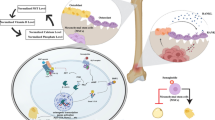
Abstract
Older women can develop osteoporosis caused by increasing osteoclast (OC) activity more than by the osteoblast (OB) activity that leads to bone fractures. Mitochondria can play an important role in energy production, calcium (Ca2+) sequestration and signaling, oxidative stress, apoptosis, and metabolism. Mitochondrial produced peptides (MPPs) include Mitochondrial-derived peptides (MDPs) and OB-activating peptide. MDPs which are secreted by mitochondria, have different roles and include humanin, and the novel mitochondrial open reading frame of the 12S rRNA-c, which is utilized to study insulin sensitivity. OB-activating peptide have been detected in the stomach, kidneys and ovaries and previously have shown a marked effect on elevating the expression of alkaline phosphatase and osteocalcin, which are considered OB differentiation markers. Many researchers have studied different mechanisms to induce osteogenesis, such as the Forkhead transcription factors of the O-class family, which maintain mature OBs through their antioxidant activities. Another approach involves the receptor activator molecules of the nuclear factor-κB ligand/osteoprotegerin (OPG) pathway, which depends on increasing the production of OPG to decrease OC activity. More research is needed to investigate the different pathways of MPPs in osteogenesis and their relationship to each other in treating various diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporosis (OP) is a common pathological condition characterized by a reduction in bone mass, demineralization, and alteration of the bone micro-architecture. OP is usually becoming worse with increasing the age and can lead to bone fractures, particularly in the wrist, femur, and spine. This condition has resulted from the reduction in bone mass either by an increase in resorption or by a decrease in ossification. During childhood, bone formation exceeds resorption. As the aging process occurs, resorption exceeds formation.
More than 9 million OP fractures occur per year globally, representing severe social, economic, public health, and clinical problems (Richards et al. 2012). The mortality rate due to hip fractures is currently estimated to be ~ 30% in the developed countries during the first year after fracture and ~ 40% in the second year (Downey et al. 2019). Moreover, fragility fractures are a leading cause of disability and mortality globally (Veronese et al. 2020).
Bone remodeling, which continues throughout the life span, is mediated by the activities of osteoclasts (OCs) and osteoblasts (OBs) (Feng and McDonald 2011). During this process, the imbalance between formation and resorption of bone leads to the loss of bone, which adversely affects bone architecture and strength (Song et al. 2015). Recent research has referred to the key role of the osteoprotegerin/receptor activator molecules of the nuclear factor-κB ligand (OPG/RANKL) (Pacifico et al. 2018) in the bone-remodeling process. At present, OPG/RANKL is considered a pivotal coupling factor between OC and OB. The main function of OPG and RANKL, which are produced from OBs and stromal cells of the bone marrow, is to prevent OC differentiation and, in turn, inhibit bone resorption activity.
An advanced study (Ikeda and Takeshita 2014) has posited that the complement component 3a (C3a) and collagen triple helix repeat containing 1 (Cthrc1) act as a channel between OC and OB. C3a is secreted from the mature OCs (mOCs) and activates the process of osteoblastogenesis. However, Cthrc1 is derived from active mOCs during bone resorption and activates OBs’ differentiation. Further research has demonstrated the regulating role of different novel transcription factors (TCFs) such as nuclear factor I-C (Lee et al. 2014), netrin-4 (Enoki et al. 2014), and omentin-1 (Yin et al. 2017) in differentiation and proliferation of OBs. Interestingly, these novel TCFs and regulators can be used in the therapeutic approach to OP.
In general, OP’s therapeutic approach is based on the inhibition of the occurrence of fragility fractures by stimulating new bone formation or reducing the rate of bone resorption. Anti-OP drugs can be used with careful supplementation with Ca2+ or vitamin D to increase their effectiveness in preventing fractures (Delmas et al. 1997; Diab and Watts 2014). Therefore, clinicians face the challenge of prescribing the best treatment option from those available and of choosing the “best drug” for everyone with OP.
OCs and Bone Resorption
Myeloid progenitor cells are the precursors of red blood cells, platelets, granulocytes (polymorphonuclear leukocytes [PMNs]: neutrophils, eosinophils, and basophils), monocyte-macrophages, dendritic cells (DCs), and mast cells and osteoclasts (Molawi and Sieweke 2013). OCs are multinucleated cells formed via fusion of the precursors of hematopoietic myeloid in the bone marrow near the bone surface. OCs can be identified in sections stained routinely with hematoxylin and eosin, as well as in the immunohistochemical sections stained for tartrate-resistant acid phosphatase (TRAP). Note that normal bone resorption is not related to TRAP expression, whereas TRAP levels in serum have shown a positive correlation with the bone resorption levels (Wang et al. 2019). The differentiation of OC from OC precursor to fully activated multinucleated OC depends primarily on RANKL, the permissive role of macrophage-colony stimulating factor (M-CSF), and a member of the tumor necrosis factor (TNF) family (Rachner et al. 2011).
OC precursors are derived in the bone marrow and then move into blood circulation via sphingosine-1 phosphate (S1P) (Meshcheryakova et al. 2017). S1P is secreted from red blood cells by large amounts and moves to the resorption lacunae of the bone marrow by RANKL (Boyce 2013), which is produced from OB and immune cells. Moreover, RANKL is produced in bone multicellular units in the bone marrow through OB and stromal cells (Sims and Martin 2015), T-lymphocytes (Boyce 2013), and osteocytes (Xiong et al. 2015). After RANKL induces RANK activation, several enzymes and key regulatory TCFs are generated to promote OCs’ proliferation, differentiation, multinucleation, activation, and survival (Rachner et al. 2011), resulting in the resorption of bone.
Osteocytes are the most abundant cell type in bone, which act as matrix-forming OBs to form bone surfaces. Normally when OBs complete the matrix-forming task, most undergo apoptosis (Blair et al. 2017), some become embedded within osteoid (the uncalcified matrix), and the remaining stay on the bone surface. When osteoid mineralization begins, the embedded OBs remains “trapped” and form osteocytes that are released in the following remodeling cycle. These sequenced events allow osteocytes to detect areas of damaged bone that need removal (Dallas et al. 2013).
OCs can produce the secreted form of RANKL; this osteocyte-derived RANKL is needed for the normal remodeling process of bone in adults (Xiong et al. 2015). Moreover, M-CSF stimulates OC activities and provides them a long survival (lifespan = ∼30 days) within the resorption lacunae. Interestingly, humans and transgenic mice with a loss of RANKL, RANK, or M-CSF have a deficiency in mOCs (Nagy and Penninger 2015) and then develop OP (Boyce 2013) due to failure to clear the mineralized matrix from the medulla of the vertebrae and the long bones during the embryonic life. The osteoporotic bones are more fragile compared with normal bones; their sclerotic picture (Teti and Econs 2017) is due to the nature of being composed of weaker woven rather than of strong lamellar bone.
OPG is a natural RANKL antagonist (Schieferdecker et al. 2014) and is characterized by its jellyfish-like shape and adhesion molecules such as integrins. OCs adhere to the bone surface to form a sealing zone and provide a highly acidic enriched microenvironment (Rachner et al. 2011).
OBs and Bone Formation
OBs, the bone-forming cells, are cuboidal cells that are embedded along the surface of the bone, representing 4%–6% of the total number of resident bone cells (Capulli et al. 2014). OBs, which originate from mesenchymal stem cells, secrete both Type I collagen (constituting 90% of the protein in bone) and bone morphogenetic proteins (BMPs) that form the initial osteoid bone (Henry and Bordoni 2020). These BMPs contain Ca2+-binding proteins such as osteonectin (ON) and osteocalcin (OCN), thrombospondins, multi-adhesive glycoproteins such as bone sialoproteins (BSP-1 [osteopontin; OPN] and BSP-2), alkaline phosphatase (ALP), and different proteoglycans. Serum levels of OCN and ALP can be used as a marker for OB activity (Szulc and Bauer 2013).
Bone formation rate can estimate a thorough evaluation of the speed and effectiveness of cell precursors from their differentiation to mature OBs (mOBs) (Rachner et al. 2011). These processes are stimulated by intermittent pulses of parathyroid hormone (PTH) and vitamin D (Rachner et al. 2011). OB differentiation requires the expression of specific genes in programmed events such as members of the Wingless (Wnt) pathways, BMPs (Houschyar et al. 2019), distal-less homeobox 5 (Dlx5), runt-related TCF 2 (Runx2), and osterix (Osx) (Capulli et al. 2014). Moreover, Runx2 plays a pivotal role in the upregulation of OB-related genes, such as ColIA1, ALP, BSP, BGLAP, and OCN (Fakhry et al. 2013).
