Abstract
Although the biology of adrenocorticotropic hormone (ACTH) protein has already been scrutinized, some functional aspects of its biology are yet to be elucidated in the context of immunological disorders. In this regard, virtual screening of a compound library was performed against the structure of Cytotoxic T-Lymphocyte Associated Protein-4 (CTLA-4) (assessed both spatially and energetically) to discover novel biological functions for ACTH. The results of virtual screening and the MD simulation demonstrated that DB01284 has high binding energy along with proper interaction orientation against CTLA-4 (FG loop) by a clamp like structure. The employed methodology was checked using confirmatory control analyses. Intriguingly, DB01284 belongs to Tetracosactide (already prescribed protein drug for clinical conditions) which is the N-terminal region of ACTH. This is the first study to reveal that ACTH protein binds to the same amino acids of CTLA-4 (FG-loop) as B7 and anti-CTLA-4 antibody binds. In light of this finding, the molecular mechanism of ACTH function in patients suffering from Cushing’s Syndrom and the immunological bases for ACTH therapy of multiple sclerosis (MS) patients could be further delineated. Moreover, this finding suggests that ACTH could also act to block CTLA-4 in the context of anticancer immune check point blockade.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aside from cytokine activation, the activation of naive T-cells needs at least two signals; the first signal is the interaction between TCR (T cell receptor) and MHC-antigen (complex molecule on the APCs) and the second signal is the interaction between CD28 (co-stimulatory molecule on T-cells) and CD80 and CD86 molecules (co-stimulatory molecule on the APCs). CTLA-4 (CD152) is a member of the immunoglobulin super family bearing up to 30% homology to CD28. CTLA-4 is expressed on activated T cells but not naive CD4+ and CD8+ T-lymphocytes (Egen et al. 2002; Fong et al. 2009; Krummel and Allison 1995; Lenschow et al. 1996; McCoy and Le Gros 1999; Mocellin and Nitti 2013). CTLA-4 and CD28 share the CD80 (B7-1) and CD86 (B7-2) molecules as their ligands. Although expression of CTLA-4 on T cell surfaces is reported to be 10- to 1000-fold lower than CD28, its affinity towards B7 molecule is 20- to 40-fold higher than CD28(Linsley et al. 1994). The interaction between CD28 and B7 stimulates the production of IL-2 and anti-apoptotic factors, which in turn induce T-cell proliferation (Krummel et al. 1996; Mocellin and Nitti 2013; Schwartz 1992). CTLA-4 blocks CD28 immune response via regulation of immune checkpoint production, increasing immunosuppressive cytokines and stopping the cell cycle. In fact, CTLA-4 prevents immune hyper activation and host tissue damage by limiting T-cell activity and increasing T-reg suppressive function (Krummel and Allison 1995).
Innate and adaptive immunity are capable of diagnosing, controlling and rejecting malignant tumor cells. However, tumor cells develop mechanisms to escape the immune system via production of inhibitory factors or activation of T-regs (Mocellin and Nitti 2013). Blocking these escape mechanisms is a compelling strategy to control or limit tumor cells. In this regard, CTLA-4 blocking has garnered a lot of attention to enhance T-cell proliferation and consequently increase immune responses against malignant tumor cells (Egen et al. 2002). Anti-CTLA-4 monoclonal antibodies have been approved by the US Food and Drug Administration (FDA) (Kyi and Postow 2014; Mocellin and Nitti 2013). The role of CTLA-4 in regulation of T-reg functions was reveal by administration of anti-CTLA-4 antibodies which could diminish the inhibitory effects of T-reg cells and brought about autoimmunity in mouse models. Anti-CTLA-4 antibodies have been demonstrated to suppress immune responses in mouse models of melanoma, colon, and prostate cancers. The rate of tumor occurrence was decreased fivefold in a mouse model of prostate cancer following combination of anti-CTLA-4 antibody and radiation therapy. Moreover, it was confirmed that the implementation of anti-CTLA-4 antibodies along with a prime boost vaccination strategy lead to more robust therapeutic results (Fong et al. 2009; Kwon et al. 2014; Selby et al. 2013; Wolchok et al. 2013).
