Abstract
Currently bioactive peptides are the main focus in attempts to identify novel angiotensin-converting enzyme inhibitors (ACEIs) for the treatment of hypertension due to their fewer side effects. In the present study we aimed to investigate the antihypertensive activity of 4 synthetic tripeptides Ornithyl-Hydroxyprolyl-Proline (Orn-Hyp-Pro OhPP) Ornithyl-Prolyl-Hydroxyproline (Orn-Pro-Hyp OPhP) Citrullyl-Hydroxyprolyl-Proline (Cit-Hyp-Pro ChPP) and Citrullyl-Prolyl-Hydroxyproline (Cit-Pro-Hyp CPhP) in vitro and in vivo. The ACE inhibitory activity and mode were analyzed with a modified spectrophotometric method and Lineweaver-Burk plots respectively. It showed that peptide Citn-Hyp-Pro (ChPP) exhibited the highest inhibition potency with an IC50 value of 40.48 μM and displayed a competitive inhibition of ACE. Molecular docking simulations suggested that ChPP could form several critical hydrogen bonds with the major residues His353 and His513 located in the S2 active site of ACE. The systolic blood pressure of spontaneously hypertensive rats monitored by the tail-cuff method was significantly reduced by 15.54% at 4 h after oral administration of 20 mg/kg BW ChPP. Also ChPP remarkably downregulated the angiotensin II receptor type 1 (agtr1) and miR-132/-212 levels in SHRs which was similar to that observed in SHRs treated with captopril. It showed us that the tripeptide ChPP could be explored as a promising ACE inhibitor (ACEI) for treatment of hypertension.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertension (HT), characterized by elevated systemic blood pressure (BP), is one of the most common chronic diseases that affects over one billion people worldwide (Mills et al. 2016). It is a major risk factor for cardiovascular disease (CVD). Angiotensin-I converting enzyme (ACE EC3.4.15.1), a zinc-containing dipeptidase, plays an important role in the regulation of BP by catalyzing the cleavage of angiotensin-I (Ang I) to the potent vasopressor angiotensin-II (Ang II) (Hayes et al. 2016) and the degradation of the vasodilator bradykinin (Regoli and Gobeil 2015). Consequently, ACE is considered as a therapeutic target for the treatment of HT.
Apart from the direct vasoconstriction, Ang II is also an elicitor for expression of some genes and miRNAs associated with HT development. Ang II induced mRNA expression of its receptor AT1R, which is a G-coupled receptor mediating many cardiac functions (Vamos et al. 2014), and expression of miR-132/212 was up-regulated in aortas from Ang II-infused mice and in Ang II-induced HT patients(Jin et al. 2012; Eskildsen et al. 2013), suggested they might play an important role in Ang II-induced hypertension.
Many potent synthetic ACE inhibitors (ACEIs) are currently used for the clinical management of HT, such as captopril, enalapril, and perindopril (Ling et al. 2018). Despite their established effectiveness, therapies with these drugs are associated with some clinically undesirable side effects, such as dry cough, skin rash, angioedema, and renal impairment (Yu et al. 2018), necessitating the need to develop new ACEIs with higher efficacy and fewer side effects.
Accordingly, bioactive peptides with ACE-inhibitory activities have attracted more and more attention due to their low toxicity. An increasing number of ACE-inhibitory peptides, either naturally derived (Lee and Hur 2017; Miralles et al. 2018) or artificially synthetic (Panyayai et al. 2018), have been identified. Among them, proline (Pro) residue is preferred at C-terminal of a potent ACE inhibitors and confers resistance to degradation by digestive enzymes (Miralles et al. 2018; Jao et al. 2012), and hydroxyproline (Hyp) in peptides, especially at the penultimate position, played an important role in interacting effectively with the active pocket of ACE (Saiga et al. 2003; Taga et al. 2018). In addition, the activity of ACE-inhibitory peptides is also related to their molecular mass, and small peptides with 2–12 amino acids long and molecular weights less than 3000 Da are considered to better fit the active sites of ACE (Sun et al. 2019; Toopcham et al. 2017). Several studies also reported that peptides having a hydrophobic amino acid at the N-terminus possess potent ACE inhibitory activity, and a basic amino acid at the N-terminus also can enhance the affinity of the peptide for ACE further increasing antihypertensive activity (Lee and Hur 2017; Sun et al. 2019; Toopcham et al. 2017).
