Abstract
Membrane active peptides are a family of peptides with ability to interact with plasma membrane. Cell penetrating peptides (CPPs) interact with membrane and enter cells via different pathways without damaging the membrane. Antimicrobial peptides (AMPs) are peptides active against microorganisms. Both CPPs and AMPs belong to membrane active peptides family. AMPs interact with membrane and some are able to translocate into cells without the need of permanent permeabilization. Thus they have can be promising source of CPPs. Moreover, some CPPs have shown antimicrobial activity against pathogens. In this review we summarize the studies with the aim of developing functional and efficient CPPs and AMPs from existing and known peptide pool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A group of peptides from different families that interact with biological membrane and causing membrane disruption or crossing through the membrane or just simply reside into it, are known as “membrane active peptides”. Cell-penetrating peptides (CPPs) and antimicrobial peptides (AMPs) are two major classes of membrane active peptides. CPPs are short peptides with the ability to interact with the membranes and usually translocate them in order to enter the cells. Since there are several remaining challenges in viral and liposomal carriers application (Stewart et al. 2016), these class of peptides have gained so much attraction as an efficient and safe delivery means. CPPs can be applied in the field of drug delivery as an alternative carrier system to directly translocate peptides, proteins, antibodies and even nucleic acid to various cell’s compartments. Application of CPPs could be further expanded by using them in functional studies including protein–protein interactions in basic research. Several studies have proven that CPPs can act as an effective delivery tools alongside the conventional delivery/carrier systems. Hence, they exhibit particular novel features that could possibly overcome some of the pitfalls of the traditional delivery approaches.
The other class of membrane active peptide are AMPs which could inactivate mainly pathogenic microbes possibly by interfering and disrupting the cell membrane. AMPs can also inhibit some cellular functions that leads to microbe cell’s death (Brogden 2005; Avci et al. 2018). According to WHO’s report in Jan 29, 2018; high levels of resistance to a number of serious bacterial infections in both high- and low-income countries is observed (High levels of antibiotic resistance 2018) emphasizing an urgent need for new antimicrobial agents to overcome multi-drug resistance infections. AMPs are thought to be promising antimicrobial compounds since they offer activity against cellular membrane and microorganisms cannot change their membrane composition easily to build resistance against these compounds (Avci et al. 2018; Moreno and Giralt 2015). Surprisingly, CPPs and AMPs are seeming to share characteristics such as sequence and structure similarity, related mode of action and look-alike physicochemical properties. These feature have led to several studies to evaluate cell penetrating and antimicrobial properties of CPPs and AMPs, interchangeably. In this review we will focus on those CPPs/AMPs and their subsequent modifications which have been applied as a substitute to the original molecule.
Cell-Penetrating Peptides
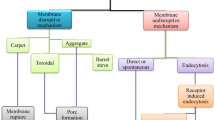
Cell-penetrating peptides or protein transduction domains (PTDs) in another term, are typically short cationic or amphipathic peptides with less than 30 amino acid residues, capable of translocating through the cell membrane. They are mainly classified based on their origin or their physicochemical properties. According to their origin, they are classified into three groups: natural derived CPPs, chimeric CPPs and synthetic CPPs. On the other hand, based on CPP’s physicochemical properties, they can be classified into cationic CPPs, amphipathic CPPs and hydrophobic CPPs. Other classification based on their secondary structure is also suggested (Milletti 2012; Radis-Baptista et al. 2017). Although CPP’s uptake mechanisms are not completely understood, two main uptake mechanisms are suggested for their internalization. CPP’s can enter cells via direct translocation (energy independent) or endocytic routes ) (Fig. 1) (Pooga and Langel 2015).
schematic illustration of CPPs entry routes. CPPs can enter cells via direct translocation or endocytosis pathways. Uptake mechanism depends on various factors such as CPP’s concentration, cell type, cargo type, cargo concentration etc. However, interaction of CPPs with cell membrane is the first step of CPPs uptake. Subsequently, CPPs may opt for direct translocation into cytoplasm by assembling and forming transient membrane permeabilization (a) or without self-assembly and damaging membrane (b). They may choose endocytosis to enter cells in an endosomal compartment (c). CPPs may use both energy independent and endocytosis mechanisms for entering the cell at the same time
CPPs can enter cells via different endocytic pathways like clathrin-mediated, caveolae-mediated and micropinocytosis. Regarding direct translocation entry pathways, inverted micelle, barrel stave, pore-forming model, carpet model, adaptive translocation, electroporation-like translocation are hypothesized for CPPs translocation into cytoplasm (Pooga and Langel 2015; Bechara and Sagan 2013; Figueiredo 2014). It should be noted that cellular uptake mechanism is dependent on various factors like, cell type, CPP concentration and cargo concentration (Saalik et al. 2011; Pae et al. 2014). Since CPPs are potent means to enter cells with no or minimal damage and cytotoxicity to the target cells, they have gained tremendous attractions to be applied as vectors and delivery tools to deliver DNA, peptides, antibodies and drugs into cells with high efficiency.
CPPs have been under intensive research for more than two decades and are largely considered safe, high efficient transfection tools in various fields of study. Development of novel and modified CPPs as drug, peptide and nucleic acid delivery vectors deems to be necessary to achieve higher efficiency, expanding the spectrum of CPPs to deliver various cargos and also to overcome current limitations.
Antimicrobial Peptides
Antimicrobial peptides or host defense peptides, belonging to innate immune systems in many multicellular and unicellular organisms, are referred to those peptides being active against a broad and diverse spectrum of microorganisms. This peptides show antimicrobial activity in micromolar concentrations and below, in addition of rapid effect in vitro, which make them promising alternatives for conventional antibiotics (Splith and Neundorf 2011). AMPs act via different mechanisms by proposing activity against bacterial membrane like pore formation, carpet-model, SHM model, electroporation model, amyloid formation model, double-belt model or by binding to nucleic acid thus inhibiting DNA replication or translation. AMPs can be produced via two main mechanisms. In the first mechanism which occurs in all life forms, AMPS are produced via transcription of the peptide’s gene and later, translation of the transcribed mRNA. In the second mechanism, the peptide is produces during multiple enzymatic processes on a precursor peptide or protein which mainly occurs in bacterial systems (Hancock and Chapple 1999; Mahlapuu et al. 2016). Similar to CPPs, there are different approaches to classify AMPs. Classification based on activity, structure, molecular target and peptide bonding patterns are suggested. Based on their activity, AMPs could be antibacterial, antifungal, antiviral and antiparasitic. Moreover, some AMPs have shown anticancer, wound healing and immune modulation properties (Hancock and Sahl 2006).