The establishment of Runx2 and ColIA1 expression during OB differentiation begins the proliferating phase during which OB progenitors stimulate ALP activity and act as pre-OBs (Capulli et al. 2014). Following this, pre-OBs mature to OBs, becoming large and cuboidal, and begin to secret BMPs such as OCN, BSP I/II, and collagen Type I (Capulli et al. 2014). Recent investigations have stated that fibroblast growth factor (FGF), microRNAs, and connexin 43 have a major role in OB differentiation (Buo and Stains 2014). Mice with a deletion of FGF-2 showed reduced bone mass and an abundance of adipocytes within the bone marrow (Gupta et al. 2010). Moreover, microRNAs can contribute to gene expression and differentiation of OBs (Hassan et al. 2012). Several miRNAs have been found to limit osteoblast differentiation, which is important for bone remodeling regulation. This physiological regulation elicited by miRNAs could be crucial for balancing the processes of bone production and resorption (Vimalraj and Selvamurugan 2013). The expression of miR-206, for example, is correlated to osteoblast differentiation. However, Runx2, a bone-specific transcription factor, controls the expression of numerous genes and is necessary for osteoblast development. On the other hands, there are miRNAs that control osteoblast differentiation in a positive way, for example, by suppressing ERB1 (TOB1) and sclerostin (SOST), miR-218 has been found to help osteoblasts differentiate into the final stage of generating mineralized tissue (Li et al. 2009). Furthermore, one study (Flenniken et al. 2005) has stated that mutation in the connexin 43 encoding gene impairs OB differentiation.
The production of the bone matrix from OBs takes place in two steps: (1) organic matrix deposition, wherein the OBs produce collagen proteins (predominantly Type I collagen), non-collagen proteins (BSP II, OCN, OPN, and ON), and proteoglycan (decorin and biglycan); and (2) mineralization, which occurs through the fibrillar and vesicular phases (Yoshiko et al. 2007). The vesicular phase is characterized by the release of matrix vesicles from the domain of OBs’ apical membrane to the newly formed bone matrix (Bottini et al. 2018), whereas the fibrillar phase is characterized by over aggregation of phosphate and Ca2+ ions within the matrix vesicles, resulting in rupture (Hasegawa et al. 2017).
In contrast, bone formation can be repressed by supplementing exogenous glucocorticoids or being less activated in some elderly individuals. Moreover, OBs can produce several non-collagenous proteins, such as ON, OPN, OCN, and an extracellular matrix containing Type-1 collagen at the resorption lacunae sites (Rachner et al. 2011).
Common Mechanisms in the Possible OP Treatment
FoxO, Oxidative Stress, and Aging
Both aging and estrogen deficiency up-regulates the generation of reactive oxygen species (ROS) (Manolagas 2008). Continuous generation of ROS during metabolism can result in lipid peroxidation, with subsequent DNA and protein damage. Moreover, a low concentration of ROS can act as signaling molecules for the differentiation and proliferation of cells (Giorgio et al. 2007). Research has shown the relationship between ROS generation and age- or gonadectomy-associated bone loss (Almeida et al. 2010). Furthermore, in murine models, osteoporotic features were related to premature aging and oxidative damage (Tyner et al. 2002).
Likewise, antioxidants can attenuate osteoclastogenesis, apoptosis of osteocytes and OBs, and gonadectomy-related bone loss (Almeida et al. 2010). In general, cells exhibit several defense mechanisms against ROS, among them the mechanism associated with the Forkhead TCFs of the O class (FoxO) family of TCFs (including four members in mammals: FoxO1/FKHR, FoxO3/FKHRL1, FoxO4/AFX, and FoxO6) (Tzivion et al. 2011). Where, FoxO1, -3, and -4 have been expressed in adults and developing tissues, FoxO6 is specific to the developing brain. Akt-mediated phosphorylation can play a role in the prevention of FoxO-mediated transcription (Lin et al. 2020).
In contrast, ROS can activate FoxO transcription via promotion of FoxO post-translational modifications, including phosphorylation of the amino-acid residues remaining after Akt phosphorylated acetylation and ubiquitylation (Calnan and Brunet 2008). This fact suggests the FoxO major protective role against ROS. The FoxO defense mechanism could be exerted via regulation of the transcription of antioxidant enzymes such as catalase and MnSOD, in addition to the regulation of genes contributing to DNA repair and the cell cycle (Salih and Brunet 2008).
Interestingly, a recent study on mice with combined deletion of FoxO1, -3, and -4 or deletion of FoxO3 has demonstrated the antioxidant activity of FoxO through its indispensable role in the survival of hematopoietic stem cell and erythropoiesis due to the attenuation of ROS (Marinkovic et al. 2007). Moreover, ß-catenin, a factor essential for the differentiation of OB (Saidak et al. 2015), is required for ROS-activated FoxO (Ma et al. 2020). In a trial conducted on the OB cell line, ROS stimulated β-catenin − FoxO association and activation of FoxO-induced transcription instead of β-catenin/T-cell specific TCF-induced transcription and differentiation of OB (Almeida et al. 2010) (Fig. 1).
Role of FoxO in osteoprogenitor cells. Reactive oxygen species (ROS) stimulates FoxO-mediated transcription and binding to β-catenin, which convert the limited β-catenin from TCF to FoxO-mediated transcription, resulting in osteoblastogenesis. However, FoxO1 enhances the transcription of runt-related transcription factor2 (Runx2) or alkaline phosphatase (ALP), leading to increased osteoblastogenesis
Moreover, FoxO can inactivate the transcription of PPARγ, which acts as a strong inactivator for osteoblastogenesis (Ma et al. 2020). In general, these findings strongly suggest the role of FoxO physiologic maintenance of mOBs through its antioxidant activities. Furthermore, FoxO can regulate the production of new OBs via modulation of their differentiation or proliferation by modulating β-catenin or PPARγ.
Calcium-Sensing Receptor (CaSR) and Bone Homeostasis
Bone remodeling, a physiological process important for skeletal integrity maintenance and Ca2+ homeostasis, occurs within BMUs and is controlled by OBs that produce the extracellular matrix and OCs that resorbs old bone (Owen and Reilly 2018). Within the BMU, the extracellular Ca2+ ([Ca2+]e) should be organized around 0.5–1.5 mm during bone production and mineralization of the extracellular matrix (Lin et al. 2020) and increased up to 2 mm or more during bone resorption (Berger et al. 2001). Several in vitro studies have stated that OBs respond to alteration in (Ca2+)e (Dvorak and Riccardi 2004). Increased concentrations of (Ca2+)e can affect many differentiation stages of OBs and enhance their maturation, proliferation, cell chemotaxis, gene expression, and mineralization of the extracellular matrix (Tang et al. 2019); therefore, (Ca2+)e can control the remodeling process.
The CaSR is a G protein-coupled receptor that responds to fluctuation in (Ca2+)e (Young et al. 2015). The CaSR has an important physiological role in kidney cells and the parathyroid gland, regulating the excretion of Ca2+ and secretion of PTH (Kallay 2018). Several trials on mice with deletion of the full-length CaSR gene have confirmed the role of the kidney and parathyroid CaSRs in the homeostasis of Ca2+ (Hendy and Canaff 2016). Recent studies have provided strong proof that CaSR is expressed in mOCs, OC precursors, OBs (Dvorak et al. 2004), and OB lineage (Dvorak et al. 2004), and it also contributes to OB survival and differentiation and to promoting bone formation and mineralization (Dvorak-Ewell et al. 2011). Moreover, CaSR plays a pivotal role in osteoclastogenesis; however, high Ca2+ concentrations prevent OC stimulation, resulting in their apoptosis (Sharan et al. 2008). In general, this evidence has suggested the role of CaSR in skeletal homeostasis via sensing alterations of Ca2+ in the skeletal microenvironment (Al‐Dujaili et al. 2016) (Fig. 2).
OPG/RANKL System and Bone
The OPG/RANKL system consists of three components: a ligand, RANKL, a cellular receptor; RANK, a soluble decoy receptor; and OPG. RANKL binding to RANK is required to differentiate OC precursors to mature cells, for the survival of OCs, and for the activation of mOCs to initiate bone resorption (Park et al. 2017). OPG, produced by the OBs and marrow stromal cells, binds to RANKL, blocking its biological activity by preventing its association with RANK. As a result, OPG inhibits RANKL-induced bone resorption (Infante et al. 2019).
