We study the influence of OK-2 scaling inhibitor based on polyhexamethyleneguanidine hydrochloride on the morphology and phase composition of calcium carbonate. It is established that the presence of inhibitor in the solution leads to an increase in the sizes of crystals in the deposit and the predominance of needle structures in it. The aragonite and calcite phases are in excess in the deposits of calcium carbonate and the presence of inhibitor in the solution increases the amount of calcite by 21%. The revealed effects are caused by the adsorption of the inhibitor on carbonate deposits, which can be described by the Langmuir isotherm. The adsorption of the inhibitor leads to the recharging of the CaCO3 surface and to the increase in the size of particles of the dispersed phase, which promotes its faster precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Calcium carbonate CaCO3, which can be in different polymorphic states, is the main component of scale formed on the heat exchange surfaces [1–4]. In the absence of inhibitors, at temperatures of 80–100°C, aragonite with small admixtures of calcite precipitates into the solid phase [5]., As compared with calcite, aragonite has is characterized by elevated adhesion to the solid surface and is not washed out with water. Therefore, it is adhered to heat exchange surfaces. The polyelectrolytes containing guanidine, carboxyl, or phosphonic groups prove to be promising inhibitors of scaling because they can form water-soluble complexes with calcium and magnesium ions and can be adsorbed on the surfaces of crystals [6]. A new OК-2 inhibitor of complex action (modification No. 4 in the TU U 21854083.009-99 of 20.11.2013) based on polyhexamethyleneguanidine hydrochloride was proposed in [7]. It decreases scaling and corrosion rate and possesses high biocide properties. If the mass concentration of OK-2 is 20 mg/liter, then the degree of protection against scaling constitutes 50 ± 5%, which is not worse than for the ZnOEDF commercial inhibitor. In this case, calcium and magnesium ions partly precipitate in the form of carbonate deposits. However, the influence of the OK-2 inhibitor on the crystallization of calcium carbonate has not been studied in the literature. Due to the intense adsorption, this inhibitor is incorporated in the deposit and then washed out. Thus, a part of the inhibitor is deposited on the surface of the equipment and another part precipitate together with the deposits and affects the sedimentation properties [8]. Clearly, if the structure of the deposit changes under the influence of inhibitors, then this can lead to a decrease in scaling on the heat exchange surfaces because the deposits formed in this case can be easily washed out by the water flow. The aim of the present work is to study the sedimentation properties and morphology of the deposits of calcium carbonate in the presence of the OK-2 inhibitor.
Method of Investigation
To estimate the influence of inhibitors on the phase composition of carbonate deposits, we used a conic flask containing 45 mliter of a 1 M CaCl2 solution and 10 ml of a 0.1 M MgCl2 solution and added to these solutions 1 mliter of the inhibitor solution with a concentration of 1 g/liter and 45 mliter of a 1 M NaHCO3 solution [6]. The solutions were held for 1 h at a temperature of 80°C, cooled down to the room temperature, and filtered by using a “blue stripe” filter. The deposit was dried at the room temperature and analyzed with the help of a DRON-3 diffractometer in the monochromatized Cu-Kα -radiation (40 kV, 20 mA; with a recording rate of 1 grad/min). The phase composition of substances was determined according to the set of interplane distances and the corresponding intensities determined from the obtained X-ray diffraction patterns [9–11]. The quantitative ratio of phases in the absence and in the presence of the inhibitor was estimated according to the intensities of the corresponding lines of calcite and aragonite by using the following approach:
where W c and W a are the amounts of calcite and aragonite phases, %, and I c and I a are the intensities of their lines.
The electron microscopic images of the deposits were obtained with the help of an REM-106I scanning electron microscope in the mode of its operation in secondary electrons.
The adsorption on the solution–calcium-carbonate boundary was studied by analyzing the changes in the surface tension of the inhibitor. The surface tension of the inhibitor on the solution–air boundary was measured by the Wilhelmy method with the help of a Vibra HT-120 balance and a platinum plate 59.7 × 0.3 × 7.4 mm in size. The role of sorbent was played by CaCO3 of the “chemically pure” grade with a specific surface of 0.21 m2/g. We placed 1 g CaCO3 in each dry flask and added 25 mliter of solutions with different concentrations of the inhibitor. At the end of the period necessary for adsorption (60 min), the solutions were filtered through the “blue stripe” paper filter. We measured the surface tension on the solution–air boundary and determined the residual concentration of the inhibitor. The rate of adsorption (Γ, mole/m2) was found as follows:
where C 1 and C 2 are the inhibitor concentrations prior to and after adsorption on calcium carbonate (in mole/m3); m is the mass of the sorbent (in g); V is the volume of solution taken for adsorption (in m3), and S sp is the specific surface of calcium carbonate (in m2/g).