Although impressive results have been achieved in antibody based treatments, their applications face several limitations. Antibody treatment, including antibody based blockade of CTLA-4, can face high production costs, inadequate pharmacokinetics and tissue accessibility, as well as impaired interactions with the immune system. Anti-CTLA-4 therapy can also cause various adverse effects due to nonspecific immune reactions, although these are reversible (Topalian and Sharpe 2014). In this regard, small molecule drugs or peptides could be used as alternative inhibitors capable of circumventing the aforementioned limitations. These molecules have been brought into spotlight owing to their expeditious ability to inhibit protein–protein interactions. However, finding or designing an amenable inhibitor for CTLA-4 blockade could be arduous and costly employing conventional empirical approaches.
Contemporary, in silico approaches have become an inevitable step in biological studies. These methods have been widely used in structural biology of molecules (Jahangiri et al. 2018b; Khalili et al. 2017b), understanding molecular mechanism (Jahangiri et al. 2017; Kazemi Moghaddam et al. 2017b; Mohammadpour et al. 2015, 2016), sequence analyses (Bazmara et al. 2019; Kazemi Moghaddam et al. 2017a; Rahbar et al. 2019) and designing various therapeutics (Jahangiri et al. 2017; 2018a; Khalili et al. 2017c; Payandeh et al. 2019). In light of these facts, we have launched an in silico study to find a suitable inhibitor to block the interactions between CTLA-4 and B7. To this end, a library of approved molecules was screened against CTLA-4 to find a molecule with high affinity and desirable interaction orientation. This molecule would be capable of blocking CTLA-4 and B7 interaction which could be used for cancer prevention or treatment. Moreover, it could bring about novel insights into the molecular biology of related diseases.
Methods
CTLA-4 Sequence and Structures
The UniProt database at http://www.uniprot.org/ was used to find the protein sequence of CTLA-4. A BLAST search against PDB (Protein Data Bank) database using NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was performed to find the most relevant resolved structure for CTLA-4. The 3D structure of CTLA-4 was prepared for docking analysis using AutoDock tools (Morris et al. 2009) of Pyrx software (Dallakyan and Olson 2015). The preparation was done by adding hydrogen atoms, merging all nonpolar hydrogens and changing the PDB files to PDBQT format. Moreover, the ConText software was used to remove all non-CTLA-4 molecules including water and ligands.
Retrieving and Preparing an Approved Library of Molecules
The DrugBank database at https://www.drugbank.ca/, a unique bioinformatics and cheminformatics resource that combines detailed drug data with comprehensive drug target information, was used to obtain a library of approved drug molecules. The Open Babel (O’Boyle et al. 2011) software was used to prepare drug molecules for docking analyses. The energy minimization run and conversion to PBDQT format was executed on all obtained molecules.
Virtual Screening of the Library
PyRex software was used to perform virtual screening of the approved drug library against the structure of CTLA-4. The AutoDock Vina (Trott and Olson 2010) interface of the PyRex software was used to do the docking analysis. The grid box size was set to 31, 29 and 26 at X, Y and Z coordinates. The set- and distance-dependent dielectric functions of the AutoDock Vina software was used to calculate the van der Waals and electrostatic terms. The method employed by (Khalili et al. 2017d; Suvannang et al. 2011) was adapted to set the other parameters of the PyRex screening software.
Energy Calculations and Visual Inspection of the Docking Results
The binding energy between CTLA-4 and all drug molecules from the library was calculated by the Autodock Vina software. Chimera software was used to visualize the spatial position of the drugs with binding affinities lower than − 20 kcal/mol. The structures were visually inspected to find a drug with the highest interference of the interaction between CTLA-4 and B7. The superimposition between the structures of CTLA-4/B7 complex and CTLA-4/drug complexes was performed using Chimera software.
Molecular Dynamics (MD) Simulation
Since the SDF file contained the planar structure of the drug molecules, the correct spatial conformation for the selected drug should have been achieved. Therefore, the selected structure was fed to VMD and NAMD2 software to prepare and execute a MD simulation. This would let the structure get more natural coordinates and proper conformation. The solvation, ionization, minimization and 10 nano-second MD run for the selected structure was carried out according the method we employed in our prior study (Khalili et al. 2017a). The root mean square deviation (RMSD) values of the structure were plotted during the MD simulation using the built in tool of the VMD software.