Osteocytes and accessory bone marrow cells produce RANKL; this production attracts OC precursors from the bloodstream side toward the resorption lacunae (Arai et al. 2012). Moreover, other TCFs such as PU.1 and microphthalmia-induced TCF (Kawakami and Fisher 2017), Wnt5a (Maeda et al. 2012), IL-34 (Chen et al. 2011), and TNF (Boyce 2013) induce the expression of RANK by OC precursors.
OPG is also produced from the bone marrow accessory cells; its primary function is to limit OC formation with subsequent bone destruction. The concentration of OPG can determine the bone resorption level (Boyce 2013). Interestingly, the U.S. Food and Drug Administration has approved the human monoclonal antibody to RANKL to treat various osteolytic disorders such as OP and multiple myeloma (Cosman 2018).
In contrast, RANK is produced from bone marrow immune cells and an increased number of cell types such as dendritic cells, which are stimulated by RANKL produced from mammary gland cells and T-cells (Nagy and Penninger 2015). Moreover, RANK is produced from human prostate and breast cancers (Nagy and Penninger 2015), and it is thought that RANKL/RANK are responsible for the metastasis of breast cancer to the bones (Sobacchi et al. 2013). In humans, the RANK encoding gene (TNFRSF11A) mutation is related to juvenile Paget’s disease and familial expansile osteolysis with an increased risk of fractures (Coudert et al. 2015).
Vital Functions of Mitochondria
Mitochondria have been considered the cell’s powerhouse because they produce most of the cellular ATP. The Krebs cycle happens within the mitochondria, causing an electrochemical gradient utilized in ATP production (Salway 2004). Furthermore, mitochondria have a vital role in apoptosis, oxidative stress, and Ca2+ signaling and sequestration (Drago et al. 2011).
Mitochondria are pivotal in the intrinsic apoptotic pathway, which is stimulated by the cellular damage of some cells and relayed on the mitochondria (Kantari and Walczak 2011). Furthermore, apoptosis is activated directly by many nucleus-encoded mitochondrial proteins when they are sent out from the mitochondria into the nucleus or cytosol. Moreover, mitochondria are the biggest dynamo of free radicals in cells (Balaban et al. 2005).
In cells, the primary source of oxidative stress is free radicals, which can damage and oxidize DNA, lipids, and proteins. In addition to the physiological role of some free radicals, elevated oxidative stress can be accompanied by various diseases, although an advantageous influence of temporary oxidative stress has been found (Ristow and Zarse 2010; Ristow et al. 2009).
Mitochondria have a comprehensive organization for simplifying the transport of Ca2+ through their inner membrane, which has an important role in hormone metabolism, ATP production, and cytoplasmic Ca2+ signaling. Derestriction of the mitochondrial Ca2+ homeostasis can deactivate the function of intra-mitochondrial enzymes and cause cell mortality. Mitochondrial Ca2+ release and uptake vary from other membrane-bound organelles in that this Ca2+ release utilizes gated channels and H+/Ca2+ or Na+ exchangers, and Ca2+ uptake is not conditional on ATP (Drago et al. 2011).
Mitochondria has a pivotal role in the keeping of osteoclast activity (Zhang et al. 2018). The ATP supply from the mitochondria are important for osteoclast maturation and differentiation (Aoki et al. 2020). ATP molecules are generated mainly by mitochondria but they produce ROS as well as an access product of ATP generation, which are damaging to cells (Liemburg-Apers et al. 2015). The activation of osteoclasts is by the interaction of RANKL with RANK receptor which is expressed on osteoclast precursors. This operation also produces oxidative stress through ROS (Lee et al. 2005). Oxidative stress might enhance the osteoclast differentiation ROS (Lee et al. 2005), extra cumulation of intracellular ROS enhances cell death by oxidation of intracellular DNA, lipids, and proteins (Liemburg-Apers et al. 2015), and in the late stages of osteoclast maturation, some results revealed a significant lowering in mitochondrial membrane potential differentiation (Aoki et al. 2020).
The theories of bone loss with age pathogenesis are elevating endogenous glucocorticoids secretion (Chiodini et al. 2007; O’brien et al. 2004) and lowering of sex hormones levels (Aitken et al. 1972; Baran et al. 1978; Fink et al. 2006; Lindsay et al. 1976). Intracellular alterations which develop with age such as, accumulating mitochondrial DNA (mtDNA) mutations, has a marked role in the declining BMD levels due to failure of bone homeostasis. The only DNA polymerase detected in mitochondria is mtDNA polymerase gamma (Polg) which is responsible for repair and replication and of mtDNA inside all cell types (Hance et al. 2005). Another proof for mitochondrial dysfunction as a possible player in osteoporosis pathogenesis is revealed in mice with a mitochondrial transcription factor A (TFAM) knockout specific to osteoclasts, the result is elevated resorption in comparison to normal osteoclasts (Miyazaki et al. 2012). The knockdown of superoxide dismutase (Sod2), an enzyme which prevents mitochondrial oxidative stress, make osteoporosis (Kobayashi et al. 2015). During aging process, the accumulation of somatic mtDNA mutations is in post mitotic and mitotic tissue, and somatic stem cell precursors (Taylor and Turnbull 2005). An investigation has revealed that the accumulation of mitochondrial defects in osteoclast at a hurried rate in PolgAmut/mut mice more than aged wild mice (Dobson et al. 2020).
During osteoblastogenesis, intracellular ATP content and oxygen consumption rate are markedly upregulated suggesting inclusion of mitochondria in lineage specification of MSCs (Shares et al. 2018). For more details about the mechanisms of modulation of osteogenic differentiation of MSCs by mitochondrial bioenergetics, researchers have detected the enhancement of canonical Wnt/β-catenin pathway which is a main organizer of bone formation by mitochondria (Scholtysek et al. 2013; Shares et al. 2018). For protection against endogenous ROS accumulation, MSCs differentiate to activate a highly efficient antioxidant defense system (Chen et al. 2008). In the mitochondria of MSCs, there is manganese superoxide dismutase (MnSOD or SOD2) which is the main antioxidant defense system. In MSCs, the MnSOD knockdown leads to impaired osteogenic differentiation (Gao et al. 2018), and it was reported osteoporosis in mice have mesenchymal conditional deficiency of MnSOD (Treiber et al. 2011).
At the ultrastructural level, the mitochondria morphological change is the main feature of osteogenic differentiation, which are characterized by mitochondrial elongation and enlargement, activation-related network formation and volume elevation (Forni et al. 2016; Palomäki et al. 2013; Shum et al. 2016). It was reported the increasing of Mitofilin through MSC-mediated osteoblastogenesis (Lv et al. 2018). In MSCs, investigations have suggested the enhancement of mitochondrial biogenesis during osteoblastogenesis with elevated protein subunits of the respiratory enzymes, increased mtDNA copy number, and upregulated crucial genes like TFAM (An et al. 2010; Chen et al. 2008). Furthermore, enhancement of MSCs osteoblastogenesis by TFAM overexpression, while MSC osteogenesis has been inhibited by treatment with mitochondrial biogenesis suppressor such as, zidovudine (An et al. 2010). Moreover, the abundance of β-catenin has been upregulated by elevated mitochondrial biogenesis, so, enhancing of Wnt-mediated osteogenic differentiation of MSCs (An et al. 2010). Further investigations are required to reveal the possible inclusion of mitochondrial mitophagy and fission to increase the knowledge about the mitochondrial organizing mechanisms related to MSC-mediated osteoblastogenesis.
In HSC-derived osteoclastogenesis, the mitochondrial significance has been gradually increased. Mature osteoclasts have many mitochondria and powerful mitochondrial respiratory activity at the end of osteoclast differentiation (Baba et al. 2018; Nishikawa et al. 2015; Zeng et al. 2015). Moreover, deletion of mitochondrial complex I subunit Ndufs4 leading to mitochondrial respiration dysfunction which inhibits osteoclastogenesis resulting in osteopetrosis (Jin et al. 2014). Suppression of mTOR signaling by genetic deletion of mTOR or Torin1 markedly inhibits osteoclastogenesis, indicating that mTOR signaling enhances the osteoclasts formation (Indo et al. 2013). Furthermore, AMPK activation blocks osteoclastogenesis, suggesting a negative role of AMPK in formation of osteoclast (Indo et al. 2013). Further studies are needed to determine the participation of mitochondria in downstream signaling of AMPK and mTOR in osteoclastogenesis.