The electrokinetic potential (ξ) of the surface of calcium carbonate is determined according to the velocity of motion of charged particles of the dispersed phase in the electric field by the formula
where u is the velocity of motion of particles (in m/sec); η is the dynamic viscosity of the medium (0.001 Pa · sec); ε is the dielectric permittivity of water (81); ε0 is the dielectric constant (8.85 · 10–12); W is the external voltage (in V), and le is the distance between the electrodes (in m).
The level of dispersed phase was recorded every 10 min for 1 h. The sign of the charge of colloid particles was determined according to the direction of motion of the sol–side-liquid boundary.
The sedimentation of CaCO3 suspensions in water (with a concentration of 3 g/liter) was studied by using a Vibra HT-120 analytic balance. The height of the suspension column was 0.140 m and the radius of the cup was equal to 0.019 m. The sedimentation ability was estimated by the value of sedimentation constant (K s ) given by the formula
where r H are the probable radii of particles of calcium carbonate (in m); ρ and ρ0 are the densities of particles of the disperse phased and dispersive medium (in kg/m3), and η is the dynamic viscosity of the medium (in Pa · sec).
In our experiments, we used CaCl2, MgCl2 · 6 H2O, and NaHCO3 salts of the “analytically pure” grade and CaCO3 of the “chemically pure” grade. The solutions were prepared by using bidistilled water.
Results and Discussion
The analysis of the obtained micrographs (Fig. 1) shows that, in the absence of inhibitors, the deposit has the form of elongated needle crystals 10–30 μm in size. The presence of the inhibitor in the solution leads to coarsening of the particles of deposit and the appearance of crystals with rhombic structure. The appearance of needle and rhombic crystal structures means that the deposit is polymorphic. Note that the introduction of inhibitor clearly leads to changes in the phase composition of the deposit.
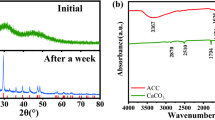
We also obtained X-ray phase diffraction patterns of the calcium-carbonate deposit both in the absence and in the presence of the inhibitor (Fig. 2). Their analysis demonstrates that calcite and aragonite are, in this case, the predominant phases.
The introduction of the inhibitor increases the amount of the calcite phase by 21%. Indeed, for C OK-2 = 0 mg/liter, we have
At the same time, for C OK-2 = 10 mg/liter, we have
It seems likely that the role of the main cause of changes in the morphology and phase composition of the deposit can be played by the adsorption of the inhibitor on the faces of the formed crystals of deposits. In order to study the process of adsorption on the surface of calcium carbonate, we preliminarily plotted the dependence of surface tension on the solution–air boundary on the concentration of the inhibitor (Fig. 3a). As a result of the adsorption of the inhibitor, its concentration in the solution decreases but the surface tension increases. By using the values of residual concentrations of the inhibitor obtained after adsorption according to formula (3), we determined its excess amounts on the surface and plotted the adsorption isotherm, which proves to be linear on the Langmuir coordinates (Fig. 3b).
This isotherm was used to determine the limiting adsorption of the inhibitor on the surface of calcium carbonate (Γ∞ = 2.6 · 10–7mole/m2) and the constant of adsorption equilibrium (B = 54.3m3/mole). The obtained values of the Gibbs free energy – 37.0 kJ/mole serve as an indication of strong adsorption of the inhibitor on the surface of calcium carbonate. Note that the OK-2 inhibitor was created on the basis of a cationic polyelectrolyte. Therefore, in the case of its adsorption on the surface of CaCO3, the charges of the crystals may change. To analyze this phenomenon, we measured the velocity of motion of the dispersed phase of calcium carbonate in aqueous media by the method of electrophoresis and computed the values of the electrokinetic potential of particles according to relation (4) (Table 1). It was established that, in the absence of inhibitors, the surfaces of the crystals of calcium carbonate have negative charges caused by the dissociation of surface compounds accompanied by the formation of HCO −3 , CO 23 , and OH− ions [12]. In the presence of the inhibitor, the surface of the deposit is recharged due to the adsorption of a positively charged polyelectrolyte, which may lead to the gluing of particles.
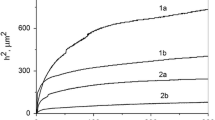
To estimate the influence of the inhibitor on the sedimentation of calcium-carbonate suspensions, we plotted the time dependences of the mass of deposits (Fig. 4a). These dependences show that the particles of calcium carbonate are polydispersed. In the presence of the OK-2 inhibitor, the mass of accumulated deposits is larger, which can be explained by the aggregation of crystals under the influence of adsorption of the inhibitor. This was promoted by a decrease in the absolute value of charge of the crystal surfaces. To confirm this assumption, we determined the values of the most probable radii of the crystal. They correspond to the maxima of differential distribution curves, i.e., of the dependences of mass distribution function F on the radii of particles (Fig. 4b).