DB01284 Docking Against CTLA-4
The Galaxy PepDock server at http://galaxy.seoklab.org/cgi-bin/submit.cgi?type=PEPDOCK, CABSdock server at http://biocomp.chem.uw.edu.pl/CABSdock, Zdock server at http://zdock.umassmed.edu/ and Hex8 software were used to analyze the possible interactions between the spatially folded drug molecule and CTLA-4 structure.
2D Interaction Diagram and Dissociation Constant (Kd) Calculation
The 2D interaction plots could demonstrate the details of the exact amino acids participating in protein–ligand interactions. The LigPlus v1.4.5 software was used to plot the amino acids which are involved in protein–eptide interactions. The Kd of the interaction between protein–ligand was calculated by PRODIGY software available at http://milou.science.uu.nl/services/PRODIGY/.
Methodology Validation
In order to evaluate the accuracy of the employed approach, we have used Zdock and Hex8 software to reestablish the interactions between an anti-CTLA-4 antibody and CTLA-4 which was resolved experimentally. Since these two software were used for final molecular docking between ACTH and CTLA-4, their success to reestablish the interactions between a previously determined anti-CTLA-4 antibody and CTLA-4 complex would indicate the accuracy of the employed methods. The structure under the PDB ID of 5 ggv belongs to CTLA-4 in complex with tremelimumab Fab. The ConTEXT software was used to extract both CTLA-4 and tremelimumab Fab structures. Then, Zdock and Hex8 software were used to dock these to structures. The structures for newly docked CTLA-4 and tremelimumab Fab complex and the original 5 ggv were superimposed using FATCAT server at http://fatcat.sanfordburnham.org/, MATRAS 1.2 at http://strcomp.protein.osaka-u.ac.jp/matras/matras_pair.html and TMalign at https://zhanglab.ccmb.med.umich.edu/TM-align/. These servers were also used for Root-mean-square deviation (RMSD) calculation between two aligned structures. Moreover, to find out if the binding energy of ACTH with CTLA-4 is comparable with the biding energy of docked CTLA-4 and tremelimumab Fab complex, energy calculation was done for both ACTH/CTLA-4 and docked CTLA-4 and tremelimumab Fab complexs using PDBePISA server at http://www.ebi.ac.uk/pdbe/pisa/.
Results
Retrieving CTLA-4 Structure and Its Preparation
The primary (amino acid) sequence of the CTLA-4 molecule (canonical isoform) from Homo sapiens was stored under the UniProt ID of P16410. This protein is 223 amino acids in length including a 35 amino acids signal peptide which is removed in the mature protein. Some glycosylation sites and disulfide bonds are also reported for the protein. The BLAST search against PBD database indicated that the resolved structures under the PDB ID of 3OSK, 1I85 and 1I8L share the highest sequence coverage (~ 70%) and identity (~ 99%) with the CTLA-4 sequence. The lower sequence coverage is due to exclusion of the transmembrane and cytoplasmic domains of CTLA-4 in the resolved structures. The obtained coordinate files belong to crystal structure of human CTLA-4 apo-homodimer (3OSK), CTLA-4/B7-2 complex (1I85) and B7-1/CTLA-4 co-stimulatory complex (1I8L). Since the 3OSK was resolved with a higher resolution (1.8 Å), the CTLA-4 structure was obtained from this crystal structure and cleared from any non-protein molecules. The PDBQT file format of the cleaned structure was prepared for the following analyses.
Preparing Approved Drug Library
A library of almost 2000 approved drugs was obtained from DrugBank. An approved drug is defined as a drug that has been approved in at least one jurisdiction, at some point in time. The library was obtained in SDF format, converted into PDBQT format and subsequently energy minimized.