CPPs and AMPs share some important physicochemical (sequence, secondary structures, etc.) properties highlighted in Table 1. In addition to that, studies have shown that certain AMPs (membrane-crossing AMPs) could transverse themselves into cytoplasm via interaction with cell membrane without any membrane damage (Matsuzaki et al. 1995; Zhang et al. 2001; Henriques et al. 2006). Observing these features has led to some speculations that defined number of AMPs could function as CPPs and vice versa or minimally researchers could use them as a platform for developing novel CPPs or AMPs. However, since some AMPs induce their antimicrobial activity by affecting the membrane directly and cause membrane integrity and disruption leading to cell death, it is necessary to engineer these peptides so they can display cell penetrating activity without damaging the membrane (Fig. 2a). As an example, Akishiba et al. successfully developed a carrier peptide called L17E by substituting L17 of M-lycotoxin, a cationic peptide isolated from spider venom, with glutamic acid. L17E peptide didn’t show any lytic activity against cell membrane. However, it was shown that this new peptide with cell penetrating property is capable of escaping endosome duo to lower pH of endosome (Fig. 2b) (Akishiba et al. 2017).
Schematic illustration of AMPs activity on plasma membrane. a Some AMPs induce their antimicrobial effect by membrane disruption activities (Stewart et al. 2016) which could be problematic for their application as CPPs. Engineering AMPs is essential in order to develop peptides that can function as CPPs and enter target cells without damaging the membrane (Brogden 2005). b L17E peptide development. An AMP called L17 with membrane lytic activity (Stewart et al. 2016) engineered by amino acid substitution and the new peptide, named L17E, was demonstrated to be non-lytic (Brogden 2005), reproduced form reference (Akishiba et al. 2017)
CPPs and Their Derivatives as AMPs
In a study conducted by Nekhotiaeva et al., antimicrobial properties of two well-known CPPs, pVEC and TP10, against 6 different microorganisms, were investigated. They assessed the antimicrobial effect of peptides by determining minimum inhibitory concentration (MIC) and zone of inhibition for Staphylococcus aureus (S. aureus). Moreover, they infected HeLa cells with S. aureus and treated the infected cells with TP10 peptide in order to evaluate the antimicrobial activity of the peptide against intracellular infections. Furthermore, they tested the peptides on rat erythrocytes to assess the hemolytic activity of the peptides. Cell permeabilization assay using SYTOX green, was performed on S. aureus, Mycobacterium smegmatis (M. smegmatis) and HeLa cells which were treated with pVEC and TP10 peptides. They have shown that these CPPs accumulated rapidly on cell surface followed by cell internalization. TP10 exhibited inhibitory activity on Candida albicans (C. albicanse) and S. aureus and pVEC was found to inhibit growth of M. smegmatis in low micromolar concentrations without any cytotoxicity to HeLa cells. They also found that these CPPs preferentially act against microbes, although they entered all cell type tested in this experiment. Infected HeLa cells with noninvasive S. aureus were cleared from bacterial infection after being treated with TP10. However, TP10 showed cytotoxicity to HeLa cells at concentrations above 15 µM. As a conclusion to this study, tested CPPs were found to enter all cell types but they were lethal only for tested microorganism (Nekhotiaeva et al. 2004).
Nan et al. studied antimicrobial properties of pVEC/pVEC analogs and found that they are effective on both Gram positive and Gram negative bacteria. Induction of near-complete membrane depolarization against S. aureus at a concentration of 4 µM was observed. BY comparing the results from dye leakage and membrane depolarization with a well characterized AMP, magainin 2, they suggested that pWEC antimicrobial properties is likely due to pore or ion channel formation in cytoplasmic membrane (Nan et al. 2011). Eriksson et al. investigated antimicrobial activity of TP10 and MAP peptides against Neisseria meningitides in human whole blood and in vivo mice models. Both TP10 and MAP peptide remained active in whole blood (which is very important for in vivo conditions) while hemolysis was so low to be considered. Moreover, in mice models infected with N. meningitidis, bacterial reduction was observed after treatment with TP10. Since MAP showed cytotoxicity in in vitro studies it didn’t enter the in vivo study. According to results of live microscopy of fluorescent dye uptake, it is suggested that TP10 acts via membrane permeabilization (Eriksson et al. 2013). Xie et al. investigated TP10 and its analogues activity against multi-drug resistant bacteria isolated from patients and standard strains. The MIC test results indicated that TP10/analogues, except TP10-8, are active against both Gram-positive and Gram negative strains. The mechanism of antimicrobial activity is due to both outer and inner membrane disruption of the bacteria and consequently, binding of TP10 to the DNA. TP10 is not cytotoxic for mammalian cells, however, some structural modifications can result in TP10 analogues with boost or loss of antimicrobial activity and cytotoxicity (Xie et al. 2015).
Tat (Tat protein residue 48–60), an Arginine-rich peptide that was derived from HIV-1 transactivator protein that is involved in the virus replication (Vives et al. 1997), was found to have antifungal activity in a study conducted by Jung et al. Antifungal activity of this peptide was examined on four fungal strains, Malassezia furfur, Saccharomyces cerevisiae, Trichosporon beigelii and Candida albicans using MTT assay. Tat peptide showed no hemolytic activity on human erythrocytes. They indicated that Tat peptide could translocate into cells without disrupting cell membrane. Peptides translocation seems to be energy and salt independent (Jung et al. 2006). Jung and colleagues also investigated antibacterial activity of Tat (Tat 2020; Shaw et al. 2008; Walrant et al. 2011; Carmona et al. 2012; Jang et al. 2012; Rydberg et al. 2012; Bahnsen et al. 2013; Oh et al. 2014; Brezden et al. 2016; Miao et al. 2016; Budagavi and Chugh 2018; Budagavi et al. 2018; Luque-Ortega et al. 2008) against Bacillus subtilis, Staphylococcus epidermidis, S. aureus, E. coli, Pseudomonas vulgaris, P. aeruginosa and Staphylococcus typhimurium; plus two clinically isolated MRSA and MRPA (multidrug-resistant Pseudomonas aeruginosa). The MIC tests results showed that this CPP has inhibitory effect on all the tested bacteria including standard Gram-positive, Gram negative and isolated bacteria. They also designed a D-enantiomer of this peptide, which L-amino acids were substituted with D-amino acids, in order to enhance peptide’s resistance to protease activity in vivo. Growth inhibitory effects of the D- enantiomer was also tested on MRSA infected HeLa cells and it was observed that D-Tat could inhibits MRSA in comparison to L-Tat that could only partially inhibit MRSA (Jung et al. 2008). Long Zhu et al. designed a Tat (2020; Shaw et al. 2008; Walrant et al. 2011; Carmona et al. 2012; Jang et al. 2012; Rydberg et al. 2012; Bahnsen et al. 2013; Oh et al. 2014; Brezden et al. 2016; Miao et al. 2016; Budagavi and Chugh 2018; Budagavi et al. 2018; Luque-Ortega et al. 2008) analogue, Tat(W), that Pro59 residue is substituted with Trp residue. Furthermore, C-terminal in this designed peptide was gone under amidation. Applied modifications were done in order to increase the antimicrobial activity. They also synthesized two dimeric Tat (W) peptides, di-Tat(W)-C and di-Tat(W)-K, with a single disulfide bond linkage and Lysine linkage, respectively. These peptides were tested on Gram-positive and Gram-negative bacteria, B. subtilis, S. aureus, S. epidermidis, E. coli, P. aeruginosa and S. thyphimurium, respectively. The results indicated that antimicrobial activity of dimeric peptides is similar to the monomeric Tat (W), but bactericidal activity is more rapid than the monomeric Tat(W). No hemolytic activity was observed on fresh human blood derived erythrocytes. Dimeric di-Tat (W)-C and di-Tat(W)-K were capable of membrane depolarization and dye leakage comparing to Tat(W). Moreover, the results from the translocation experiments, in comparison to Tat (W), indicated that these dimeric peptides couldn’t effectively translocate through the lipid bilayer (Long Zhu and Shin 2009). Bourre and colleagues tested porphyrin (acting as a photosensitizer that kills microorganisms by producing free radicals or monoxides) conjugated Tat peptide on different bacteria with the aim of a delivery of a photosensitizer with CPP and study the efficacy of antimicrobial photodynamic therapy. The results indicated that antimicrobial properties of Tat-porphyrin are dependent on the concentration of the CPP and light dose given to treated cells. Expectedly, Gram positive strains are more susceptible to the conjugate due to their membrane structure which lacks outer membrane and lipopolysaccharide (LPS) (Bourré et al. 2010).