The sedimentation results were processed under the assumption that the crystals mainly have the spherical shape. The analysis of the values of r H (see Table 1) shows that, in the presence of OK-2, the size of the crystals of calcium carbonate increases due to the coagulation of particles. The increase in the radii of calciumcarbonate particles leads to a faster precipitation of the dispersed phase, which is confirmed by the growth of the sedimentation constant.
As follows from the results of electron microscopy, the obtained crystals of calcium and magnesium carbonates are larger in the presence of the inhibitor than in its absence (see Fig. 1). Clearly, this is explained by the intensification of coagulation of particles. Moreover, in the presence of the inhibitor, the deposit visually looks less compact. Thus, the OK-2 inhibitor affects the structure of the deposit and increases the calcite phase. The deposit is better washed out with water, which leads to the decrease in the amount of scale on the surfaces of the equipment.
Conclusions
The action of the OK-2 inhibitor results in the increase in the sizes of crystals of deposits of calcium and magnesium carbonates and in the appearance of crystals with rhombic structure. It is shown that the deposit mainly consists of the calcite and aragonite phases. In the presence of the inhibitor, the content of calcite phase increases by 21%.
The adsorption of the inhibitor on the crystal surfaces is the main cause of changes in the morphology and phase composition of the deposit. The results of investigations of adsorption show that it can be adequately described by the Langmuir isotherm. Moreover, the high value of the Gibbs energy reveals good adsorption of the inhibitor on the surfaces of crystals of the deposit, which leads to the recharging of the CaCO3 surface and increases the sizes of particles of the dispersed phase. The increase in the radii of particles results in a faster precipitation of the dispersed phase. The deposits formed in the presence of OK-2 are easily washed out with water.
The present work was carried out within the framework of the State-Budget Project No. 0113U000016.
References
J. W. Morse, R. S. Arvidson, and A. Luttge, “Calcium carbonate formation and dissolution,” Chem. Rev., 107, No. 2, 342–381 (2007).
P. G. Shanmukha and V. K. Subramanian, “Polymorphism in CaCO3—effect of temperature under the influence of EDTA (di sodium salt),” Desalination, 297, 38–47 (2012).
F. Manoli and E. Dalas, “Calcium carbonate over growth on elastic substrate,” J. Cryst. Growth, 204, No. 3, 369–375 (1999).
C. Perdikouri, A. Kasioptas, T. Geisler, et al., “Experimental study of the aragonite to calcite transition in aqueous solution,” Geochim. Cosmochim. Acta, 75, No. 20, 6211–6224 (2011).
S. V. Ben’kovskii, S. M. Kruglyi, and S. K. Sekstanov, Technology of Soda Products [in Russian], Khimiya, Moscow (1972).
I. V. Shestak, P. D. Vorob’ev, D. V. Cherednichenko, et al., “Influence of polyacrylic acid and polyethylene glycol on the crystallization of calcium carbonates in the presence of magnesium ions,” Zh. Neorg. Khim., 56, No. 2, 213–217 (2011).
V. Obraztsov, E. Rubl’ova, O. Zhyhalova, and Z. Pakina, “A new inhibitor of corrosion and scaling with bactericide properties,” Fiz.-Khim. Mekh. Mater., Special Issue No. 10, 379–383 (2014).
V. V. Goncharuk, V. F. Vakulenko, Yu. O. Shvadchina, and L. M. Oleinik, “Influence of polyhexamethyleneguanidine on the process of coagulation cleaning of river waters,” Khim. Tekhnol. Vody, No. 5, 552–566 (2008).
Powder Diffraction File, Int. Center for Diffraction Data, Swarthmore, Penn., Sets 1–45.
S. S. Gorelik, Yu. A. Skakov, and L. N. Rastorguev, X-Ray Diffraction and Electron-Optic Analyses [in Russian], MISIS, Moscow (1994).
L. I. Mirkin, A Handbook of X-Ray Structural Analysis of Polycrystals [in Russian], Fiz.-Mat. Lit., Moscow (1961).
V. A. Poluéktova, N. A. Shapovalov, and L. N. Balyatinskaya, “Adsorption of oxyphenolfurfural oligomers on dispersed materials,” Fundam. Issled., No. 11, Part 6, 1470–1474 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 51, No. 5, pp. 56–61, September–October, 2015.
V. B. Obraztsov is Deceased.
Rights and permissions
About this article
Cite this article
Obraztsov, V.B., Rubl’ova, E.D. & Baskevych, O.S. Influence of the Scaling Inhibitor on the Phase Composition, Morphology, and Sedimentation Properties of CaCO3 Deposits. Mater Sci 51, 652–658 (2016). https://doi.org/10.1007/s11003-016-9887-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-016-9887-3