Mitochondria share in lineage specification and stem cell pluripotency based on signaling and metabolic modulations (Shadel and Horvath 2015). This organization is significant and prominent in the skeletal system of HSCs and MSCs, which has a pivotal role the homeostatic maintenance, development, and regenerative repair of bone (Hsu et al. 2016; Snoeck 2017). Therefore, the therapeutic handlings of mitochondria are studied to oppose bone pathologies and aging and to assist in bone healing (Coleman et al. 2018; Lv et al. 2018). Some key matters still to be studied in related to general identifications of disease-associated and health-associated and mitochondria-targeted intervention in translational investigations are highly required. Interestingly, nanoparticles designed for mitochondria-targeted drug delivery systems, gene editing technologies for mitochondria containing mitochondrially targeted zinc-finger nucleases (mtZFN) and mitochondrial-targeted transcription activator-like effector nucleases (mitoTALEN) have supplied high therapeutic trends (Bacman et al. 2018; Gammage et al. 2018; Wongrakpanich et al. 2014).
Short Anabolic Peptides Used in OP Treatment
Osteoporosis is an age-related skeletal condition that causes bone fragility and an increased frequency of fractures, both of which are associated with high expenses and significant morbidity and mortality. The majority of osteoporosis drugs work by lowering osteoclastic activity and hence lowering bone resorption. The focus has recently shifted to bone-anabolic agents. Such substances can boost bone mass and strength, potentially reversing structural damage. Because of their high target binding specificity, which translates to powerful activity with few side effects, short peptides are an important alternative for the development of novel bone-anabolic drugs (Fig. 3).
OB-Activating Peptide (OBAP)
OBAP, a new 24-amino-acid peptide (Fig. 4) that forms a tight hairpin structure, with one leg being an alpha helix, has no Cys, so it is not forming disulfide bonds. OBAP has only five hydrophobic amino acids that are dispersed (Fig. 1) and induces an increase in many OB differentiation markers, such as OCN and ALP, as reported by Fukushima et al. (2010) in Sprague–Dawley rats. Furthermore, the recovery rate against OP caused by gastrectomy was markedly elevated by OBAP supplementation (Fukushima et al. 2010). The sequence of amino acids of OBAP encodes the C-terminal side domain of NADH dehydrogenase (ubiquinone) flavoprotein 3 (Ndufv3) transcript variant 2, which is localized at the mitochondrial membrane and has been indicated to be a preprotein of OBAP (de Coo et al. 1997; Kitahara et al. 1996; Pilkington and Walker 1989; Runswick et al. 1989).
The function of Ndufv3 is unknown, but OBAP has been found to be efficient in curing OP stimulated by gastrectomy. Therefore, OBAP is considered a strong candidate against OP (Fukushima et al. 2010). OBAP has been found to be distributed in the parietal cells of the rat stomach (Noreldin et al. 2016). OP is stimulated by gastrectomy in young females and males or ovariectomy in adult females (Andersson et al. 2002). A lack of estrogen explains the influence of ovariectomy. However, the mechanism by which gastroectomy stimulates OP is still unknown.
Parietal cells have been found to secrete estrogen, which has a pivotal role in OP (Ueyama et al. 2002). Gastrin induces parietal cells to secret hydrochloric acid (Kidd et al. 2007), and OP happens in mice defective in the gastrin receptor due to the negative influence on acid secreted by the parietal cells (Schinke et al. 2009). One study has mentioned that gastrin stimulates a hypocalcemic agent from the rat stomach, creating the possibility that it might be OBAP (Persson et al. 1989). Fukushima et al. (2010) have reported that OBAP was efficient against OP in a gastroectomized model. Moreover, OBAP is secreted by the parietal cells (Noreldin et al. 2016). Therefore, parietal cells can have a vital role in maintaining a normal level of Ca2+ balance in the blood, which might be a cause for OP from gastrectomy.
OBAP is localized at the distal convoluted tubule (DCT) and connecting tubule (CT) (Noreldin et al. 2018); calbindin 28 kDa transports Ca2+ actively in these sites (Bindels et al. 1991) under the effects of parathyroid and calcitonin hormones (Hemmingsen 2000). This finding suggests the role of OBAP in Ca2+ transportation. Therefore, OBAP can be a sharing factor in the passage of Ca2+ into the cells of the CT and DCT, where active Ca2+ reabsorption occurs through the transcellular route.
The CaSR is highly expressed in the basolateral membrane and apical surface of the Henle loop (HL) and DCT of the nephrons (Graca et al. 2016) (Fig. 5) and in the kidney organizes (Ca2+)e concentrations and regulates Ca2+ reabsorption (Vezzoli et al. 2009). Moreover, increased (Ca2+)e levels stimulate the CaSR of the DCT, which induce (calcitonin and gastrin) and decrease secretion (parathyroid and insulin hormones) (Riccardi 1999). Furthermore, CaSR is expressed in the mOB precursors and OBs (Dvorak et al. 2004) and is required to improve bone mineralization and formation (Chattopadhyay et al. 2004). Fukushima et al. (2010) have mentioned OBAP’s stimulation and elevation in OB differentiation, and we have detected the distribution of OBAP in the HL and DCT overlapping areas where CaSR previously has been shown to be expressed (Graca et al. 2016), indicating a possible direct role of OBAP in Ca2+ regulation.
The ovarian interstitial endocrine cells (IC) revealed the highest distribution of OBAP, followed by the oocytes of mature Graafian follicles (MGF) and the mature corpus luteum. There was a powerful negative correlation between OBAP and aromatase. Strong positive correlations with receptor activator of nuclear factor-κB (except IC), 3β-hydroxysteroid dehydrogenase (except MGF), and calmodulin (except MGF and IC) were detected. Moreover, OBAP revealed a partial positive correlation between estrogen receptor and progesterone receptor in the corpus luteum and with IC and calbindin in the MGF (Noreldin et al. 2021).
OBAP could have other advantages as well. More studies of the OBAP receptor types localized at the OB and the distribution of OBAP receptors in all organs are necessary to understand further the different mechanisms of OBAP, which can help determine new applications and roles.
Humanin
The first discovered MDP, humanin (HN), found by Hashimoto et al. (2001), is interesting. HN was revealed by screening protective proteins against amyloid-β, which is a potential cause of Alzheimer’s disease, and recently has been shown to have both the capability to connect IGF binding protein 3 (IGFBP3) and an anti-apoptotic influence (Ikonen et al. 2003), and its analogs can influence metabolism, such as lowering visceral fat and body weight gain and elevating glucose-induced insulin release (Gong et al. 2015; Kim et al. 2017).
HN is a small amino-acid peptide (NH2-Met-Ala-Pro-Arg-Gly-Phe-Ser-Cys-Leu-Leu-Leu-Leu-Thr-Ser-Glu-Ile-Asp-Leu-Pro-Val-Lys-Arg-Arg-Ala-COOH) (Fig. 6) encoded from a 75- bp ORF sequence within the 1567 bp cDNA, which yields either a 21- or 24-amino-acid polypeptide depending on the location of the translation machinery (Gong et al. 2014). HN’s biological activities can act as a signal peptide and do not require a signal peptide for secretion (Yamagishi et al. 2003). HN is the first novel peptide detected within the mitochondrial genome since 1981 (Anderson et al. 1981).
HN relieved renal microvascular remodeling, apoptosis, and inflammation in the early stage of kidney disease in hypercholesterolemic mice. This peptide can act as a new treatment to attenuate kidney damage at the beginning of atherosclerosis (Zhang et al. 2012).
Kang et al. (2019) have shown that HN suppresses RANKL-stimulated OC formation and decreases the expression of the genes included in osteoclastogenesis, involving OC-associated receptor, nuclear factor of activated T-cells cytoplasmic 1, TRAP, and cathepsin K. Furthermore, HN elevated the levels of phosphorylated adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK); compound C, an AMPK inhibitor, recovered HN-stimulated OC differentiation. In addition, Kang et al. (2019) have determined that HN markedly lowers the levels of RANKL-stimulated ROS in BMMs. Therefore, in osteoclastogenesis, HN acts as a suppressor of bone disorders by AMPK activation. An association among life span, HN levels, and growth hormone/IGF-1 (GH/IGF axis) has been detected utilizing different mouse models having mutations in the GH/IGF axis (Gong et al. 2014).