Besides 20 standard amino acids, there are many non-standard amino acids, among which citrulline (l-Cit) has no net charge and increases the hydrophobicity of protein with its content increase, and ornithine (l-Orn) is a basic amino acid. They all possess antioxidant activity and vascular protective properties (Allerton et al. 2018; Coles 2007; Butterworth and Canbay 2019), which might be beneficial in reducing side effects (Sun et al. 2017). Therefore, in this study, based on these ideas above, four tripeptides containing an N-terminal l-Cit or l-Orn, with Pro-Hyp or Hyp-Pro dipeptide motifs at the C-terminus were designed. Then, their ACE inhibitory activity, inhibition mode, antihypertensive effects, and interaction with ACE were analyzed. The results suggested that ChPP is a potent candidate for hypertension prevention.
Materials and Methods
Materials
The tripeptides Ornithyl-Hydroxyprolyl-Proline (Orn-Hyp-Pro, OhPP), Ornithyl-Prolyl-Hydroxyproline (Orn-Pro-Hyp, OPhP), Citrullyl-Hydroxyprolyl-Proline (Cit-Hyp-Pro, ChPP), and Citrullyl-Prolyl-Hydroxyproline (Cit-Pro-Hyp, CPhP) were synthesized and provided by GL Biochem (Shanghai) Ltd (Shanghai, China) with purity > 95%. Hippuryl-l-histidyl-l-leucine (HHL) and Hippuric acid (HA) were purchased from Sigma Chemical Co. (Louis, USA). All chemicals used were chemically pure. Spontaneously hypertensive rats (SHRs) were bought from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China).
Ethics Statement
All animal studies were performed in conformance with the Guide for the Care and Use of Laboratory Animals of the Jiangsu Vocational College of Medicine and approved by the Committee on the Ethics of Animal Experiments of the Jiangsu Vocational College of Medicine (Permit Number: 20,178,102).
ACE inhibition Assay
Prior to ACE inhibition assay, the ACE extracts from rabbit lung were prepared and its activity was determined according to our previous study (Qian et al. 2019). Subsequently, the tripeptides OhPP, OphP, ChPP, and CPhP were dissolved in 100 mmol/l borate buffered saline (BBS) containing 10% Triton X-100 and 0.1% DMSO as cosolvents at pH of 8.3. ACE inhibitory activity was assayed according to the procedure (listed in Table 1) described by Gao et al. (2011). After reaction stopped by HCl, 1.5 ml of ethyl acetate was added and mixed with a vigorous shaking for 1 min followed by a centrifugation at 3000 rpm for 5 min. Point five milliliter of the upper lipid phase was transferred into a new tube, and 2.5 ml of 0.5% diaminobenzidine (DAB) was added in and mixed well by vortex. Afterwards the tubes were heated to 40 °C and placed for 30 min in dark for chromogenic reaction between HA and DAB. Finally optical density value of the reacts were detected at 456 nm using a spectrophotometer (UV-160A, Shimadzu Corporation, Kyoto, Japan). Mixture of 0.5 ml of ethyl acetate and 2.5 ml of 0.5% DAB was used as a blank to eliminate background noise. Each assay was finished with triplicates.
ACE inhibition rate (%) was calculated according to Eq. 1. The IC50 value was defined as the concentration of inhibitor required to inhibit 50% of the ACE activity.
where A was the absorbance of the reaction with the buffer instead of tested samples, S was the absorbance of the reaction with tested sample, and C was the absorbance of the reaction in which HCl was added prior to HHL.
Kinetics of ACE Inhibition
The substrate HHL (0.5, 1, 2 and 4 mmol/l) was mixed with ACE (80 U/l) and a series of synthetic tripeptides, respectively, and incubated at 37 °C for 10 min. Lineweaver–Burk plots were constructed for determining the mode of inhibition. The Km and Ki were calculated based on the amount of HA released.
Molecular Docking
The 3D structures of the four peptides were generated and energy-minimized with ChemOffice 2004 (CambridgeSoft Co., MA, USA). The crystal structure of human ACE was obtained from the RCSB Protein Data Bank (1O86.pdb). Before docking, the ACE protein was first extracted along with its cofactors chloride and zinc, and the polar hydrogens were then added. Afterwards, Molecular docking simulations were then performed using the AutoDock Vina software (v1.1.2) (Morris et al. 2009) in order to predict the binding affinities and conformations of each compound with ACE. The ranked docking pose of the peptide tested in the active site of ACE was determined according to the binding affinity (kcal/mol).
