-2 cells (control, lane 2), RL95-2 cells were treated with L-selectin antibody (lane 3). RL95-2 cells were treated with heparin (lane 4)
Discussion
At the implantation window, one of the essential features of competent embryo which has undergone differentiation, maturation, and activation is the expression of many adhesion molecules [24]. Similarly, the preparations of the uterine endometrium from a refractory state to the receptive state include not only the alterations in the apical structures, but also the adhesion molecule reservoir [25]. Through the ligand–receptor interactions of these molecules at the fetal–maternal interface, the initial adhesion is accomplished. Because of not accessible assay in early human in vivo implantation, the application of in vitro implantation model composed of JAR cells and RL95-2 cells is of importance for the study [19, 20], and we applied it to analyze sLeX/L-selectin adhesion system at the fetal–maternal interface.
Glycans carried by the glycoconjugates on the cell surface are dynamically changed in the different stages of embryogenesis, embryo implantation and invasion and placentation, such as LeX, LeY, and sLeX [26, 27]. Genbacev et al. [16] found that strong L-selectin staining observed over the trophoblast surface, while L-selectin ligands, sLeX epitopes, were detected in the human endometrium throughout the implantation [28]. The sLeX/L-selectin adhesion system is also involved in the early placentation. In this process, the trophoblastic cells co-express sLeX and L-selectin epitopes, and take part in cell–cell interactions which are important for trophoblastic cell aggregates [29]. Although there are still less reports related to the expression of sLeX on human blastocyst and L-selectin on the human uterine epithelium until now, here, we found that sLeX was also expressed on JAR cells and L-selectin expressed on RL95-2 cells (Figs. 1, 2).
The function of sLeX/L-selectin adhesion system in implantation can be regulated by changing sLeX or L-selectin level functionally available participate the interactions at the fetal–maternal interface. FUT7 is the key enzyme for sLeX synthesis [10, 30, 31]. Our previous data showed that FUT7 gene expression on the mouse embryo of different development stage could be regulated by an essential cytokine, leukemia inhibitory factor (LIF) [32]. In this study, the results indicate that the levels of FUT7 gene and protein, as well as sLeX were increased in JAR cells when they were transfected with FUT7 cDNA. Therefore, FUT7 is a critical enzyme that controls sLeX synthesis during embryo adhesion and implantation. Moreover, the elevated sLeX on JAR cells increased the adhesion of JAR cells to RL95-2 cell monolayer. Therefore, sLeX synthesis on embryo can be up-regulated, which contributes to the interactions of embryo–uterine endometrium, therefore, the enhanced adhesion.
The stage specifically high expression of L-selectin indicates its implantation importance in the interaction with sLeX ligand. To explore whether the strongly expressed L-selectin, instead of E- and P-selectin, on RL95-2 cells was functional in mediating the adhesion between embryo and uterine endometrium, adhesion inhibition assay was employed. When RL95-2 cells pre-incubated with L-selectin antibody, the L-selectin was blocked, and the adhesion percent of JAR cells to RL95-2 cells was significantly reduced, compared with the untreated control. Heparin has functions in modulating embryo implantation. It is an effective inhibitor of L-selectin [21, 22] and acts as a role in assisted conception. It is reported that treatment of the tumor cells with heparin led to the attenuation of metastasis primarily by inhibition of L-selectin-mediated interactions [22]. Here, the results showed that the supplement of heparin in the culture medium of RL95-2 cells could significantly reduce the percent adhesion. The results indicate that heparin could decrease the embryo adhesion by binding with L-selectin expressed on RL95-2 cells.
The apoptosis of uterine epithelium is one of the cascade events induced after embryo adhered to uterine epithelium. It is required for further embryo implantation and invasion, as well as placenta formation [1, 2, 18]. Fas was localized at the apical cell surface of uterine epithelium. There is evidence suggesting that trophoblasts could induce the uterine epithelium apoptosis after being co-cultured with the latter. Therefore, Fas may play an important role in embryo implantation. In this study, we detected the change of Fas in RL95-2 cells after JAR cells or RL95-2 cells pretreated, respectively, before JAR cell delivery. We found that JAR cells transfected with FUT7 cDNA could activate endometrium to express more Fas than the control, whereas when JAR cells pre-incubated with anti-sLeX antibody could down-regulate the expression of Fas. Furthermore, when RL95-2 cells pre-incubated with anti-L-selectin antibody or heparin, the Fas expression was decreased compared with the control (Fig. 5). We conclude that regulating the expression of sLeX on JAR cells or L-selectin on RL95-2 cells could control the adhesion, and effectively triggers the apoptosis of uterine epithelium.
In conclusion, our results demonstrated the expression of sLeX on embryonic cells and L-selectin on uterine epithelial cells contributes the adhesion at the fetal–maternal interface. sLeX/L-selectin adhesion system not only mediates the initial adhesion of embryo and uterine epithelium, but also functional induces the apoptosis of uterine epithelium, which is required for further embryo implantation. It may offer a new insight about the embryo implantation and strategy in assisting or impairing embryo implantation.