HN is distributed within all stages of maturation in the rat testis (Moretti et al. 2010) and interacts with insulin-like growth factor 1 (IGF-1) to stimulate DNA synthesis that reflects on the steroidogenesis and enhanced Leydig cells’ survival in culture. This result suggests HN’s role as a novel testicular anti-apoptotic factor (Colon et al. 2006).
HN has neuroprotective influences; it is located in many tissues having an elevated metabolic rate, and its expression lowers with age. In physiological conditions of the brain, HN has been detected in the glial cells. Zárate et al. (2019) have mentioned that surgical menopause stimulates hippocampal mitochondrial dysfunction that imitates an aging phenotype. Humanin expression was lower in the hippocampus of ovariectomized rats and its immunoreactivity colocalized with astroglial markers. Moreover, ovarian hormones increased HN’s intracellular content in the astrocytes. Therefore, the relationship between ovarian hormones and HN can provide cues for understanding its role in the aging process.
Many studies have accepted the positive influences of HN in various domains. Thummasorn et al. have affirmed that HN therapy can treat ischemia–reperfusion injury and indicated that this might be because of a reduction in ROS generation (Thummasorn et al. 2016). Two other articles have revealed the significance of HN in neurocognition by revealing that it can avert diazepam-induced memory dysfunction and acts as an anxiolytic agent (Murakami et al. 2017). Gidlund et al. (2016) have detected elevated muscle HN levels during strength training as compared with aerobic exercise (Nordic walking) and control, but circulating levels were not influenced, concluding that this change could be because of HN’s role in glucose metabolism. Recently, for the first time, von Walden et al. (2021) discovered that acute endurance exercise (EE) significantly increased circulation levels of HN but not resistance exercise (RE); nonetheless, MOTS-C levels showed a trend to rise after EE. These findings suggest that whereas plasma MDP levels are unrelated to fitness, acute EE is. In addition, the findings of Woodhead et al. (2020) revealed that humanin is an exercise-sensitive mitochondrial peptide, that acute exercise-induced humanin responses in muscle are non-transcriptionally controlled, and that this may partially explain the observed increase in plasma concentrations. As the first MDP detected, HN has been the most inclusively studied, and both functional and structural features have been revealed. Its function as an anti-apoptotic peptide has been thoroughly studied, but its function in cognition is still being investigated.
MOTS-c
The detection of a short open reading frame (sORF) in the mitochondrial DNA (mtDNA) that encodes a signaling peptide, HN, indicates the possible existence of further sORFs in the mtDNA (Lee et al. 2015). Another sORF within the mitochondrial 12S rRNA encoding a 16-amino-acid peptide (NH2-Met-Arg-Trp-Gln-Glu-Met-Gly-Tyr-Ile-Phe-Tyr-Pro-Arg-Lys-Leu-Arg-COOH) (Fig. 7) nominated MOTS-c (mitochondrial open reading frame of the 12S rRNA-c) has been discovered (Lee et al. 2016).
Moreover, MOTS-c has been shown to enhance glucose metabolism via the skeletal muscle and has important functions in organizing obesity and diabetes, representing a novel mitochondrial-signaling mechanism to regulate the metabolism between and within cells (Kim et al. 2017; Lee et al. 2016). MOTS-c organizes both metabolic homeostasis and insulin sensitivity via AMPK, stimulates AMPK in HEK293 cells, and elevates 5-aminoimidazole-4-carboxamide ribonucleotide levels. Lowering this AMPK stimulation by siRNA or chemical compounds cancels the induced glucose-stimulated glycolytic response. In vivo MOTS-c injection markedly elevated insulin-stimulated glucose-discarding rate and glucose disposal in clamp studies and a glucose tolerance test. Furthermore, MOTS‐c prohibits insulin resistance and high-fat diet (HFD)-stimulated obesity in CD-1 mice and prohibited HFD-stimulated obesity independent of caloric intake in C57BL/6 J mice (Lee et al. 2016).
More investigations on whether MDPs modify mitochondrial uncoupling will provide a greater understanding of the reasons for this elevation in oxygen-consuming rate. Because mitochondrial oxygen consuming is linked to ATP production, the elevation in energy production and its TCA cycle metabolites can improve mitochondrial metabolism. The circulating levels of MOTS-c decline with age, similar to HN (Lee et al. 2016), suggesting that they are possible aging organizers. As these peptides are derived from the mitochondrial genome, they are included in metabolism and apoptosis; however, there is some overlapping function. Clearly, each MDP also has a unique signaling signature resulting in an individual response. For instance, Kumagai et al. (2021) proposed that MOTS-c could be a possible therapeutic for insulin resistance-induced skeletal muscular atrophy as well as other muscle wasting phenotypes such as sarcopenia by lowering myostatin expression. MOTS-c impact on myostatin expression is mediated by the CK2-PTEN-mTORC2-AKT-FOXO1 pathways. However, (Kong et al. 2021) found that MOTS-c alleviated the development of hyperglycemia and reduced islet-infiltrating immune cells by modulating TCR/mTOR complex 1 (mTORC1) signaling. Future investigations are needed to determine these signaling pathways.
MOTS-c significantly suppressed RANKL-stimulated OC differentiation (Ming et al. 2016). Moreover, MOTS-c can suppress OP via the AMPK-dependent suppression of osteoclastogenesis (Ming et al. 2016). Yan et al. (2019) have studied the influences of MOTS-c on bone metabolism and immune cells, revealing in an ultra-high molecular weight polyethylene (UHMWPE) particle-stimulated osteolysis mouse model that MOTS-c treated inflammation and bone erosion. MOTS-c elevated the OPG/RANKL ratio in osteocytes, resulting in the suppression of osteoclastogenesis (Fig. 8). In primary bone marrow macrophages (BMMs), MOTS-c alleviated NF-κB and STAT1 phosphorylation stimulated by UHMWPE particles. Enhancing ROS production or suppressing peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1α (PGC-1α) by AMPK repression prevented these anti-inflammatory influences. Therefore, MOTS-c can suppress osteoclastogenesis by organizing osteocyte OPG/RANKL secretion and inhibit inflammation by limiting the STAT1 and NF-κB pathways. Furthermore, MOTS-c influences on NF-κB activation are dependent on the AMPK − PGC-1α − ROS axis, indicating its possible use for osteolysis. To understand the fundamental mechanism for the impact of MOTS-c on STAT1, Yan et al. (2019) studied the expression of its upstream proteins, IFN-γ and JAK1, in macrophages. MOTS-c treatment resulted in a drop in JAK1 levels, as well as a considerable downregulation of IFN- mRNA and protein levels, implying that STAT1 suppression is linked to the effects of MOTS-c on IFN- and JAK1(Fig. 8).
Conclusion
OP remains a challenge for older adults in developed countries. MPPs such as HN, MOTS-c, and OBAP can enhance OB activity and build and remodel bones. These peptides hold promise for novel therapeutic candidates for OP treatment. More investigations are needed to understand their mechanisms of action in OP and value for the treatment of other bone-related diseases.