Cells Viability Assay
Human umbilical vein endothelial cells (HUVECs) (BNCC347734) were obtained from BeNa Culture Collection (Jiangsu, China) and cultured according to the description of Villars et al. (2002). The procedure is to determine the cell viability in 96 well plates using a Cell Counting Kit-8 (VICMED, Jiangsu, China) after 24 h stimulation with the candidate tripeptides, consulting the method described by Qian et al. (2017). Briefly, the HUVEC cells frozen at − 80 °C were removed and quickly melted in a water bath at 37 °C. Then, the cells were centrifuged at 1000 rpm for 3 min. After removing the supernatant, the cells were resupended with ECM medium and seeded into a 9 cm culture dish containing the prepared medium (ECM medium supplemented with 1% ECGS, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) and cultured in the incubator with 5% CO2 and 95% humidity at 37 °C. To investigate cytotoxity, 100 l cell suspension, about 1 × 105 was seeded in a 96-well plate and cultured in an incubator for 24 h so that they sticked to the bottom. Subsequently, 10 μl of ChPP, CPhP, OhPP or OPhP solutions with different concentrations (1.25, 2.5 and 5.0 μg/ml) were added to each well of the 96-well plates, and then incubated in the incubator for 12 h, followed by adding 10 μl ccK-8 solution to each well. After incubation in the incubator for 4 h, the absorbance at 450 nm was determined by a microplate reader. The viability were evaluated with the following equation (Eq. 2):
Antihypertensive Tests In Vivo
Twelve-week-old male SHRs (about 320 g body weight BW SPF) with tail systolic blood pressure (SBP) of over 180 mmHg were employed. They were housed in cages and cared according to standards for experimental rat care (Qian et al. 2019). SHRs were acclimatized for 7 days prior to experiment and randomly divided into three groups: ChPP group (n = 6), captopril group (n = 6), and control group (normal saline n = 6). A standard laboratory rat diet (SLACOM, Shanghai, China) and tap water were freely available. After administration with 10 or 20 mg tripeptide/kg BW, or 2 mg/kg BW captopril, or normal saline with equal volume by gavage, the SBP of each rat was measured by the tail-cuff method with a programmable electrosphygmomanometer (model Softron BP-98A; Softron Beijing Incorporated, Beijing, China) at 0.5 h interval. For an accurate SBP reading, rats were placed in a chamber maintained at 37 °C to adapt to the SBP measurement device environment for at least 10 min prior to measurement. Data were expressed as means ± SD (n = 6).
Effects of Candidate Peptides on Transcriptional Levels of miR-132 miR-212 and Agtr1
To further determine the ACE inhibitory activity of these designed tripeptides, transcriptional levels of agtr1 and miR-132/-212 regulated by Ang II were assayed, respectively. Following administration by oral gavage, the microRNA and large fragment RNA were isolated from the hearts of SHRs at timepoint of the lowest blood pressure using E.Z.N.A™ miRNA kit (OMEGA Biotek, Norcross, GA, USA). Reverse transcription of mRNA and miRNA was completed with the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) following methods reported by Qian et al. (2019), and the transcriptional levels were evaluated by qRT-PCR with AceQ™ qPCR SYBR Green Master Mix (Vazyme Biotech, Nanjing, China). Primers for reverse transcription and qRT-PCR were listed in supplemental Table S1. GAPDH gene and small nuclear RNA (SnRNA) U6 were used as endogenous control to determine relative mRNA and miRNA expression, respectively. All reactions were run in triplicate and presented as means ± SD (n = 6). The relative quantification analysis of expression levels of mRNA and miRNA was done by 2−ΔΔCT method.
Statistical Analysis
Values are presented as the mean ± SD or SE. Statistical significance of differences was evaluated by Student’s t test (p < 0.05) using the software SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
ACE Inhibitory Activities of the Tripeptides
ACE was prepared as the crude extracts from rabbit lung and its activity was determined as 0.747 ± 0.043 U/ml. For exploring novel ACE-inhibitors, 4 l-proline based derivates were designed and chemically synthesized (Table 2). It suggested that the hydrophobicity of the peptides with N-terminal Citn residue were less than those with N-terminal Orn residue based on the ClogP and TSPA values. As shown in Table 2, the activity of ChPP (IC50 = 40.48 ± 0.47 μmol/l) was significantly higher than that of CPhP (IC50 = 139.15 ± 2.69 μmol/l). Meanwhile, OhPP (IC50 = 212.35 ± 4.90 μmol/l) exhibited a significantly higher activity than OPhP (IC50 = 1272.35 ± 187.69 μmol/l).