Virtual Screening and Analyzing the Obtained Results
The docking analyses between the drugs of the library and the CTLA-4 structure resulted in almost 2000 drug/CTLA-4 complexes. The binding energy calculation used to analyze the affinity of the drugs towards the CTLA-4 structure resulted in a wide range of energies from − 0.7 kcal/mol (DrugBank ID of DB11135) to − 37.5 kcal/mol (DrugBank ID of DB08896). The lower the calculated binding energy, the stronger the established interaction. In order to select drugs with the highest binding energies, 35 structures with binding energies lower than − 20 were selected for further investigation (Table 1). Visual inspection of the docked drug/CTLA-4 complexes revealed that most of the drugs in the analyzed library preferably interact with non-binding regions of CTLA-4. However, some of the drugs could establish interactions with partial interference of the CTLA-4/B7 interaction (Fig. 1). Among the visually analyzed drugs the drug with the DrugBank ID of DB01284 has the highest interference of the CTLA-4/B7 complex. This molecule prefers to interact with CTLA-4 in its B7 binding region with a binding energy of − 25.4 kcal/mol (Fig. 2).
Superimposed structures of CTLA-4/B7 complex and CTLA-4/drug complexes. The CTLA-4/B7 complex is in green and the CTLA-4/drug complexes are in red/atom type coloring. Flat molecules are 35 selected drugs that are docked to the CTLA-4 molecule. Most of the selected 35 drugs are populated within the region between two subunits of the CTLA-4 molecule (Color figure online)
The interaction between DB01284 and CTLA-4. The DB01284 molecule interacts with the CTLA-4 molecule in the B7 binding region of the molecule. B7 is in green, CTLA-4 is in red and the DB01284 is colored by atom type (Carbon: gray, Oxygen: red, Nitrogen: blue and hydrogen: white) (Color figure online)
MD Simulation Results
MD simulation provides enough time for the structures to interact with their own and surrounding atoms and improved their folding to more stable and more natural coordination. The 10 ns MD on the DB01284 changed the molecule from a planar form to a spatially folded structure (Fig. 3). Typically, the RMSD plot of an equilibrated system in MD simulation converges after a period of time. Our results indicate that advancing in MD simulation time leads the structure to an equilibrated state in which the atoms do not have extreme movements and the fluctuations in RMSD value become minor.
MD simulation results. The MD simulation altered the planar DB01284 structure into folded structure. The structures include the planar DB01284 (a), the solvated and ionized structure during MD (water molecules are in red and NaCl molecule is illustrated as red sphere) (b) and the final structure after MD (c) (Color figure online)
Folded DB01284 Interactions with CTLA-4
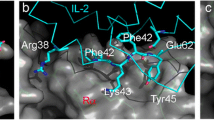
The docking results between folded DB01284 and CTLA-4 indicated that the interactions occur exactly in the B7 binding region of CTLA-4. This suggests that the folded structure of DB01284 is highly capable of interfering with CTLA-4 and B7 interactions. Figure 4 illustrates the interactions between the DB01284 and CTLA-4 molecules. As depicted in the Fig. 4 the DB01284 peptide encompasses the FG loop of the CTLA-4 molecule by a clamp like structure.
Docking results between DB01284 and CTLA-4. a Shows the interaction between the DB01284 and CTLA-4 subunit (DB01284 is shown as sticks along with amino acid labels, and CTLA-4 is shown as surface presentation). b Shows the interaction between the DB01284 and CTLA-4 subunit while it’s superimposed with the structure of 1I85 (blue is 1I85, red is the DB01284 and green is CTLA-4) (Color figure online)
2D Interaction Diagram and Kd
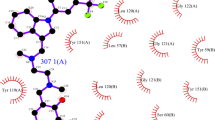
The interactions between CTLA-4 and DB01284 were predicted using the LigPlot software. The details of possible interaction between the CTLA-4 and DB01284 molecules on the amino acid level are depicted in Fig. 5. The majority of interactions are hydrophobic interactions, while 2 hydrogen bonds also exist between two molecules. The FG loop (amino acids from 95 to 105) of CTLA-4 structure is mainly involved in interactions. The Kd of the interaction was calculated to be 8.5e-06.