Pep-1 is a chimeric 21-amino acids residue cell penetrating peptide with a hydrophobic tryptophan-rich domain, a hinge and a hydrophilic lysine-rich domain (Morris et al. 2001). Although this CPP shares features with AMPs but when tested by Zhu et al. it showed weak antibacterial activity but a wide spectrum. This issue leads Zhu et al. to develop Pep-1-K, a Pep-1 analogue. In Pep-1-K all Glu residues were replaced by Lys. Pep-1-K was tested on three Gram-positive and three Gram-negative bacteria plus MRSA (methicillin resistant Staphylococcus aureus) and multidrug-resistant Pseudomonas aeruginosa obtained from clinical isolates, using MIC assay. Pep-1-K was found to have strong antibacterial activity on both Gram-positive and Gram-negative standard bacterial strains as well as clinical isolate. No hemolysis on human erythrocytes was observed at inhibitory concentrations. The tryptophan fluorescence studies suggested that antimicrobial activity of this peptide is due to small channels formation in the membrane that allows ions to efflux (Zhu et al. 2006). Park et al. tested Pep-1 peptide on Chlamydia. They concluded that Pep-1 peptide has anti-chlamydial effect for the intracellular stage of chlamydial infection, but pre-incubated elementary bodies (EBs) with Pep-1 before infection, didn’t seem to inhibit inclusion formation. They also tested this peptide on E. coli and S. aureus and Toxoplasma gondii, but no antimicrobial activity was observed (Park et al. 2009). This group also conducted another study in which, they tested antibacterial activity of designed derivatives, PK-17, PK-12, PK-15, PK-12KK and PK-12KKP on both Gram positive and Gram negative bacteria. Unlike PK-17, PK-12 KK and PK-12KKP; PK-15 and PK-12 showed 5% hemolysis and two-fold antimicrobial decrease. The greater antimicrobial activity in PK-17, PK-12 KK and PK-12KKP is assumed to be due to the presence of two lysine residues in the C-terminal region of these peptides which enhance the antimicrobial activity (Zhu et al. 2009). In previous studies it was indicated that Pep-1-K possess better antimicrobial effects rather than its mother peptide; Pep-1. In a separate study, Bobone and her colleagues evaluated antimicrobial potency of Pep-1-K. They designed model membranes and used fluorescence spectra plus confocal laser scanning microscopy to show Pep-1-k peptide-membrane interactions. The results indicated that Pep-1-K is capable of perturbing membrane, thus inducing ion leakage; but these result weren’t observed in the case of Pep-1. It was postulated that Pep-1-K has higher affinity towards anionic membranes that mimic bacterial membranes and that’s the reason for having higher antimicrobial potency in comparison to Pep-1 (Bobone et al. 2011).
ppTG20 is a synthetic 20 amino acid long CPP with an amino acid sequence of GLFRALLRLLRSLWRLLLRA (Rittner et al. 2002). In a study, P7 peptide, a ppTG20 analogue, was tested on Salmonella typhimurium to determine its antimicrobial activity in comparison to its parent peptide; ppTG20. P7 is derived from ppTG20 by substituting Phe and Trp in positions 3 and 14 respectively with Arg in order to increase the hydrophobicity. Results of this study, showed that this peptide has a great antimicrobial activity against S. typhimurium and its mechanism of action was found to be membrane disruption and ion channels formation that causes potassium ions leakage. However, this peptide also show cytotoxicity against HT29 and MDA-MB231 mammalian cell lines which could potentially prevent its use as a antimicrobial peptide in vivo (Li et al. 2012). Having said that, it is still possible to introduce some modifications in the peptide to decrease toxicity on mammalian cells to the accepted levels. In another study on P7, Li et al. tested peptide against different bacteria in order to identify a new food preservative for food industry. The electron microscopy results indicated that this peptide could induces pore on the cell membrane. However, pore formation was not the sole reason for cell death but high DNA binding affinity and probably DNA damage of P7 also plays critical role. Moreover, the cell cycle analysis and RT-PCR showed that the P7 induce a decrease in gene expression of the genes involved in DNA damage repair pathways (Li et al. 2015). Li and colleagues investigated the antifungal activity of P7 peptide on C. albicans and other fungal species. They observed that this ppTG20 analogue acts via permeabilization and depolarization of the plasma membrane that would leads to binding to the DNA and ultimately cell death (Li et al. 2016). In another study antimicrobial activity of APP peptide was investigated by Li et al. APP is an analogue of a CPP; ppTG20. Growth inhibition in both Gram negative and Gram positive strains treated with this peptide was observed while hemolysis was decreased in comparison to ppTG20. It’s suggested that APP acts via ion channels formation which subsequently leads to DNA binding, DNA damage and cell death (Li et al. 2013). In a similar study by Li et al. the other ppTG20 derivative, APP was tested on C. albicans as the fungus model and other fungal species and found quite similar antifungal activity to P7 (Li et al. 2016).
Holm et al. performed a study in which 21 peptides (both AMP and CPP) were tested on Malassezia sympodialis which is a yeast that cause skin abnormalities. By performing colony count and microdilution assay, the results showed that among all different tested peptides six peptide, Arg9, Tat, Histatin5, Penetratin, pVEC and scrambled pVEC (five CPP and one AMP) were found to be antifungal in submicromolar concentrations. Although the mechanism of action remains unclear it was observed that six peptides showing antifungal activity are the least hydrophobic peptides among all 21 tested peptides. It seems that peptide with hydrophobic nature cannot interact well enough with the yeast cell wall and cell membrane. Moreover no membrane damage was observed in keratinocytes (Holm et al. 2012).