ACE Inhibitory Kinetics
The ACE inhibition mode of the synthetic tripeptides was evaluated using the Lineweaver–Burk plots. As it can be seen in Fig. 1, for the designed peptides, all the lines crossed at the same y-intercept, but at different slopes and x-intercepts, suggesting a competitive inhibition pattern towards ACE.
Besides, the inhibition constant (Ki), an indicator of binding affinity to ACE enzyme, was calculated for these competitive inhibitor. As listed in Table 2, the Ki value followed an order: ChPP (0.59 μmol/l) < CPhP (0.81 μmol/l) < OhPP (1.85 μmol/l) < OPhP (12.23 μmol/l), which was consistent with their ACE inhibitory activity.
ACE Molecular Docking
To further understand the inhibition mechanism of the tripeptides, molecular docking studies were performed by the AutoDock Vina software (v1.1.2) (Trott and Olson 2010). The binding affinities of ChPP, CPhP, OhPP, and OPhP were shown in Table 2. It is noted that ChPP exhibits the highest binding activity with a binding affinity value of − 7.6 kcal/mol, followed by CPhP (− 7.5 kcal/mol), OhPP (− 6.4 kcal/mol), and OPhP (− 6.4 kcal/mol), respectively.
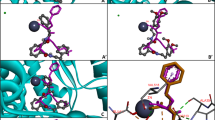
The active site of ACE is composed of three active pockets: S1 (Ala354 Glu384 and Tyr523), S2 (Gln281 His353 Lys511 His513 and Tyr520), and S1′ (Glu162 residue) (Rohit et al. 2012). Additionally, zinc ion (Zn2+) in the active site plays an essential role in ACE catalytic activity and is tetrahedrally coordinated by His383, His387, and Glu411 residues in ACE (Spyroulias et al. 2004). The optimal docking pose of the tripeptides in relation to ACE is displayed in Fig. 2. The carbonyl group at N-terminal of ChPP could coordinate with His353 and His513 in S2 active pocket by hydrogen bonds with distance of 2.167 and 2.142 Å, respectively (Fig. 2a, b). The interaction between CPhP and ACE was mediated through hydrogen bonds between carboxyl group at C-terminal and carbonyl group at N-terminal of CPhP and Tyr523 and Ala354 in S1 active pocket of ACE with distance of 2.216 and 2.073 Å, respectively, and hydrogen bonds between amine group at N-terminal of CPhP and Lys511 in S2 active pocket of ACE with distance of 2.224 Å (Fig. 2c, d). The 2-amine group of Ornithine residue of OhPP formed hydrogen bond interaction with Glu384 in S1 active pocket of ACE with distance of 2.196 Å (Fig. 2e, f). Interaction of OPhP with ACE was same as that exhibited between OhPP and ACE with distance of 2.113 Å (Fig. 2g, h).
Effect of ACE Inhibitory Peptides on HUVECs Viability
The effect of candidate tripeptides on the viability of HUVECs was estimated using the CCK-8 assay. The results showed that all candidate tripeptides slightly reduced the viability of HUVECs by 1.80–8.12% compared with control group (Fig. 3), suggesting that the concentration of candidate tripeptides (5 μg/ml) showed no cellular toxicity.
Docking confirmation of candidate tripeptides (red) and ligand compound captopril (yellow) with tACE. Zinc ion is shown as blue sphere (a, c, e, g). Interactions between peptides (sticks) and the residues of tACE (lines) (b, d, f, h). Green dotted lines indicates the formation of hydrogen bonds. Image obtained with AutoDock Tools software.
Effect of ACE Inhibitory Peptides on the SBP of SHRs
Based on the above results, the antihypertensive activity of the tripeptide ChPP was further investigated in acute antihypertensive tests using SHRs. Male SHRs were administrated intragastrically with the ChPP. Captopril was used as a positive control. ChPP (20 mg/kg BW) induced a significant reduction of SBP, with the largest decline of SBP by about 15.54% occurring at 4 h (p < 0.05), which was slightly less than that of captopril (10 mg/kg BW) (Fig. 4). SBP in the ChPP group then began to recover and almost returned to the basal level at 5 h. Increasing doses of ChPP to 20 mg/kg BW reduced SBP to a greater extent. Nevertheless, the changes did not reach statistical significance (Fig. 4).