The 2D interaction diagram between CTLA-4 and DB01284 molecules. The upper line of amino acids belongs to DB01284, while the lower line belongs to CTLA-4. There are two hydrogen bonds between two molecules. One bond is between Tyr100 from the CTLA-4 and Ser3 from the DB01284 and the seconds one is between Tyr105 from the CTLA-4 and Tyr23 from the DB01284. The lengths of the hydrogen bonds are 3.3 and 2.81 angstroms for the first and second bonds respectively
Methodology Evaluation
The RMSD for structural superimposition between docked CTLA-4 and tremelimumab Fab complex and the original 5 ggv complex was calculated to be 2.66 angstroms. The obtained low RMSD indicated that there is a high structural similarity between these two complexes (Fig. 6). This means that the employed method is capable of accurate reestablishment of the original interactions between CTLA-4 and tremelimumab Fab bearing correct orientation. Binding affinity for the interactions of ACTH and docked tremelimumab Fab with CTLA-4 were calculated to be − 9.6 and − 14.8 kcal/mol which indicated higher affinity of the ACTH for CTLA-4.
Discussion
Immune regulation is mainly exerted by different immune checkpoints that control T-cell activation and regulatory T cells (TR). Immunopathology or autoimmunity and T-cell exhaustion or dysfunction could be the consequences of impaired immune regulation against tumors or pathogens. T-cell exhaustion is characterized by overexpression of multiple inhibitory immune checkpoints, including CTLA-4 (Pauken and Wherry 2015). Specific blockade of this immunosuppressive checkpoint could restore T cell functions (Postow et al. 2015). In this regard, using an integrative structural approach, our study has introduced DB01284 from DrugBank data base to be an amenable inhibitor to block CTLA-4. Our results indicated that DB01284 is capable of a high affinity interaction with CTLA-4. Moreover, this interaction occurs at the region which CTLA-4 interacts with B7-1 molecule and specific anti-CTLA-4 antibodies. It has been reported that the interactions between CTLA-4 and B7-1 is dominated by the amino acids of the FG loop of CTLA-4 which contributes 400 A2 of protein surface to the binding interface (Stamper et al. 2001). The FG loop which contains a hydrophobic sequence spanning the 99-105 amino acids (MYPPPYY) is highly conserved in CTLA-4 and CD28. A largely nonpolar surface of B7-1 consisting of Tyr 31, Met 38, Thr 41, Met 43, Val 83, Leu 85, Ala 91, Phe 92 and Leu 97 participate in hydrophobic contacts with the FG loop. Mutating FG loop amino acids reduced or abolished the binding to B7-1 (Metzler et al. 1997). The amino acids of FG loop are also involved in interaction with tremelimumab. It has been shown that a large number of residues including A2, E33, R35, S44, Q45, V46, E48, L91, I93, M99, P102, P103, Y104, Y105, L106, I108, and N110, contribute to van der Waals contact with tremelimumab (Lee et al. 2016). Our results indicated that the same FG loop is in tight interaction with DB01284, forming hydrophobic interactions and hydrogen bonds. The binding of DB01284 to CTLA-4 could efficiently compete with B7-1/2 binding to CTLA-4, thereby it could block the suppressive functions of CTLA-4 in cancer and infections. Harboring these properties, DB01284 could be deemed as an alternative drug candidate for immune check point blockade strategies.