The cell-penetration of three different antimicrobial peptides were investigated by Bahnsen et al. in order to identify a cell-penetrating antimicrobial peptide to target intracellular infections. This group tested three AMPs, PK-12-KKP, SA-3 and TPk and a cell-penetrating peptide, penetratin labeled with 5 (Milletti 2012)-carboxytetramethylrhodamin. The antimicrobial activity of these peptides were tested on S. aureus and E. coli. The results of their study showed that TPk exhibit a strong antimicrobial activity and could be a great cell-penetrating peptide candidate with the ability to target intracellular bacteria as a result of its penetration ability into eukaryotic cells without any significant cytotoxicity against them (Bahnsen et al. 2015). Horvati et al. investigated the antimicrobial activity of 11 selected cationic peptides (including three cell penetrating peptides, Tat (Horváti et al. 2017; Subbalakshmi et al. 1996; Sitaram et al. 2003; National Center for Biotechnology Information 2020; Shaw et al. 2008; Walrant et al. 2011; Carmona et al. 2012; Jang et al. 2012; Rydberg et al. 2012; Bahnsen et al. 2013; Oh et al. 2014; Brezden et al. 2016; Miao et al. 2016), Penetratin and Transportan), six antimicrobial peptides (including Magainin II, Buforin II (Moreno and Giralt 2015; Milletti 2012; Radis-Baptista et al. 2017; Pooga and Langel 2015; Bechara and Sagan 2013; Figueiredo 2014; Saalik et al. 2011; Pae et al. 2014; Splith and Neundorf 2011; Hancock and Chapple 1999; xxxx; Hancock and Sahl 2006; Matsuzaki et al. 1995; Zhang et al. 2001; Henriques et al. 2006; Akishiba et al. 2017; Wang et al. 2016), GranF2, Dhvar4, Crot (Stewart et al. 2016; Brogden 2005; Avci et al. 2018; High levels of antibiotic resistance found worldwide 2018; Moreno and Giralt 2015; Milletti 2012; Radis-Baptista et al. 2017; Pooga and Langel 2015; Bechara and Sagan 2013; Li et al. 2012, 2015, 2016, 2013, 2016), CM15), Melittin as a positive control and OT20 as a negative control, in a comprehensive manner. These peptides were tested for both their antimicrobial activity against S. pneumonia and M. tuberculosis and their internalization into MonoMac6 cells was also assessed by using Cf-labelled peptides. The rate of internalization was determined by flow cytometer using 488 nm laser. Furthermore, their cytotoxicity against human PBMC cells and the hemolytic activity were assessed. It was observed that peptides that showed higher cytotoxicity against PBMC cells (including Melittin, Transportan, CM15 and Dhvar4) also exhibited higher hemolytic activity. GranF2, Magainin and Penetratin showed modest cytotoxicity and Tat (Sitaram et al. 2003; National Center for Biotechnology Information 2020; Shaw et al. 2008; Walrant et al. 2011; Carmona et al. 2012; Jang et al. 2012; Rydberg et al. 2012; Bahnsen et al. 2013; Oh et al. 2014; Brezden et al. 2016; Miao et al. 2016), Buforin II, Crot (Stewart et al. 2016; Brogden 2005; Avci et al. 2018; High levels of antibiotic resistance found worldwide 2018; Moreno and Giralt 2015; Milletti 2012; Radis-Baptista et al. 2017; Pooga and Langel 2015; Bechara and Sagan 2013; Li et al. 2012, 2015, 2016, 2013, 2016) and OT20 peptides did not show any cytotoxicity up to 300 μM. It’s noteworthy that Melittin, Transportan and Dhvar4 that showed higher cytoxicity and hemolytic activity, also exhibited higher internalization into MonoMac6 cells. Penetratin also exhibited high internalization as well as Melittin, Transportan and Dhvar4, however it showed lower cytotoxicity to both PBMC cells and RBCs. The results demonstrated that among all tested peptides, Penetratin is the promising antibacterial peptide with high selectivity and less cytotoxicity for mammalian cells that could be further applied as a delivery system for antibacterial agents delivery (Horváti et al. 2017).Over all, the results of this study emphasized the importance of combinational therapy in which different mode of actions provided by multiple active elements target various active sites within the microbe which lead to enhanced antimicrobial activity with lower chance of induction of resistance.
Shaw et al. conducted a study in which antimicrobial activity of three peptides, indolicidin and its analogues ILF and ILA, melittin and Tat was tested on Bacillus subtilis. Indolicidin is a short peptide with 13 amino acid residues (ILPWKWPWWPWRR-NH2) which was isolated from cytoplasmic granules of bovine neutrophils. The ILA analogue of this peptide has three alanines in its sequence instead of three prolines and the ILF analogue has five phenylalanines instead of tryptophans (Subbalakshmi et al. 1996; Sitaram et al. 2003). Melittin, is a 26 amino acids peptide that is isolated from honey bee venom (Apis mellifera) and is a well-known cytolytic peptide National Center for Biotechnology Information (2020). Results showed that melittin, indolicidin and its analogues exhibit great antimicrobial properties at micromolar concentrations against both Gram-positive/Gram negative bacteria and fungi. In contrast, Tat did not inhibit Gram-positive B. subtilis growth. No hemolysis was observed on fresh human derived erythrocytes neither for Tat peptide, nor other three peptides (Shaw et al. 2008).
Walrant et al. investigated antimicrobial properties of three CPPs; RL9, R9 and RW9 on E. coli and S. aureus. RL9 and R9 exhibited no antimicrobial activity against neither E.coli nor S. aureus. RW9 was observed to inhibit growth up to 99% on E. coli and S. aureus in 100 µM concentration. Interestingly, it was observed that E. coli is capable of re-growth when it was incubated with 25 µM and 50 µM of RW9 after 24 h, thus suggesting that RW9 is bacteriostatic not bactericidal. It is suggested that the antibacterial activity is due to the tendency of RL9, RW9 and R9 peptides to interact with anionic lipids and bacterial membrane is mainly composed of negatively charged lipids. Whereas Eukaryotic cell membrane is composed of zwitterionic lipids (Walrant et al. 2011).
Carmona et al. studied PAF26 peptide fungicidal effects on Saccharomyces cerevisiae and its mutant strain with YAP1 deletion to determine if endogenous ROS accumulation and nitric oxide (induced by the peptide) are the fungicidal causes. The data indicated that the mutant strain didn’t show sensitivity to oxidative stress and ROS accumulation wasn’t different from its parent strain. Thus ROS are not the primary mechanism of antifungal activity of PAF26 and it was revealed that nitric oxide production is the mechanism of antifungal action (Carmona et al. 2012). Jang et al. designed analogues of buforin IIb, buforin III in order to enhance the antimicrobial activity, with amino acid substitutions in proline hinge and α-helixes of buforin IIb. These peptides were tested on B. subtilis, S. aureus, E. coli, S. cerevisiae, candida albicans, pseudomonas putida, salmonella enteritidis and Cryptococcus neoformans. The antimicrobial activity of buforin III peptides is much higher than buforin IIb and among them Buf IIIb and Buf IIIc were found to have antimicrobial activity nearly sevenfold higher than Buf IIb. In vitro studies showed that these peptides are capable of binding to DNA and DNA binding affinity of the peptides is nearly correlated with their antimicrobial activity (Jang et al. 2012). With these findings, it is likely that the mode of action of buforin peptides is to cause DNA nicking and damage.