Effects of Tripeptides on Transcriptional Levels of Agtr1 and miR-132/-212
To further illuminate the ACE inhibitory effect of the tripeptides, the effects of tripeptides on expression levels of Agtr1 mRNA and miR-132/212 were assayed by qRT-PCR in the aorta of SHRs (Fig. 5). As depicted in Fig. 5, ChPP significantly downregulated the transcriptional levels of Agtr1 and miR-132/-212 (p < 0.05). It is noted that Agtr1 level was significantly lower in captopril group than that of ChPP group (p < 0.05), while the levels of miR-132/-212 were comparable between the two groups.
Discussion
Acting as a vital player in the renin-angiotensin system (RAS) by activating vasoconstrictor Ang II and inactivating vasodilator bradykinin, the overactivity of ACE results in high blood pressure, which makes it become an important target for clinical drugs, such as captopril, lisinopril, and enalapril, belonging to ACEIs structurally containing C-terminal proline residue. Recently, natural peptides composed of amino acids are preferred because of the side effects of synthetic peptidomimetic drugs.
In this study, we designed four tripeptides and evaluated their ACE inhibitory activity in vitro and antihypertensive effect in vivo. Our results are consistent with previous studies reporting that the presence of hydrophobic amino acids at the C-terminus could potentiate the ACE inhibitory activity of the peptide (Miralles et al. 2018; Jao et al. 2012; Taga et al. 2018; Ruiz et al. 2004). Ruiz et al. (2004) revealed that ACE only binds weakly with peptide inhibitors that have penultimate Pro residues while the presence of Pro at the C-terminal has demonstrated to enhance binding to the enzyme (Ruiz et al. 2004). In addition, it is also demonstrated that substitution of Hyp with Pro in “X-Hyp-Gly” type tripeptides dramatically decreased the ACE-inhibitory activity of tripeptides (Taga et al. 2018). In contrast, the ACE inhibitory activity was dramatically decreased by prolyl hydroxylation in the case of C-terminal Pro-containing “Gly-X-Pro” type tripeptide (Taga et al. 2018). Our results exhibited the same pattern that the ACE-inhibitory activity of “X-Hyp-Pro” type tripeptide was stronger than that of “X-Pro-Hyp” type ones. Table 2 also shows that the ACE-inhibitory activity of ChPP is significantly higher than that of OhPP. It might be due to that Orn is more hydrophilic than Citn, leading to the greater hydrophilicity of the peptide OhPP than that of ChPP (as reflected by the Clog/TPSA values in Table 2), which increased the solubility of OhPP in the aquae phase, but on the other hand, restricts the entry of the peptide to the ACE active site as reported by prior studies (Li et al. 2017; Ashok and Aparna 2017), thus reducing the ACE inhibitory activity.
The result of ACE inhibition mode analysis revealed that all the designed peptides here can compete with the substrates for the catalytic site of ACE or can alter the ACE conformation thereby reducing the enzymatic activity (Jao et al. 2012). It also indicated that the peptides can bind to the ACE at positions including both the active and other regulatory sites, consequently preventing catalysis.
The Ki value of ChPP (0.59 μmol/l) was lower than that of other three peptides indicating higher binding ability of ChPP to ACE which is in line with their respective IC50 values. Many Peptides are found to be competitive inhibitors for ACE, such as commercial tripeptides IPP and VPP (FitzGerald et al. 2004). However, there were also some mixed inhibition peptides, such as Phe-Glu-Asp-Tyr-Val-Pro-Leu-Ser-Cys-Phe (FEDYVPLSCF) and Phe-Asn-Val-Pro-Leu-Tyr-Glu (FNVPLYE ) with IC50 of 10.77 and 7.72 μmol/l, respectively (Ahn et al. 2012). Forghani et al. (2016) also obtained three peptides (EVSQGRP, VSRHFASYAN, and SAAVGSP) from Stichopus horrens, exhibiting mixed inhibition patterns with IC50 of 50, 80, and 210 μmol/l, respectively (Forghani et al. 2016).
The binding affinity of all four tripeptides are in line with their in vitro ACE inhibitory activity (IC50) in this study. It is known that His353 and His513 were conserved in various ACE homologues (Sturrock et al. 2004) and played a vital role in ACE activity. The stability of the ACE-captopril complex was mainly attributed to the hydrogen bonds between the carbonyl group of captopril and His353 and His513 residues of ACE, respectively (Bhuyan and Mugesh 2011). The best ACE inhibitory activity of ChPP might be due to its interaction with His353 and His513 in ACE S2 pocket, even though the interaction between C=O groups of ChPP and Zn2+ at optimal state (with distance > 3 Å) was not a potential active site, compareed with the interaction between the S–H group of captopril and Zn2+ (with distance about 2.5 Å) (data not shown).