References
Aitken JM, Armstrong E, Anderson JB (1972) Osteoporosis after oophorectomy in the mature female rat and the effect of oestrogen and-or progestogen replacement therapy in its prevention. J Endocrinol 55:79–87
Al-Dujaili SA, Koh AJ, Dang M, Mi X, Chang W, Ma PX, McCauley LK (2016) Calcium sensing receptor function supports osteoblast survival and acts as a co-factor in PTH anabolic actions in bone. J Cell Biochem 117:1556–1567
Almeida M, Martin-Millan M, Ambrogini E, Bradsher R 3rd, Han L, Chen XD, Roberson PK, Weinstein RS, O’Brien CA, Jilka RL, Manolagas SC (2010) Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha. J Bone Miner Res 25:769–781
An JH, Yang J-Y, Ahn BY, Cho SW, Jung JY, Cho HY, Cho YM, Kim SW, Park KS, Kim SY, Lee HK, Shin CS (2010) Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone 47:140–150
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465
Andersson N, Surve VV, Lehto-Axtelius D, Ohlsson C, Håkanson R, Andersson K, Ryberg B (2002) Drug-induced prevention of gastrectomy- and ovariectomy-induced osteopaenia in the young female rat. J Endocrinol 175:695–703
Aoki S, Shimizu K, Ito K (2020) Autophagy-dependent mitochondrial function regulates osteoclast differentiation and maturation. Biochem Biophys Res Commun 527:874–880
Arai A, Mizoguchi T, Harada S, Kobayashi Y, Nakamichi Y, Yasuda H, Penninger JM, Yamada K, Udagawa N, Takahashi N (2012) Fos plays an essential role in the upregulation of RANK expression in osteoclast precursors within the bone microenvironment. J Cell Sci 125:2910–2917
Baba M, Endoh M, Ma W, Toyama H, Hirayama A, Nishikawa K, Takubo K, Hano H, Hasumi H, Umemoto T, Hashimoto M, Irie N, Esumi C, Kataoka M, Nakagata N, Soga T, Yao M, Kamba T, Minami T, Ishii M, Suda T (2018) Folliculin regulates osteoclastogenesis through metabolic regulation. J Bone Miner Res 33:1785–1798
Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, Williams SL, Larsson N-G, Stewart JB, Moraes CT (2018) MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 24:1696–1700
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Baran DT, Bergfeld MA, Teitelbaum SL, Avioli LV (1978) Effect of testosterone therapy on bone formation in an osteoporotic hypogonadal male. Calcif Tissue Res 26:103–106
Berger CE, Rathod H, Gillespie JI, Horrocks BR, Datta HK (2001) Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J Bone Miner Res 16:2092–2102
Bindels RJ, Timmermans JA, Hartog A, Coers W, van Os CH (1991) Calbindin-D9k and parvalbumin are exclusively located along basolateral membranes in rat distal nephron. J Am Soc Nephrol 2:1122–1129
Blair HC, Larrouture QC, Li Y, Lin H, Beer-Stoltz D, Liu L, Tuan RS, Robinson LJ, Schlesinger PH, Nelson DJ (2017) Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue engineering. Part b, Reviews 23:268–280
Bottini M, Mebarek S, Anderson KL, Strzelecka-Kiliszek A, Bozycki L, Simão AMS, Bolean M, Ciancaglini P, Pikula JB, Pikula S, Magne D, Volkmann N, Hanein D, Millán JL, Buchet R (2018) Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta 1862:532–546
Boyce BF (2013) Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J Bone Miner Res 28:711–722
Buo AM, Stains JP (2014) Gap junctional regulation of signal transduction in bone cells. FEBS Lett 588:1315–1321
Calnan DR, Brunet A (2008) The FoxO code. Oncogene 27:2276–2288
Capulli M, Paone R, Rucci N (2014) Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys 561:3–12
Chattopadhyay N, Yano S, Tfelt-Hansen J, Rooney P, Kanuparthi D, Bandyopadhyay S, Ren X, Terwilliger E, Brown EM (2004) Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145:3451–3462
Chen C-T, Shih Y-RV, Kuo TK, Lee OK, Wei Y-H (2008) Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes During Osteogenic Differentiation of Human Mesenchymal Stem Cells. STEM CELLS 26:960–968
Chen Z, Buki K, Vääräniemi J, Gu G, Väänänen HK (2011) The critical role of IL-34 in osteoclastogenesis. PLoS One 6:e18689
Chiodini I, Mascia ML, Muscarella S, Battista C, Minisola S, Arosio M, Santini SA, Guglielmi G, Carnevale V, Scillitani A (2007) Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med 147:541–548
Coleman MC, Goetz JE, Brouillette MJ, Seol D, Willey MC, Petersen EB, Anderson HD, Hendrickson NR, Compton J, Khorsand B, Morris AS, Salem AK, Fredericks DC, McKinley TO, Martin JA (2018) Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aan5372
Colon E, Strand ML, Carlsson-Skwirut C, Wahlgren A, Svechnikov KV, Cohen P, Soder O (2006) Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in leydig cells during the first wave of spermatogenesis. J Cell Physiol 208:373–385
Cosman F (2018) Long-term treatment strategies for postmenopausal osteoporosis. Curr Opin Rheumatol 30:420–426
Coudert AE, de Vernejoul MC, Muraca M, Del Fattore A (2015) Osteopetrosis and its relevance for the discovery of new functions associated with the skeleton. Int J Endocrinol 2015:372156
Dallas SL, Prideaux M, Bonewald LF (2013) The osteocyte: an endocrine cell … and more. Endocr Rev 34:658–690
de Coo RF, Buddiger P, Smeets HJ, van Oost BA (1997) Molecular cloning and characterization of the human mitochondrial NADH:oxidoreductase 10-kDa gene (NDUFV3). Genomics 45:434–437
Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C (1997) Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 337:1641–1647
Diab DL, Watts NB (2014) Denosumab in osteoporosis. Expert Opin Drug Saf 13:247–253
Dobson PF, Dennis EP, Hipps D, Reeve A, Laude A, Bradshaw C, Stamp C, Smith A, Deehan DJ, Turnbull DM, Greaves LC (2020) Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci Rep 10:11643
Downey C, Kelly M, Quinlan JF (2019) Changing trends in the mortality rate at 1-year post hip fracture - a systematic review. World Journal of Orthopedics 10:166–175
Drago I, Pizzo P, Pozzan T (2011) After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J 30:4119–4125
Dvorak MM, Riccardi D (2004) Ca2+ as an extracellular signal in bone. Cell Calcium 35:249–255
Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D (2004) Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A 101:5140–5145
Dvorak-Ewell MM, Chen T-H, Liang N, Garvey C, Liu B, Tu C, Chang W, Bikle DD, Shoback DM (2011) Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res 26:2935–2947
Enoki Y, Sato T, Tanaka S, Iwata T, Usui M, Takeda S, Kokabu S, Matsumoto M, Okubo M, Nakashima K, Yamato M, Okano T, Fukuda T, Chida D, Imai Y, Yasuda H, Nishihara T, Akita M, Oda H, Okazaki Y, Suda T, Yoda T (2014) Netrin-4 derived from murine vascular endothelial cells inhibits osteoclast differentiation in vitro and prevents bone loss in vivo. FEBS Lett 588:2262–2269
Fakhry M, Hamade E, Badran B, Buchet R, Magne D (2013) Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells 5:136–148
Feng X, McDonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol 6:121–145
Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, Orwoll ES (2006) Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91:3908–3915
Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GI, Voronina I, Vukobradovic I, Wood GA, Zhu Y, Zirngibl RA, Aubin JE, Bai D, Bruneau BG, Grynpas M, Henderson JE, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J (2005) A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132:4375–4386
Forni MF, Peloggia J, Trudeau K, Shirihai O, Kowaltowski AJ (2016) Murine mesenchymal stem cell commitment to differentiation is regulated by mitochondrial dynamics. Stem Cells 34:743–755
Fukushima N, Hiraoka K, Shirachi I, Kojima M, Nagata K (2010) Isolation and characterization of a novel peptide, osteoblast activating peptide (OBAP), associated with osteoblast differentiation and bone formation. Biochem Biophys Res Commun 400:157–163
Gammage PA, Viscomi C, Simard M-L, Costa ASH, Gaude E, Powell CA, Van Haute L, McCann BJ, Rebelo-Guiomar P, Cerutti R, Zhang L, Rebar EJ, Zeviani M, Frezza C, Stewart JB, Minczuk M (2018) Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med 24:1691–1695
Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, Xu J, Chen L, Cao K, Long J, Li Z, Shen W, Liu J (2018) SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ 25:229–240
Gidlund EK, von Walden F, Venojärvi M, Risérus U, Heinonen OJ, Norrbom J, Sundberg CJ (2016) Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep. https://doi.org/10.14814/phy2.13063
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8:722–728
Gong Z, Tas E, Muzumdar R (2014) Humanin and age-related diseases: a new link? Front Endocrinol. https://doi.org/10.3389/fendo.2014.00210