Rydberg et al. studied antimicrobial activity of six designed tryptophan/arginine peptides and melittin. It was observed that these designed peptides have antimicrobial activity against Gram-positive S. aureus and S. pyogenes while no growth inhibition was reported against Gram negative species like P. mirabilis and P. aeruginosa. There was no obvious connection between membrane interaction and the antimicrobial activity of these peptides, suggesting a different molecular mechanism (Rydberg et al. 2012). Bahnsen et al. studied antimicrobial activity of penetratin on E. coli and S. aureus as their models and the results showed that antimicrobial activity against Gram-negative E. coli was higher than Gram-positive S. aureus (Bahnsen et al. 2013).Again this study suggest that the antibacterial action of this peptide probably is a mechanism other than membrane damage as Gram negative bacteria are usually more resistant to AMPs due to complex cell wall structure in compare to Gram positive bacteria.
Oh et al. conducted a study to evaluate antimicrobial activity of 14 different synthesized cell-penetrating peptides with linear and cyclic structures against MRSA and P. aeruginosa. They showed that cyclic R4W4 is active against MRSA and generally the cyclic peptides offered higher activity against MRSA and P. aeruginosa than the linear counterparts. Cytotoxicity assay was performed on three eukaryotic cell lines, SK-OV-3, CCRF-CEM and HEK 293 T. The cell viability was more than 84% at a concentration of 15 µM (Oh et al. 2014).
A cell penetrating peptide-antibiotic conjugate to target intracellular pathogens was developed by Brezden et al. They conjugated kanamycin, an aminoglycoside antibiotic, to P14RLL, a cell penetrating peptide with antimicrobial activity, by tether with or without disulfide bond. They hypothesized that the disulfide bond will be reduced and cleaved in the intracellular environment and the kanamycin will be released and act against the pathogen. This dual conjugate was found to be effective in in vitro studies, in situ studies (the J774A.1 cells infected with pathogens) and in vivo study (in vivo C. elegans model). It was shown that this conjugate possesses selective activity towards bacteria which is crucial for in situ and in vivo treatments (Brezden et al. 2016).Dual antibiotic strategy, where one of the compound has CPP properties, could help many antibiotics to reach to their therapeutic concentrations inside the mammalian cells in treatment against intracellular bacteria.
Miao et al. isolated a cell penetrating peptide from kefir with a low molecular weight called F3. Surprisingly, this peptide was revealed to be effective on both bacterial and fungal species. The cell penetration studies on E. coli and S. aureus showed that F3can penetrate through cell membrane, accumulate in the cell and change the cellular morphology of the bacteria (Miao et al. 2016).
Budagavi et al. investigated the antimicrobial activity of latarcin derived peptide (LDP) and LDP-NLS on different bacteria and MRSA as a MDR bacteria. The data showed that LDP-NLS exhibit higher antibacterial effect on MRSA activity and less cytotoxicity to HeLa cells even at the high concentrations of the peptide, While LDP was cytotoxic for HeLa cells as it induces damages to the membrane. They also showed that bactericidal activity of LDP is mainly due to membrane damage on the MRSA cell membrane, while LDP-NLS may have intracellular targets (Budagavi and Chugh 2018). In another study, they tested these peptides on a fungus model, Fusarium solani. Both peptide exhibited excellent antifungal activity but LDP penetration into the hyphae and spores was very low which could limit their use (Budagavi et al. 2018).
AMPs (or Their Derivatives) as CPPs
In search for new CPPs, AMPs have received significant attention by many researchers as they share some important physicochemical properties, and hence possibly same function, with classic CPPs.
Luque-Ortega et al. investigated cell penetration activity of a human salivary antimicrobial peptide called Histatin 5 on leishmania. The results showed that this peptide penetrates well into the cell cytoplasm in a receptor-independent manner and then accumulates into the mitochondria as it does in fungi. Furthermore, it’s shown that this peptide inhibits F0F1-ATPase function and presumably, leading to cell death. This peptide showed no cytotoxicity against peritoneal mouse macrophages however it exhibited leishmanicidal activity on promastigotes. Moreover, the parasite burden was reduced significantly in macrophages infected with L. pifanoi axenic amastigotes. High selectivity of Histatin 5 towards leishmania, high penetration and accumulation in pathogen and targeting mitochondria, can make this peptide a potential candidate for efficient transportation of anti-leishmania drugs into pathogen (Luque-Ortega et al. 2008).
Fang et al. studied the cell penetrating activity of bovine lactoferricin derivative, bLFcin6, that is an AMP with only 6 amino acid residues. Accumulation of this peptide in cytoplasm and nucleus shown to be dose dependent. At a concentration of 1 µM peptide was seen mainly in cytoplasm of Hela cells while at the concentration of 10 µM the peptide distributes between cytoplasm and nucleus evenly. It was shown that bLFcin6 enters into the cells via lipid-raft mediated endocytosis pathways followed by destabilization of macropinosomes and release into the cytoplasm. The ability of this peptide, as a CPP, to deliver siRNA, as a cargo, into the cell was also investigated. Upon delivery of siRNA which target GAPDH, it was observed that it is functional as it shows knockout activity at both mRNA and protein levels. The results were comparable with the complex of Tat/ siRNA as a control. Of the utmost important is that the peptide didn’t show any significant cytotoxicity on HeLa cells making it promising for future use (Fang et al. 2013).
BP100 is an antimicrobial peptide originally developed to act against plant pathogens. In a study conducted by Eggenberger et al. the cellular uptake and functional cargo delivery of this peptide was assessed. Primarily, peptide was capable of entering plant cells and accumulated in the cytosol according to microscopy images via endocytosis independent pathways compared to fluorescently labeled 4-kDa dextran that permeates into target cells via endocytosis. As a proof of concept, they fused BP100 to a peptide which targets actin filament named Lifeact, in order to investigate whether BP100 as a cargo delivery tool is able to transport the functional cargo into tobacco BY-2 cells. They showed that the cargo entered cells and maintained its function. Actin filaments are central elements for intra and intercellular trafficking in plant cells. Conventional methods for live imaging of actin filaments possesses some major pitfalls like the need for cell fixation or the need to genetically modification of target cells. This new study proposes a promising new strategy for live imaging using CCP assisted actin targeting and detection with Lifeact peptide that is independent from fixation and transformation (Eggenberger et al. 2011).