The in vivo antihypertensive activity of ChPP in SHRs in our study was comparable or even better than antihypertensive peptides under similar conditions reported in previous studies (Jung et al. 2006; Ko 2012). Jung et al. (2006) found that administration of 10 mg/kg BW peptides MIFPGAGGPEL caused SBP reduction of 22 mmHg (about reduction of 11%) in SHRs at 3 h after administration, and the effect was maintained for 9 h. Ko (2012) described that administration of the peptide VEGY (dose: 10 mg/kg BW) led to a reduction of SBP by about 11.2% and activities were maintained for 6 h. Thus, our results clearly indicated that ChPP can be explored as a peptide candidate for the hypertension management.
In RAS, Ang II is the major executor downstream of ACE. Ang II not only elevates blood pressure but also stimulates expression of some genes and miRNAs involved in cardiovascular system (Vamos et al. 2014; Mitra et al. 2010; Jin et al. 2012; Eskildsen et al. 2013). Mitra et al. (2010) described that expression of Ang II type 1 receptors (agtr1) in cardiovascular regions of the brain was increased as increasing level of Ang II or prolonging exposure to Ang II (Mitra et al. 2010). Besides, chronic infusion of Ang II could up-regulate level of miRNA-132/-212 in the heart and aorta of in hypertensive rats (Jin et al. 2012; Eskildsen et al. 2013). From the data, we could find that the mRNA expression of agtr1 significantly was suppressed by captopril more than by Peptide, but the expression of miRNA-132/-212 were same. It might be attributed to the direct interaction between Ang II and agtr1, but the intermediation between Ang II and miRNA-132/-212. And, there were other factors involved in the expression regulation of miRNA-132/-212 (Kumarswamy et al. 2014). Totally, we could conclude that ChPP could serve as a potent bioactive agent, inhibiting ACE to produce Ang II to lower blood pressure, although endogenous level of Ang II was not detected. Furthermore more studies should be executed to evaluate its safety compared to current ACEIs.
Conclusions
This study demonstrated that ChPP, one of 4 synthetic tripeptidic derivatives of proline, exhibited the most potent in vitro ACE inhibitory activity with a competitive inhibition mode. What‘s more, molecular docking simulation confirmed its interaction with ACE S2 active pocket, and it reduced the SBP almost to a similar extent to that of captopril. The down-regulation of Agtr1 mRNA and miR-132/-212 further suggested that ChPP might be explored as a potential antihypertensive candidate for further utilization.
References
Ahn CB, Jeon YJ, Kim YT, Je JY (2012) Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process Biochem 47(12):2240–2245. https://doi.org/10.1016/j.procbio.2012.08.019
Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA (2018) l-Citrulline supplementation: impact on cardiometabolic health. Nutrients 10(7):921. https://doi.org/10.3390/nu10070921
Ashok NR, Aparna HS (2017) Empirical and bioinformatic characterization of buffalo (Bubalus bubalis) colostrum whey peptides and their angiotensin I-converting enzyme inhibition. Food Chem 228:582–594. https://doi.org/10.1016/j.foodchem.2017.02.032
Bhuyan BJ, Mugesh G (2011) Effect of peptide-based captopril analogues on angiotensin converting enzyme activity and peroxynitrite-mediated tyrosine nitration. Org Biomol Chem 9(14):5185–5192. https://doi.org/10.1039/C1OB05148B
Butterworth RF, Canbay A (2019) Hepatoprotection by l-ornithine l-aspartate in non-alcoholic fatty liver disease. Dig Dis 37:63–68. https://doi.org/10.1159/000491429
Coles KE (2007) Investigation into the antioxidant capacity of l-arginine and l-citrulline in relation to their vascular protective properties. PhD Thesis, Cardiff University. http://orca.cf.ac.uk/id/eprint/55641
Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP (2013) Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 14(6):11190–11207. https://doi.org/10.3390/ijms140611190
FitzGerald RJ, Murray BA, Walsh DJ (2004) Hypotensive peptides from milk proteins. J Nutr 134:980S-988S. https://doi.org/10.1093/jn/134.4.980S