The DrugBank ID of DB01284 belongs to a peptide drug known as Tetracosactide (also known as Cosyntropin) which is a synthetic peptide encompassing the 24-amino acid segment (sequence: SYSMEHFRWGKPVGKKRRPVKVYP) at the N-terminus of adrenocorticotropic hormone (ACTH). Tetracosactide exhibits the full corticosteroidogenic activity of natural ACTH. Melanocortin receptors (MCRs) were previously introduced as the specific receptors of ACTH. There are five known MCR subtypes (MC1R–MC5R) with differential tissue distribution throughout the body. Although each melanocortin hormone is recognized by exclusive MCR affinities, ACTH acts as universal agonist for all MCRs and it is reported to be the only MC peptide recognized by MC2R (Brzoska et al. 2008; Catania et al. 2004). To best of our knowledge, we are the first group to report the possible affinity of ACTH towards CTLA-4 molecules. ACTH is approved by the US FDA for treatment of relapsing forms of multiple sclerosis (MS). The ability of ACTH to increase endogenous corticosteroid production has long been assumed as the mechanism underlying its therapeutic efficacy. However, in light of evidence from recent studies it has been suggested that ACTH could exert multiple actions due to wide distribution of MCRs. Corticosteroid-independent melanocortin pathways including direct anti-inflammatory and immune-modulating actions via ACTH binding to MCRs (involving T and B lymphocytes, macrophages, sympathetic nervous system involved in inflammatory processes, reduction of pro-inflammatory cytokines, inflammatory nitric oxide, adhesion molecules, production of anti-inflammatory IL-10 for which IL-10 producing B cells are reported to be deficient in MS) are among other pronounced ACTH functions (Arnason et al. 2013; Berkovich and Agius 2014). Since the interaction between ACTH and CTLA-4 is not considered studying the ACTH functions, we hypothesize that a more complicated network of processes is ongoing.
T-cell-mediated autoimmune etiology (Pender and Greer 2007) and the role of CTLA-4 (Fukazawa et al. 1999; Kantarci et al. 2003) in MS has already been established. Prior studies have revealed that the interaction between CTLA-4 and B7 inhibits IL-2 production which in turn leads to reversible clonal anergy and tolerance to auto-antigens (Gimmi et al. 1993; Krummel and Allison 1996). However, it has been reported that CTLA-4 blockade could induce a Th1 or Th2 favored response (Alegre et al. 1998; Anderson et al. 2000) depending upon the strength of delivered signal via the T-cell receptor (TCR) and the T-cell activation state. Thus, in contrast to conventional expectation, blocking this negative signal may paradoxically inhibit immune responses for whole T-cell populations. In this regard, Khoury et al. and Cross et al. described that disruption of CTLA-4/B7 interaction inhibits Th-1 responses (IL-2 and IFN-gamma), while sparing Th-2 responses (IL-4, IL-10, and IL-13) (Cross et al. 1995; Khoury et al. 1995). In view of these facts, it could be concluded that the CTLA-4/B7 co-stimulatory pathway may play a pivotal role in peripheral tolerance of auto-reactive T cells that escape thymic selection (Kantarci et al. 2003). Herein, we have unveiled that, aside from MCRs binding, ACTH shares a common binding region (FG loop of CTLA-4) with B7 and anti-CTLA-4 revealing its potential use as an effective inhibitors of CTLA-4. In view of this fact, we hypothesized that ACTH could have the potential to act as a double agent in MS treatment. It should be noted that like other CTLA-4 inhibitors, depending on the strength of delivered signal through TCR and the T-cell activation state, ACTH could lead to anti-inflammatory and immune-modulating actions (favoring Th2 responses which helps MS treatment) as well as activation of auto-reactive T-cells (favoring Th1 responses which could exacerbate the MS condition). Our hypothesis is corroborated by a study conducted by Brod et al. They demonstrated that oral ingestion of ACTH, in mouse experimental autoimmune encephalomyelitis (EAE, a mouse model for MS) model, leads to decreased IL-17 (Teff), Th1-like encephalitogenic cytokines, IL-2, IFN-γ, and increased secretion of immune-regulatory IL-4 (Brod and Hood 2011). This observation is in concordance with the immune modulatory consequences that have arisen from inhibiting CTLA-4 and B7 interactions via anti-CTLA-4 antibodies (Cross et al. 1995; Khoury et al. 1995). On the other hand, ACTH administration could impede the B7 molecule from CTLA-4 binding and consequently preclude CTLA-4 from its immune-modulating functions which is the case in CTLA-4 blockade with antibodies in immunotherapy of cancer and infectious diseases (Postow et al. 2015; Wykes and Lewin 2017).