Hu et al. studied a cationic 13-amino acids long antimicrobial peptide from bovine called indolicidin to assess its ability to deliver plasmid cargo into the cell as an anionic cargo. They tested DNA transfection using plasmid-indolicidin, plasmid-PEI and plasmid-PEI-indolicidin complexes. As a positive control they used Polyetheylenamin (PEI) which is a cationic polymer that is able to deliver oligonucleotide cargo to the cells at different C/P ratio (Taranejoo et al. 2015). They indicated that transfection rate using plasmid/indolicidin/PEI is higher than the other two complexes. Results also revealed that this complex displays greater loading efficiency than PEI and indolicidin alone (Hu et al. 2013).
[D]-K6L9 is an antimicrobial peptide previously proved to be able to kill cancer cells by membrane disruption mechanism. Zhang et al. designed and constructed a new peptide by adding a stearyl moiety to the N-terminal of [D]-K6L9 in order to enhance the ability of peptide to condense DNA. It was shown that this peptide can transfect nearly 100% of the cells while the parent peptide, [D]-K6L9, was unable to transfer plasmid cargo into cells. The main mechanism of DNA/peptide penetration was found to be caveolin-mediated since the uptake was decreased by 82% in cells treated with methyl-β-cyclodextrin, an inhibitor of caveolin-mediated endocytosis, in comparison to control cells (Zhang et al. 2013). A histone-derived antimicrobial peptide, called hipposin, has a sequence consisting of two other antimicrobial peptides; parasin and buforin II plus a C-terminal region. Bustillo et al., found that the C-terminal region is a cell-penetrating peptide capable of entering into the bacterial cells without any membrane disruption or cell death. While the parasin fragment of hipposin is bactericidal and showed activity on both Gram-positive and Gram negative bacterial strains by the means of membrane permeabilization mechanism. Surprisingly,, hipposin didn’t show any activity against eukaryotic cell lines, suggesting that it targets bacterial cell membrane specifically (Bustillo et al. 2014).
Do et al. studied the capacity of melittin for penetrating through skin as a vector ex vivo. They tested melittin on skin samples and found that after 24 h of peptide exposure the peptide penetrated into epidermis and dermis. They used penetratin and LMWP as controls and achieved same results as melittin. However, penetratin and LMWP penetration into viable epidermis and dermis layers were affected and decreased by cutaneous enzymes inhibitor. These results suggest that enzymatic cleavage of these peptides may has a role in penetration into the skin. Since AMPs and CPPs share many features, it can be postulated that cutaneous enzymes may have similar effects on melittin penetration into skin. This peptide left skin cells intact since no significant increase in IL-6 and IL-8 levels were observed. Anticancer effect of melittin was also investigated and it exhibited strong anticancer properties (Do et al. 2014).
Soler et al. introduced a novel cell-penetrating peptide from CECMEL11 library, called BP16. The CECMEL11 library contains peptides with a potent activity against plant pathogens. BP16 peptide was found to be non-toxic at a concentration of 200 µM against mammalian MCF-7, CAPAN 1 and 3T3 cell lines. Flow cytometry and confocal microscopy assays showed that this peptide is internalized into the cell and accumulates in the cytoplasm via clathrin dependent pathways. Moreover, BP16 potency as an anti-cancer drug delivery vector, chlorambucil, was investigated. Conjugates of chlorambucil-BP10 showed a significant increase in drug efficiency towards malignant cell types (MCF-7, 3T3 and CAPAN-1), and flow cytometry results indicated no significant difference in internalization of BP16 conjugate and BP16. These data show BP16 can effectively transport the therapeutic agents while maintaining its internalization efficiency in conjugated forms (Soler et al. 2014).
Tachyplesin is an antimicrobial peptide with two disulfide bonds in its structure, isolated from Tachypleus tridentatus. Jain et al. studied this peptide as a potential cell penetrating peptide and hence, a delivery tool. Uptake studies in plant and mammalian cells showed that this peptide can enter both types of cells via an endocytosis-independent pathway, since uptake rates showed no significant difference in response to endocytosis inhibitors. This peptide showed no significant cytotoxicity in HeLa cells while protoplasts viability was reduced after 14–15 h of peptide exposure. Further, they studied the cargo delivery efficacy in both cell types by delivery of the β-galactosidase enzyme followed by X-gal staining. It was observed that the cargo was delivered in more than 90% of the HeLa cells, 30% of the protoplasts and 99% of wheat root tips, coleoptiles and hypocotyls (Jain et al. 2015).
Zhang et al. developed a hybrid peptide with potent antimicrobial activity based on substance P called NS. Substance P is a 11 residue peptide which belongs to tachykinin family which has a role in important biological activities like cancer, wound healing, exocrine gland secretion, neuroendocrine, and immune regulation (Łazarczyk et al. 2007). Anticancer properties and peptide’s ability as a plasmid delivery tool were studied. Upon translocation NS was trafficked to the cell nucleus and it showed antitumor properties. Growth inhibition of cancerous cells seems to be by membrane disruption and DNA synthesis inhibition mechanisms. More analysis revealed that the peptide could be a potent DNA vector and as it was expected, the transfection rate was increased when the peptide modified to bear a stearyl moiety in its N-terminal (Zhang et al. 2014).
Conclusion
AMPs and CPPs, as they belong to Membrane Active Peptides family, share key characteristics such as charge and the ability of interaction with plasma membrane. However, in AMPs the ability of interacting with membrane finally leads to cell death or cell cycle arrest but in CPPs, peptide-membrane interactions result in penetration of the peptide into the cytoplasm and nucleus. Numerous studies that have been reviewed in this paper, indicate that some CPPs or their derivative could act as AMPs. CPPs possessing anti-microbial properties (Table 2) can be great candidates for clinical applications as both an efficient vector to transport therapeutic agents and acting as an anti-microbial agent itself. Given the importance of emerging of drug resistance among many clinically important bacterial pathogens, there is an urgent need to discover and develop novel anti-microbial compounds and exploiting CPPs in this regard could be one of them.
Similarly, AMPs that could function as CPPs (Table 3) are also summarized in this review. Since microbial cell membrane (and particularly bacterial cell wall) are different in composition in compare to mammalian cell membrane (especially the presence of cholesterol in animal cell membrane), many AMPs which usually act on membrane, could potentially interact with animal plasma membrane with minimal damage compare to microbial cell membrane. As many studies have shown, interaction of AMPs and their derivative with animal plasma membrane could lead to translocation of the peptide, and even the cargo of the peptide, into the cells, thus operating as CPPs. As a matter of fact, the search for new delivery tools including CPPs is an endless one as the transfection efficacy of many delivery tools varies depending of the cell type, cargo nature, experimental conditions and etc. In addition to that, CPPs have some shortcomings such as short circulating half-life, lack of oral bioavailability, being prone to degradation and short duration of action and thus introducing novel CPPs with biological source, i.e. AMPs, might cover some of the limitations. Further, some AMPs have intracellular targets which means their mechanism of action is not involved membrane damage and hence they are able to penetrate through to cell membrane and enter the cells. These kind of AMPs can be used as CPPs with minimal modifications.