Gong Z, Su K, Cui L, Tas E, Zhang T, Dong HH, Yakar S, Muzumdar RH (2015) Central effects of humanin on hepatic triglyceride secretion. Am J Physiol Endocrinol Metab 309:E283-292
Graca JA, Schepelmann M, Brennan SC, Reens J, Chang W, Yan P, Toka H, Riccardi D, Price SA (2016) Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am J Physiol Renal Physiol 310:F518-533
Gupta RR, Yoo DJ, Hebert C, Niger C, Stains JP (2010) Induction of an osteocyte-like phenotype by fibroblast growth factor-2. Biochem Biophys Res Commun 402:258–264
Hance N, Ekstrand MI, Trifunovic A (2005) Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum Mol Genet 14:1775–1783
Hasegawa T, Yamamoto T, Tsuchiya E, Hongo H, Tsuboi K, Kudo A, Abe M, Yoshida T, Nagai T, Khadiza N, Yokoyama A, Oda K, Ozawa H, de Freitas PHL, Li M, Amizuka N (2017) Ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization. Jpn Dent Sci Rev 53:34–45
Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I (2001) Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci 21:9235–9245
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2012) miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem 287:42084–42092
Hemmingsen C (2000) Regulation of renal calbindin-D28K. Pharmacol Toxicol 87:5
Hendy GN, Canaff L (2016) Calcium-sensing receptor gene: regulation of expression. Front Physiol 7:394
Henry JP, Bordoni B (2020) Histology, Osteoblasts, StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL
Houschyar KS, Tapking C, Borrelli MR, Popp D, Duscher D, Maan ZN, Chelliah MP, Li J, Harati K, Wallner C (2019) Wnt pathway in bone repair and regeneration–what do we know so far. Front Cell Develop Biol 6:170
Hsu Y-C, Wu Y-T, Yu T-H, Wei Y-H (2016) Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. Semin Cell Dev Biol 52:119–131
Ikeda K, Takeshita S (2014) Factors and mechanisms involved in the coupling from bone resorption to formation: how osteoclasts talk to osteoblasts. J Bone Metab 21:163–167
Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P (2003) Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A 100:13042–13047
Indo Y, Takeshita S, Ishii K-A, Hoshii T, Aburatani H, Hirao A, Ikeda K (2013) Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res 28:2392–2399
Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A (2019) RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Experiment Clin Cancer Res 38:12–12
Jin Z, Wei W, Yang M, Du Y, Wan Y (2014) Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab 20:483–498
Kallay E (2018) Physiology and pathophysiology of the extracellular calcium-sensing receptor. Front Physiol 9:413
Kang N, Kim KW, Shin DM (2019) Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation. Korean J Physiol Pharmacol 23:411–417
Kantari C, Walczak H (2011) Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 1813:558–563
Kawakami A, Fisher DE (2017) The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab Invest 97:649–656
Kidd M, Modlin IM, Black JW, Boyce M, Culler M (2007) A comparison of the effects of gastrin, somatostatin and dopamine receptor ligands on rat gastric enterochromaffin-like cell secretion and proliferation. Regul Pept 143:109–117
Kim SJ, Xiao J, Wan J, Cohen P, Yen K (2017) Mitochondrially derived peptides as novel regulators of metabolism. J Physiol 595:6613–6621
Kitahara T, Takeda N, Kubo T, Kiyama H (1996) Molecular cloning of the rat NADH:ubiquinone oxidoreductase subunit and its up-regulation in the facial muscle after denervation: detected by means of differential display. Neurol Res 18:329–336
Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, Koike M, Asou Y, Shirasawa T, Yokote K, Kaneko K, Shimizu T (2015) Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep 5:9148
Kong BS, Min SH, Lee C, Cho YM (2021) Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep 36:109447
Kumagai H, Coelho AR, Wan J, Mehta HH, Yen K, Huang A, Zempo H, Fuku N, Maeda S, Oliveira PJ, Cohen P, Kim SJ (2021) MOTS-c reduces myostatin and muscle atrophy signaling. Am J Physiol Endocrinol Metab 320:E680-e690
Lee NK, Choi YG, Baik JY, Han SY, Jeong D-w, Bae YS, Kim N, Lee SY (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859
Lee DS, Choung HW, Kim HJ, Gronostajski RM, Yang YI, Ryoo HM, Lee ZH, Kim HH, Cho ES, Park JC (2014) NFI-C regulates osteoblast differentiation via control of osterix expression. Stem Cells 32:2467–2479
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21:443–454
Lee C, Kim KH, Cohen P (2016) MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2016.05.015
Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2009) Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284:15676–15684
Liemburg-Apers DC, Willems PH, Koopman WJ, Grefte S (2015) Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol 89:1209–1226
Lin X, Patil S, Gao Y-G, Qian A (2020) The bone extracellular matrix in bone formation and regeneration. Front Pharmacol. https://doi.org/10.3389/fphar.2020.00757
Lindsay R, Aitken J, Anderson L, Hart D, MacDonald E, Clarke A (1976) Long-term prevention of postmenopausal osteoporosis by oestrogen: evidence for an increased bone mass after delayed onset of oestrogen treatment. The Lancet 307:1038–1041
Lv YJ, Yang Y, Sui BD, Hu CH, Zhao P, Liao L, Chen J, Zhang LQ, Yang TT, Zhang SF, Jin Y (2018) Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics 8:2387–2406
Ma X, Su P, Yin C, Lin X, Wang X, Gao Y, Patil S, War AR, Qadir A, Tian Y, Qian A (2020) The roles of FoxO transcription factors in regulation of bone cells function. Int J Mol Sci 21:692
Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N (2012) Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18:405–412
Manolagas SC (2008) De-fense! De-fense! De-fense: scavenging H2O2 while making cholesterol. Endocrinology 149:3264–3266
Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S (2007) Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117:2133–2144
Meshcheryakova A, Mechtcheriakova D, Pietschmann P (2017) Sphingosine 1-phosphate signaling in bone remodeling: multifaceted roles and therapeutic potential. Expert Opin Ther Targets 21:725–737
Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, Chengming X, Banjun R, Li W, Zifan L (2016) Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2016.05.135
Miyazaki T, Iwasawa M, Nakashima T, Mori S, Shigemoto K, Nakamura H, Katagiri H, Takayanagi H, Tanaka S (2012) Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. J Biol Chem 287:37808–37823
Molawi K, Sieweke MH (2013) Chapter Ten - Transcriptional Control of Macrophage Identity, Self-Renewal, and Function. In: Murphy KM, Merad M (eds) Advances in Immunology. Academic Press, pp 269–300
Moretti E, Giannerini V, Rossini L, Matsuoka M, Trabalzini L, Collodel G (2010) Immunolocalization of humanin in human sperm and testis. Fertil Steril 94:2888–2890
Murakami M, Nagahama M, Maruyama T, Niikura T (2017) Humanin ameliorates diazepam-induced memory deficit in mice. Neuropeptides 62:65–70
Nagy V, Penninger JM (2015) The RANKL-RANK Story. Gerontology 61:534–542
Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S-i, Tsujita T, Nakamura T, Kato S, Yamamoto M, Takayanagi H, Ishii M (2015) DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine–producing metabolic pathway. Nat Med 21:281–287
Noreldin AE, Sogabe M, Yamano Y, Uehara M, Mahdy MA, Elnasharty MA, Sayed-Ahmed A, Warita K, Hosaka YZ (2016) Spatial distribution of osteoblast activating peptide in the rat stomach. Acta Histochem 118:109–117
Noreldin AE, Elewa YHA, Kon Y, Warita K, Hosaka YZ (2018) Immunohistochemical localization of osteoblast activating peptide in the mouse kidney. Acta Histochem 120:323–328
Noreldin AE, Gewaily MS, Saadeldin IM, Abomughaid MM, Khafaga AF, Elewa YH (2021) Osteoblast-activating peptide exhibits a specific distribution pattern in mouse ovary and may regulate ovarian steroids and local calcium levels. Am J Transl Res 13:5796–5814
O’brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841
Owen R, Reilly GC (2018) In vitro models of bone remodelling and associated disorders. Front Bioeng Biotechnol 6:134–134
Pacifico L, Andreoli GM, D’Avanzo M, De Mitri D, Pierimarchi P (2018) Role of osteoprotegerin/receptor activator of nuclear factor kappa B/receptor activator of nuclear factor kappa B ligand axis in nonalcoholic fatty liver disease. World J Gastroenterol 24:2073–2082
Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P (2013) HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells 31:1902–1909
Park JH, Lee NK, Lee SY (2017) Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells 40:706–713