Forghani B, Zarei M, Ebrhimpour A, Philip R, Bakar J, Hamid AA, Saari N (2016) Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens: stability study against the ACE and inhibition kinetics. J Funct Foods 20:276–290. https://doi.org/10.1016/j.jff.2015.10.025
Gao DD, Cao YS, Mai X (2011) Modified spectrophotometric method for assay of angiotensin I-converting enzyme inhibitory activity of food-derived peptides. J Zhejiang Univ Agric Life Sci 37(2):219–223. https://doi.org/10.3785/j.issn.1008-9209.2011.02.015
Hayes M, Mora L, Hussey K, Aluko RE (2016) Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innov Food Sci Emerg 37:253–260. https://doi.org/10.1016/j.ifset.2016.03.014
Jao CL, Huang SL, Hsu KC (2012) Angiotensin I-converting enzyme inhibitory peptides: inhibition mode bioavailability and antihypertensive effects. BioMedicine 2(4):130–136. https://doi.org/10.1016/j.biomed.2012.06.005
Jin W, Reddy MA, Chen Z, Putta S, Lanting L, Kato M, Park JT, Chandra M, Wang C, Tangirala RK, Natarajan R (2012) Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J Biol Chem 287(19):15672–15683. https://doi.org/10.1074/jbc.M111.322669
Jung WK, Mendis E, Je JY, Park PJ, Son BW, Kim HC, Choi YK, Kim SK (2006) Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem 94(1):26–32. https://doi.org/10.1016/j.foodchem.2004.09.048
Ko SC (2012) A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem 47:2005–2011. https://doi.org/10.1016/j.procbio.2012.07.015
Kumarswamy R, Volkmann I, Beermann J, Napp LC, Jabs O, Bhayadia R, Melk A, Ucar A, Chowdhury K, Lorenzen JM, Gupta SK, Batkai S, Thum T (2014) Vascular importance of the miR-212/132 cluster. Eur Heart J 35(45):3224–3231. https://doi.org/10.1093/eurheartj/ehu344
Lee SY, Hur SJ (2017) Antihypertensive peptides from animal products marine organisms and plants. Food Chem 228:506–517. https://doi.org/10.1016/j.foodchem.2017.02.039
Li B, Qiao L, Li L, Zhang Y, Li K, Wang L, Qiao Y (2017) Novel Antihypertensive peptides derived from Adlay (Coix larchryma-jobi L. var. ma-yuen Stapf) glutelin. Molecules 22(4):E534. https://doi.org/10.3390/molecules22010123
Ling Y, Sun LP, Zhuang YL (2018) Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: inhibition kinetics and molecular docking. Food Funct 9(10):5251–5259. https://doi.org/10.1039/C8FO00569A
Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J (2016) Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 134(6):441–450. https://doi.org/10.1161/CIRCULATIONAHA.115.018912
Miralles B, Amigo L, Recio I (2018) Critical review and perspectives on food-derived antihypertensive peptides. J Agric Food Chem 66(36):9384–9390. https://doi.org/10.1021/acs.jafc.8b02603
Mitra AK, Gao L, Zucker IH (2010) Angiotensin II-induced upregulation of AT1-receptor expression: sequential activation of NF-κB and Elk-1 in neurons. Am J Physiol Cell Physiol 299:C561–C569. https://doi.org/10.1152/ajpcell.00127.2010
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J Comput Chem 16:2785–2791. https://doi.org/10.1002/jcc.21256
Panyayai T, Sangsawad P, Pacharawongsakda E, Sawatdichaikul O, Tongsima S, Choowongkomon K (2018) The potential peptides against angiotensin-I converting enzyme through a virtual tripeptide-constructing library. Comput Biol Chem 77:207–213. https://doi.org/10.1016/j.compbiolchem.2018.10.001
Qian BJ, Shen SQ, Zhang JH, Jing P (2017) Effects of vitamin B6 deficiency on the composition and functional potential of T cell populations. J Immunol Res 2017:2197975. https://doi.org/10.1155/2017/2197975
Qian BJ, Tian CC, Huo JH, Ding ZW, Xu R, Zhu J, Yu LL, Villarreal OD (2019) Design and evaluation of four novel tripeptides as potent angiotensin converting enzyme (ACE) inhibitors with anti-hypertension activity. Peptides 122:170171. https://doi.org/10.1016/j.peptides.2019.170171
Regoli D, Gobeil FJr (2015) Critical insights into the beneficial and protective actions of the kallikrein-kinin system. Vasc Pharmacol 64:1–10. https://doi.org/10.1016/j.vph.2014.12.003
Rohit AC, Sathisha K, Aparna HS (2012) A variant peptide of buffalo colostrums β-lactoglobulin inhibits angiotensin I-converting enzyme activity. Eur J Med Chem 53:211–219. https://doi.org/10.1016/j.ejmech.2012.03.057