Indeed, both AMPs and CPPs can be engineered by many approaches such as amino acid substitutions or conjugation with other peptides to achieve improved function with the least possible adverse effects.
References
Akishiba M, Takeuchi T, Kawaguchi Y, Sakamoto K, Yu H-H, Nakase I et al (2017) Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat Chem 9(8):751
Avci FG, Akbulut BS, Ozkirimli E (2018) Membrane active peptides and their biophysical characterization. Biomolecules 8(3):77
Bahnsen JS, Franzyk H, Sandberg-Schaal A, Nielsen HM (2013) Antimicrobial and cell-penetrating properties of penetratin analogs: effect of sequence and secondary structure. Biochimica et Biophysica Acta - Biomembranes 1828(2):223–232
Bahnsen JS, Franzyk H, Sayers EJ, Jones AT, Nielsen HM (2015) Cell-penetrating antimicrobial peptides - prospectives for targeting intracellular infections. Pharm Res 32(5):1546–1556
Bechara C, Sagan S (2013) Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett 587(12):1693–1702
Bobone S, Piazzon A, Orioni B, Pedersen JZ, Nan YH, Hahm KS et al (2011) The thin line between cell-penetrating and antimicrobial peptides: the case of pep-1 and pep-1-K. J Pept Sci 17(5):335–341
Bourré L, Giuntini F, Eggleston IM, Mosse CA, MacRobert AJ, Wilson M (2010) Effective photoinactivation of gram-positive and gram-negative bacterial strains using an HIV-1 Tat peptide-porphyrin conjugate. Photochem Photobiol Sci 9(12):1613–1620
Brezden A, Mohamed MF, Nepal M, Harwood JS, Kuriakose J, Seleem MN et al (2016) Dual targeting of intracellular pathogenic bacteria with a cleavable conjugate of kanamycin and an antibacterial cell-penetrating peptide. J Am Chem Soc 138(34):10945–10949
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Budagavi DP, Chugh A (2018) Antibacterial properties of Latarcin 1 derived cell-penetrating peptides. Eur J Pharm Sci 115:43–49
Budagavi DP, Zarin S, Chugh A (2018) Antifungal activity of Latarcin 1 derived cell-penetrating peptides against Fusarium solani. Biochim Biophys Acta 1860(2):250–256
Bustillo ME, Fischer AL, Labouyer MA, Klaips JA, Webb AC, Elmore DE (2014) Modular analysis of hipposin, a histone-derived antimicrobial peptide consisting of membrane translocating and membrane permeabilizing fragments. Biochim Biophys Acta 1838(9):2228–2233
Carmona L, Gandía M, López-García B, Marcos JF (2012) Sensitivity of saccharomyces cerevisiae to the cell-penetrating antifungal peptide PAF26 correlates with endogenous nitric oxide (NO) production. Biochem Biophys Res Commun 417(1):56–61
de Figueiredo IR, Freire JM, Flores L, Veiga AS, Castanho MA (2014) Cell-penetrating peptides: a tool for effective delivery in gene-targeted therapies. IUBMB Life 66(3):182–194
Do N, Weindl G, Grohmann L, Salwiczek M, Koksch B, Korting HC et al (2014) Cationic membrane-active peptides - anticancer and antifungal activity as well as penetration into human skin. Exp Dermatol 23(5):326–331
Eggenberger K, Mink C, Wadhwani P, Ulrich AS, Nick P (2011) Using the peptide Bp100 as a cell-penetrating tool for the chemical engineering of actin filaments within living plant cells. ChemBioChem 12(1):132–137
Eriksson OS, Geörg M, Sjölinder H, Sillard R, Lindberg S, Langel UL et al (2013) Identification of cell-penetrating peptides that are bactericidal to neisseria meningitidis and prevent inflammatory responses upon infection. Antimicrob Agents Chemother 57(8):3704–3712
Fang B, Guo HY, Zhang M, Jiang L, Ren FZ (2013) The six amino acid antimicrobial peptide bLFcin6 penetrates cells and delivers siRNA. FEBS J 280(4):1007–1017
Hancock RE, Chapple DS (1999) Peptide antibiotics. Antimicrob Agents Chemother 43(6):1317–1323
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557
Henriques ST, Melo MN, Castanho MARB (2006) Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J 399(1):1–7
High levels of antibiotic resistance found worldwide, new data shows 2018 available from https://www.who.int/mediacentre/news/releases/2018/antibiotic-resistance-found/en/.
Holm T, Bruchmann J, Scheynius A, Langel U (2012) Cell-penetrating peptides as antifungals towards Malassezia sympodialis. Lett Appl Microbiol 54(1):39–44
Horváti K, Bacsa B, Mlinkó T, Szabó N, Hudecz F, Zsila F et al (2017) Comparative analysis of internalisation, haemolytic, cytotoxic and antibacterial effect of membrane-active cationic peptides: aspects of experimental setup. Amino Acids 49(6):1053–1067
Hu WW, Lin ZW, Ruaan RC, Chen WY, Jin SLC, Chang Y (2013) A novel application of indolicidin for gene delivery. Int J Pharm 456(2):293–300
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:2559
Jain A, Yadav BK, Chugh A (2015) Marine antimicrobial peptide tachyplesin as an efficient nanocarrier for macromolecule delivery in plant and mammalian cells. FEBS J 282(4):732–745
Jang SA, Kim H, Lee JY, Shin JR, Kim DJ, Cho JH et al (2012) Mechanism of action and specificity of antimicrobial peptides designed based on buforin IIb. Peptides 34(2):283–289
Jung HJ, Jeong KS, Lee DG (2008) Effective antibacterial action of tat (47–58) by increased uptake into bacterial cells in the presence of trypsin. J Microbiol Biotechnol 18(5):990–996
Jung HJ, Park Y, Hahm KS, Lee DG (2006) Biological activity of Tat (47–58) peptide on human pathogenic fungi. Biochem Biophys Res Commun 345(1):222–228
Łazarczyk M, Matyja E, Lipkowski A (2007) Substance P and its receptors – a potential target for novel medicines in malignant brain tumour therapies (mini-review). Folia Neuropathol 45(3):99–107
Li L, Shi Y, Cheng X, Xia S, Cheserek MJ, Le G (2015) A cell-penetrating peptide analogue, P7, exerts antimicrobial activity against Escherichia coli ATCC25922 via penetrating cell membrane and targeting intracellular DNA. Food Chem 166:231–239
Li L, Shi Y, Cheserek MJ, Su G, Le G (2013) Antibacterial activity and dual mechanisms of peptide analog derived from cell-penetrating peptide against salmonella typhimurium and streptococcus pyogenes. Appl Microbiol Biotechnol 97(4):1711–1723
Li L, Shi Y, Su G, Le G (2012) Selectivity for and destruction of salmonella typhimurium via a membrane damage mechanism of a cell-penetrating peptide ppTG20 analogue. Int J Antimicrob Agents 40(4):337–343
Li L, Song F, Sun J, Tian X, Xia S, Le G (2016) Membrane damage as first and DNA as the secondary target for anti-candidal activity of antimicrobial peptide P7 derived from cell-penetrating peptide ppTG20 against Candida albicans. J Peptide Sci 22:427–433