Abbreviations
- sLeX:
-
Sialyl Lewis X
- FUT7:
-
Fucosyltransferase VII
- SNARF-1:
-
5-Chloromethy carboxyseminapthorhodofluor-1
- DAPI:
-
4,6-Diamino-2-phenyl indole
- TRITC:
-
Tetramethylrhodamine isothiocyanate
- FITC:
-
Fluoresceinisothiocyanate
- HRP:
-
Horseradish peroxidase
- PE:
-
Phycoerythrin
- FBS:
-
Fetal bovine serum
- BSA:
-
Bovine serum albumin
- ECL:
-
Enhanced chemiluminescence
References
Guzeloglu-Kayisli O, Basar M, Arici A (2007) Basic aspects of implantation. Reprod Biomed Online 15:728–739
Makrigiannakis A, Minas V (2007) Mechanisms of implantation. Reprod Biomed Online 14:102–109
Aplin JD (1997) Adhesion molecules in implantation. Rev Reprod 2:84–93
Dey SK, Lim H, Das SK et al (2004) Molecular cues to implantation. Endocr Rev 25:341–373
Lai TH, Shih IeM, Vlahos N, Ho CL, Wallach E, Zhao Y (2005) Differential expression of L-selectin ligand in the endometrium during the menstrual cycle. Fertil Steril 83:1297–1302
Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF (2008) High expression of L-selectin ligand in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol 60:127–134
Läubli H, Stevenson JL, Varki A, Varki NM, Borsig L (2006) L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res 66:1536–1542
Smith PL, Gersten KM, Petryniak B et al (1996) Expression of the alpha (1, 3) fucosyltransferase Fuc-TVII in lymphoid aggregate high endothelial venules correlates with expression of L-selectin ligands. J Biol Chem 271:8250–8259
Zhang Y, Liu S, Liu Y, Wang Z, Wang X, Yan Q (2009) Overexpression of fucosyltransferase VII (FUT7) promotes embryo adhesion and implantation. Fertil Steril 91:908–914
Liu S, Zhang Y, Liu Y, Qin H, Wang X, Yan Q (2008) FUT7 antisense sequence inhibits the expression of FUT7/sLeX and adhesion between embryonic and uterine cells. IUBMB Life 60:461–466
Uchimura K, Rosen SD (2006) Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol 27:559–565
Jackson LA, Drevets DA, Dong ZM, Greenfield RA, Murphy JW (2005) Levels of L-selectin (CD62L) on human leukocytes in disseminated cryptococcosis with and without associated HIV-1 infection. J Infect Dis 191:1361–1367
Kawashima H (2006) Roles of sulfated glycans in lymphocyte homing. Biol Pharm Bull 29:2343–2349
Resto VA, Burdick MM, Dagia NM, McCammon SD, Fennewald SM, Sackstein R (2008) L-selectin-mediated lymphocyte–cancer cell interactions under low fluid shear conditions. J Biol Chem 283:15816–15824
McEver RP (1997) Selectin–carbohydrate interactions during inflammation and metastasis. Glycoconj J 14:585–591
Genbacev OD, Prakobphol A, Foulk RA et al (2003) Trophoblast L-selectin-mediated adhesion at the maternal–fetal interface. Science 299:405–408
Fazleabas AT, Kim JJ (2003) What makes an embryo stick? Science 299:355–356
Galan A, O’Connor JE, Valbuena D et al (2000) The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol Reprod 63:430–439
Thie M, Denker HW (2002) In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs 172:237–252
Tinel H, Denker HW, Thie M (2000) Calcium influx in human uterine epithelial RL95-2 cells triggers adhesiveness for trophoblast-like cells. Model studies on signalling events during embryo implantation. Mol Hum Reprod 6:1119–1130
Nelson SM, Greer IA (2008) The potential role of heparin in assisted conception. Hum Reprod Update 14:623–645
Stevenson JL, Varki A, Borsig L (2007) Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb Res 120:107–111
Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G (2004) First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod 10:55–63
Staun RE, Shalev E (2005) Human trophoblast function during the implantation process. Reprod Biol Endocrinol 20:56
Achache H, Revel A (2006) Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 12:731–746
Carson DD (2002) The glycobiology of implantation. Front Biosci 1:1535–1544
Wang XQ, Zhu ZM (1998) Role for cell surface oligosaccharide in cell-cell recognition during implantation. Mol Hum Reprod 4:735–738
Shamonki MI, Kligman I, Shamonki JM (2006) Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil Steril 86:1365–1375
Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ (2006) A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol 298:107–117
Becker DJ, Lowe JB (2003) Fucose: biosynthesis and biological function in mammals. Glycobiology 13:41–53
Taniguchi N, Honke K, Fukuda M (2002) Handbook of glycosyltransferases and related genes. In: Oriol R, Mollicone R, Narimatsu H (eds) Fucosyltransferases. Springer, Tokyo, pp 205–231
Zhang Q, Liu S, Zhu Z, Yan Q (2009) Regulating effect of LIF on the expression of FuT7: probe into the mechanism of sLe(x) in implantation. Mol Reprod Dev 76(8):692
Acknowledgments
This work is supported by National Natural Science Foundation of China Research Grant (30670465), Specialized Research Fund for the Doctoral Program of Higher Education of China (20060161001), and Fund for Creative Research Groups of Liaoning Province (2007T021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Yang, X., Liu, Y. et al. sLeX/L-selectin mediates adhesion in vitro implantation model. Mol Cell Biochem 350, 185–192 (2011). https://doi.org/10.1007/s11010-010-0697-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0697-x