Persson P, Håkanson R, Axelson J, Sundler F (1989) Gastrin releases a blood calcium-lowering peptide from the acid-producing part of the rat stomach. Proc Natl Acad Sci U S A 86:2834–2838
Pilkington SJ, Walker JE (1989) Mitochondrial NADH-ubiquinone reductase: complementary DNA sequences of import precursors of the bovine and human 24-kDa subunit. Biochemistry 28:3257–3264
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Riccardi D (1999) Cell surface, Ca2+ (cation)-sensing receptor (s): one or many? Cell Calcium 26:77–83
Richards JB, Zheng HF, Spector TD (2012) Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 13:576–588
Ristow M, Zarse K (2010) How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45:410–418
Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106:8665–8670
Runswick MJ, Gennis RB, Fearnley IM, Walker JE (1989) Mitochondrial NADH:ubiquinone reductase: complementary DNA sequence of the import precursor of the bovine 75-kDa subunit. Biochemistry 28:9452–9459
Saidak Z, Le Henaff C, Azzi S, Marty C, Da Nascimento S, Sonnet P, Marie PJ (2015) Wnt/β-catenin signaling mediates osteoblast differentiation triggered by peptide-induced α5β1 integrin priming in mesenchymal skeletal cells. J Biol Chem 290:6903–6912
Salih DAM, Brunet A (2008) FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20:126–136
Salway J (2004) Metabolism at a glance, 3rd edn. Blackwell Publishing Ltd, Malden, MA
Schieferdecker A, Voigt M, Riecken K, Braig F, Schinke T, Loges S, Bokemeyer C, Fehse B, Binder M (2014) Denosumab mimics the natural decoy receptor osteoprotegerin by interacting with its major binding site on RANKL. Oncotarget 5:6647–6653
Schinke T, Schilling AF, Baranowsky A, Seitz S, Marshall RP, Linn T, Blaeker M, Huebner AK, Schulz A, Simon R, Gebauer M, Priemel M, Kornak U, Perkovic S, Barvencik F, Beil FT, Del Fattore A, Frattini A, Streichert T, Pueschel K, Villa A, Debatin KM, Rueger JM, Teti A, Zustin J, Sauter G, Amling M (2009) Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med 15:674–681
Scholtysek C, Katzenbeisser J, Fu H, Uderhardt S, Ipseiz N, Stoll C, Zaiss MM, Stock M, Donhauser L, Böhm C, Kleyer A, Hess A, Engelke K, David J-P, Djouad F, Tuckermann JP, Desvergne B, Schett G, Krönke G (2013) PPARβ/δ governs Wnt signaling and bone turnover. Nat Med 19:608–613
Shadel Gerald S, Horvath Tamas L (2015) Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 163:560–569
Sharan K, Siddiqui J, Swarnkar G, Chattopadhyay N (2008) Role of calcium-sensing receptor in bone biology. Ind J Med Res 127:274–286
Shares BH, Busch M, White N, Shum L, Eliseev RA (2018) Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J Biol Chem 293:16019–16027
Shum LC, White NS, Mills BN, de Mesy Bentley KL, Eliseev RA (2016) Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Develop 25:114–122
Sims NA, Martin TJ (2015) Coupling signals between the osteoclast and osteoblast: how are messages transmitted between these temporary visitors to the bone surface? Front Endocrinol (lausanne) 6:41
Snoeck H-W (2017) Mitochondrial regulation of hematopoietic stem cells. Curr Opin Cell Biol 49:91–98
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 9:522–536
Song L, Xie XB, Peng LK, Yu SJ, Peng YT (2015) Mechanism and treatment strategy of osteoporosis after transplantation. Int J Endocrinol 2015:280164
Szulc P, Bauer DC (2013) Biochemical markers of bone turnover in osteoporosis, osteoporosis. Elsevier, Amsterdam, pp 1573–1610
Tang KC, Pan W, Doschak MR, Alexander RT (2019) Increased FoxO3a expression prevents osteoblast differentiation and matrix calcification. Bone Rep 10:100206–100206
Taylor RW, Turnbull DM (2005) Mitochondrial DNA mutations in human disease. Nat Rev Genet 6:389–402
Teti A, Econs MJ (2017) Osteopetroses, emphasizing potential approaches to treatment. Bone 102:50–59
Thummasorn S, Apaijai N, Kerdphoo S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N (2016) Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc Ther 34:404–414
Treiber N, Maity P, Singh K, Kohn M, Keist AF, Ferchiu F, Sante L, Frese S, Bloch W, Kreppel F, Kochanek S, Sindrilaru A, Iben S, Högel J, Ohnmacht M, Claes LE, Ignatius A, Chung JH, Lee MJ, Kamenisch Y, Berneburg M, Nikolaus T, Braunstein K, Sperfeld A-D, Ludolph AC, Briviba K, Wlaschek M, Scharffetter-Kochanek K (2011) Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell 10:239–254
Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park SH, Thompson T, Karsenty G, Bradley A, Donehower LA (2002) p53 mutant mice that display early ageing-associated phenotypes. Nature 415:45–53
Tzivion G, Dobson M, Ramakrishnan G (2011) FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochimica et Biophysica Acta 1813:1938–1945
Ueyama T, Shirasawa N, Numazawa M, Yamada K, Shelangouski M, Ito T, Tsuruo Y (2002) Gastric parietal cells: potent endocrine role in secreting estrogen as a possible regulator of gastro-hepatic axis. Endocrinology 143:3162–3170
Veronese N, Kolk H, Maggi S (2020) Epidemiology of Fragility Fractures and Social Impact. In: Veronese N, Kolk H, Maggi S (eds) Orthogeriatrics. Springer, Cham, pp 19–34
Vezzoli G, Soldati L, Gambaro G (2009) Roles of calcium-sensing receptor (CaSR) in renal mineral ion transport. Curr Pharm Biotechnol 10:302–310
Vimalraj S, Selvamurugan N (2013) MicroRNAs: synthesis, gene regulation and osteoblast differentiation. Curr Issues Mol Biol 15:7–18
von Walden F, Fernandez-Gonzalo R, Norrbom J, Emanuelsson EB, Figueiredo VC, Gidlund EK, Norrbrand L, Liu C, Sandström P, Hansson B, Wan J, Cohen P (1985) Alkner B (2021) Acute endurance exercise stimulates circulating levels of mitochondrial-derived peptides in humans. J Appl Physiol 131:1035–1042
Wang F, Zhang C, Ge W, Zhang G (2019) Up-regulated CST5 inhibits bone resorption and activation of osteoclasts in rat models of osteoporosis via suppression of the NF-κB pathway. J Cell Mol Med 23:6744–6754
Wongrakpanich A, Geary SM, M-lA J, Anderson ME, Salem AK (2014) Mitochondria-targeting particles. Nanomedicine 9:2531–2543
Woodhead JST, D’Souza RF, Hedges CP, Wan J, Berridge MV, Cameron-Smith D, Cohen P, Hickey AJR, Mitchell CJ (1985) Merry TL (2020) High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J Appl Physiol 128:1346–1354
Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O’Brien CA (2015) Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 10:e0138189
Yamagishi Y, Hashimoto Y, Niikura T, Nishimoto I (2003) Identification of essential amino acids in Humanin, a neuroprotective factor against Alzheimer’s disease-relevant insults. Peptides 24:585–595
Yan Z, Zhu S, Wang H, Wang L, Du T, Ye Z, Zhai D, Zhu Z, Tian X, Lu Z, Cao X (2019) MOTS-c inhibits Osteolysis in the Mouse Calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol Res 147:104381
Yin L, Huang D, Liu X, Wang Y, Liu J, Liu F, Yu B (2017) Omentin-1 effects on mesenchymal stem cells: proliferation, apoptosis, and angiogenesis in vitro. Stem Cell Res Ther 8:224–224
Yoshiko Y, Candeliere GA, Maeda N, Aubin JE (2007) Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 27:4465–4474
Young SH, Rey O, Rozengurt E (2015) Intracellular Ca(2+) oscillations generated via the extracellular Ca(2+)-sensing receptor (CaSR) in response to extracellular Ca(2+) or L-phenylalanine: Impact of the highly conservative mutation Ser170Thr. Biochem Biophys Res Commun 467:1–6
Zárate SC, Traetta ME, Codagnone MG, Seilicovich A, Reinés AG (2019) Humanin, a mitochondrial-derived peptide released by astrocytes, prevents synapse loss in hippocampal neurons. Front Aging Neurosci 11:123
Zeng R, Faccio R, Novack DV (2015) Alternative NF-κB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J Bone Miner Res 30:2287–2299
Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO (2012) Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci 91:199–206
Zhang Y, Rohatgi N, Veis DJ, Schilling J, Teitelbaum SL, Zou W (2018) PGC1β organizes the osteoclast cytoskeleton by mitochondrial biogenesis and activation. J Bone Miner Res 33:1114–1125
Acknowledgements
This work was supported by the Ministry of Science and ICT through the Brain Pool program (grant no: 2021H1D3A2A02040098) to Islam M. Saadeldin.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noreldin, A.E., Saadeldin, I.M., Khalifa, N.E. et al. Emerging Therapeutic Potential of Short Mitochondrial-produced Peptides for Anabolic Osteogenesis. Int J Pept Res Ther 28, 40 (2022). https://doi.org/10.1007/s10989-021-10353-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-021-10353-2