Li L, Sun J, Xia S, Tian X, Cheserek MJ, Le G (2016) Mechanism of antifungal activity of antimicrobial peptide APP, a cell-penetrating peptide derivative, against Candida albicans: intracellular DNA binding and cell cycle arrest. Appl Microbiol Biotechnol 100(7):3245–3253
Long Zhu WL, Shin SY (2009) Effects of dimerization of the cell-penetrating peptide Tat analog on antimicrobial activity and mechanism of bactericidal action. J Pept Sci 15(5):345–352
Luque-Ortega JR, Van’t Hof W, Veerman ECI, Saugar JM, Rivas L (2008) Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J 22(6):1817–1828
Mahlapuu M, Håkansson J, Ringstad L, Björn C (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Matsuzaki K, Murase O, Fujii N, Miyajima K (1995) Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry 34(19):6521–6526
Miao J, Guo H, Chen F, Zhao L, He L, Ou Y et al (2016) Antibacterial effects of a cell-penetrating peptide isolated from kefir. J Agric Food Chem 64(16):3234–3242
Milletti F (2012) Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today 17(15–16):850–860
Moreno M, Giralt E (2015) Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins 7(4):1126–1150
Morris MC, Depollier J, Mery J, Heitz F, Divita G (2001) A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 19:1173
Nan YH, Park IS, Hahm KS, Shin SY (2011) Antimicrobial activity, bactericidal mechanism and LPS-neutralizing activity of the cell-penetrating peptide pVEC and its analogs. J Pept Sci 17(12):812–817
National center for biotechnology information. PubChem Database. Melittin, CID=16133648 available from https://pubchem.ncbi.nlm.nih.gov/compound/Melittin. Accessed 6 Jan 2020
Nekhotiaeva N, Elmquist A, Rajarao GK, Hällbrink M, Langel U, Good L (2004) Cell entry and antimicrobial properties of eukaryotic cell-penetrating peptides. FASEB J 18(2):394–396
Oh D, Sun J, Nasrolahi Shirazi A, Laplante KL, Rowley DC, Parang K (2014) Antibacterial activities of amphiphilic cyclic cell-penetrating peptides against multidrug-resistant pathogens. Mol Pharm 11(10):3528–3536
Pae J, Säälik P, Liivamägi L, Lubenets D, Arukuusk P, Langel Ü et al (2014) Translocation of cell-penetrating peptides across the plasma membrane is controlled by cholesterol and microenvironment created by membranous proteins. J Control Release 192:103–113
Park N, Yamanaka K, Tran D, Chandrangsu P, Akers JC, De Leon JC et al (2009) The cell-penetrating peptide, pep-1, has activity against intracellular chlamydial growth but not extracellular forms of chlamydia trachomatis. J Antimicrob Chemother 63(1):115–123
Pooga M, Langel U (2015) Classes of cell-penetrating peptides. Methods Molecular Biol 1324:3–28
Radis-Baptista G, Campelo IS, Morlighem JRL, Melo LM, Freitas VJF (2017) Cell-penetrating peptides (CPPs): from delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis. J Biotechnol 252:15–26
Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R et al (2002) New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo. Mol Ther 5(2):104–114
Rydberg HA, Carlsson N, Nordén B (2012) Membrane interaction and secondary structure of de novo designed arginine-and tryptophan peptides with dual function. Biochem Biophys Res Commun 427(2):261–265
Saalik P, Niinep A, Pae J, Hansen M, Lubenets D, Langel U et al (2011) Penetration without cells: membrane translocation of cell-penetrating peptides in the model giant plasma membrane vesicles. J Control Release 153(2):117–125
Shaw JE, Epand RF, Hsu JCY, Mo GCH, Epand RM, Yip CM (2008) Cationic peptide-induced remodelling of model membranes: direct visualization by in situ atomic force microscopy. J Struct Biol 162(1):121–138
Sitaram N, Subbalakshmi C, Nagaraj R (2003) Indolicidin, a 13-residue basic antimicrobial peptide rich in tryptophan and proline, interacts with Ca(2+)-calmodulin. Biochem Biophys Res Commun 309(4):879–884
Soler M, González-Bártulos M, Soriano-Castell D, Ribas X, Costas M, Tebar F et al (2014) Identification of BP16 as a non-toxic cell-penetrating peptide with highly efficient drug delivery properties. Org Biomol Chem 12(10):1652–1663
Splith K, Neundorf I (2011) Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J 40(4):387–397
Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF (2016) In vitro and ex vivo strategies for intracellular delivery. Nature 538(7624):183–192
Subbalakshmi C, Krishnakumari V, Nagaraj R, Sitaram N (1996) Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett 395(1):48–52
Taranejoo S, Liu J, Verma P, Hourigan K (2015) A review of the developments of characteristics of PEI derivatives for gene delivery applications. J Appl Polym Sci. https://doi.org/10.1002/app.42096
Vives E, Brodin P, Lebleu B (1997) A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272(25):16010–16017
Walrant A, Correia I, Jiao CY, Lequin O, Bent EH, Goasdoué N et al (2011) Different membrane behaviour and cellular uptake of three basic arginine-rich peptides. Biochim Biophys Acta 1808(1):382–393
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44(D1):D1087–D1093
Xie J, Gou Y, Zhao Q, Li S, Zhang W, Song J et al (2015) Antimicrobial activities and action mechanism studies of transportan 10 and its analogues against multidrug-resistant bacteria. J Pept Sci 21(7):599–607
Zhang L, Rozek A, Hancock RE (2001) Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem 276(38):35714–35722
Zhu WL, Hahm KS, Shina SY (2009) Cell selectivity and mechanism of action of short antimicrobial peptides designed from the cell-penetrating peptide pep-1. J Pept Sci 15(9):569–575
Zhu WL, Lan H, Park IS, Kim JI, Jin HZ, Hahm KS et al (2006) Design and mechanism of action of a novel bacteria-selective antimicrobial peptide from the cell-penetrating peptide Pep-1. Biochem Biophys Res Commun 349(2):769–774
Zhang W, Song J, Liang R, Zheng X, Chen J, Li G et al (2013) Stearylated antimicrobial peptide [D]-K6L9 with cell penetrating property for efficient gene transfer. Peptides 46:33–39
Zhang Y, Song J, Zhang W, Liang R, Ma Y, Zhang L et al (2014) Functional properties of a novel hybrid antimicrobial peptide NS: Potent antitumor activity and efficient plasmid delivery. J Pept Sci 20(10):785–793
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nooranian, S., Oskuee, R.K. & Jalili, A. Antimicrobial Peptides, a Pool for Novel Cell Penetrating Peptides Development and Vice Versa. Int J Pept Res Ther 27, 1205–1220 (2021). https://doi.org/10.1007/s10989-021-10161-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-021-10161-8