Ruiz JÁG, Ramos M, Recio I (2004) Angiotensin converting enzyme-inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int Dairy J 14(12):1075–1080. https://doi.org/10.1016/j.idairyj.2004.04.007
Saiga A, Okumura T, Makihara T, Katsuta S, Shimizu T, Yamada R, Nishimura T (2003) Angiotensin I-converting enzyme inhibitory peptides in a hydrolyzed chicken breast muscle extract. J Agric Food Chem 51(697):1741–1745. https://doi.org/10.1021/jf020604h
Spyroulias GA, Galanis AS, Pairas G, Manessi-Zoupa E, Cordopatis P (2004) Structural features of Angiotensin-I converting enzyme catalytic sites: conformational studies in solution homology models and comparison with other zinc metallopeptidase. Curr Top Med Chem 4:403–429. https://doi.org/10.2174/1568026043451294
Sturrock ED, Natesh R, van Rooyen JM, Acharya KR (2004) Structure of angiotensin I-converting enzyme. Cell Mol Life Sci 61(21):2677–2686. https://doi.org/10.1007/s00018-004-4239-0
Sun Y, Bai YJ, He XR, Liu P, Zhao ZF, Chen XF, Zheng XH (2017) Design, synthesis, and evaluation of novel phenolic acid/dipeptide/borneol hybrids as potent angiotensin converting enzyme (ACE) inhibitors with anti-hypertension activity. Molecules 22:1739. https://doi.org/10.3390/molecules22111739
Sun S, Xu X, Sun X, Zhang X, Chen X, Xu N (2019) Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar Drugs 17(3):179. https://doi.org/10.3390/md17030179
Taga Y, Hayashida O, Ashour A, Amen Y, Kusubata M, Ogawa-Goto K, Shimizu K, Hattori S (2018) Characterization of angiotensin-converting enzyme inhibitory activity of X-Hyp-Gly-type tripeptides: importance of collagen-specific prolyl hydroxylation. J Agric Food Chem 66(33):8737–8743. https://doi.org/10.1021/acs.jafc.8b03648
Toopcham T, Mes JJ, Wichers HJ, Roytrakul S, Yongsawatdigul J (2017) Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem 220:190–197. https://doi.org/10.1016/j.foodchem.2016.09.183
Trott O, Olson AJ (2010) AutoDock Vina improving the speed and accuracy of docking with a new scoring function efficient optimization and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Vamos Z, Cseplo P, Ivic I, Matics R, Hamar J, Koller A (2014) Age determines the magnitudes of angiotensin II-induced contractions mRNA and protein expression of angiotensin type 1 receptors in rat carotid arteries. J Gerontol A Biol Sci Med Sci 69(5):519–526. https://doi.org/10.1093/gerona/glt128
Villars F, Guillotin B, Amédée T, Dutoya S, Bordenave L, Bareille R, Amédée J (2002) Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol 282:C775–C785. https://doi.org/10.1152/ajpcell.00310.2001
Yu F, Zhang Z, Luo L, Zhu J, Huang F, Yang Z, Tang Y, Ding G (2018) Identification and molecular docking study of a novel angiotensin-II converting enzyme inhibitory peptide derived from enzymatic hydrolysates of Cyclina sinensis. Mar Drugs 16(11):411. https://doi.org/10.3390/md16110411
Funding
This research was sponsored by the Jiangsu Provincial Medical Youth Talent (QNRC2016804), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJB320004), Qing Lan Project, the Jiangsu Provincial Population and Family Planning Commission (J201605), Project of Jiangsu Provincial Department of Education (2018GRFX023, 2018GRFX024), and Yancheng Medical Science and Technology Development Project (YK2016045). The funders played no role in the study design data collection and analysis, the decision to publish or in the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Bingjun Qian designed the experiments, analyzed the data, and wrote the paper; Lili Yu and Oscar D. Villarreal finished Molecule docking; Siyi Huang and Jianghua Huo finished analysis of ACE inhibitory activity and blood pressure monitoring; Chongchong Tian finished RT-PCR analysis and revised the paper.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, B., Yu, L., Tian, C. et al. Citrullyl-Hydroxyprolyl-Proline (ChPP): An Artificially Synthesized Tripeptide as Potent ACE Inhibitor. Int J Pept Res Ther 27, 967–976 (2021). https://doi.org/10.1007/s10989-020-10142-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10142-3