It’s quit surprising that our hypotheses could be a possible explanation for the immunological complications observed in patients suffering from Cushing’s syndrome (CS). CS is mostly (80–85%) the result of chronic exposure to excess ACTH usually produced by a pituitary corticotroph adenoma. The prevalence of autoimmune disorders is reported to be 0–20% during the active phase of the CS and up to 60% during the remission phase (Colao et al. 2000; da Mota et al. 2011; Pivonello et al. 2007; Takasu et al. 1993). Glucocorticoid excess leads to immunosuppression during the active phase of the disease. Consequently, an immune rebound occurs after DS remission which is responsible for development or exacerbation of autoimmune disorders. Although the exact mechanism underlying the Glucocorticoids functions is yet to be elucidated, it has been reported that they suppress Th1 responses and promote Th2 responses (Fareau and Vassilopoulou-Sellin 2007; Kovalovsky et al. 2000). These effects are similar to the results attained from disruption of CTLA-4/B7 interaction (Cross et al. 1995; Khoury et al. 1995). Given our results, it could be assumed that the excessing ACTH interacts with CTLA-4 receptor and disrupts the CTLA-4/B7 interaction which leads to immunological consequences. It has already been demonstrated that CTLA-4 is involved in inhibition of auto-reactive T-cells (Ueda et al. 2003). CTLA-4 is constitutively expressed on TR cells. A part of TR function could be exerted by CTLA-4 binding to CD80/CD86 on immature dendritic cells which in turn could increase tryptophan catabolism and downregulate Teff activity (Grohmann et al. 2002; Manzotti et al. 2002). Since depletion of TR cells causes autoimmune disease, the possible inhibitory interactions of CTLA-4 and the excess ACTH in CS provides the ground for autoimmune complications.
The ability of two individual proteins to interact with the same receptor in a similar orientation with high binding energies could only be rationalized by high structural identity between those proteins in their interacting interfaces. This identity brings to mind the idea of possible interactions between the anti-CTLA-4 antibodies and MCRs. It could be imagined that the anti-CTLA-4 antibodies and ACTH share common structural properties in their interacting surfaces which should enable them to have interchangeable interactions with the other molecule’s specific receptors. MCRs are highly distributed throughout the body and they differ in their tissue specificity (Abdel-Malek 2001). Patients treated with anti-CTLA-4 antibodies have shown a wide spectrum of immune-related adverse events (IRAEs) (Boasberg et al. 2010; Torino et al. 2012). We believe that unprecedented interactions between anti-CTLA-4 antibodies and MCRs have molecular consequences due to this interaction that could contribute to the extent of emerging IRAEs. However, the possibility and probable intricacies of this hypothesis remains to be investigated (Fig. 7).
In conclusion, it should be noted that the molecular mechanism underlying the ACTH and anti-CTLA-4 antibodies could be more complicated than initially assumed. Our findings could pave the way for better comprehension of these mechanism. The attained results have introduced a novel therapeutic application in the context of immune check point blockade for a previously prescribed drug (ACTH). Moreover, these results could be employed to engineer more effective therapeutics in fight against cancer and infectious diseases.
References
Abdel-Malek Z (2001) Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci 58:434–441
Alegre M-L, Shiels H, Thompson CB, Gajewski TF (1998) Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol 161:3347–3356
Anderson DE, Bieganowska KD, Bar-Or A, Oliveira EM, Carreno B, Collins M, Hafler DA (2000) Paradoxical inhibition of T-cell function in response to CTLA-4 blockade; heterogeneity within the human T-cell population. Nat Med 6:211–214
Arnason BG, Berkovich R, Catania A, Lisak RP, Zaidi M (2013) Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler J 19:130–136
Bazmara H, Rasooli I, Jahangiri A, Sefid F, Astaneh SDA, Payandeh Z (2019) Antigenic properties of iron regulated proteins in Acinetobacter baumannii: an in silico approach. Int J Pept Res Ther 25:205–213
Berkovich R, Agius MA (2014) Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther Adv Neurol Disord 7:83–96
Boasberg P, Hamid O, O’Day S (2010) Ipilimumab: unleashing the power of the immune system through CTLA-4 blockade. In: Seminars in oncology, vol 5. Elsevier, Amsterdam, pp 440–449
Brod SA, Hood ZM (2011) Ingested (oral) ACTH inhibits EAE. J Neuroimmunol 232:131–135
Brzoska T, Luger TA, Maaser C, Abels C, Böhm M (2008) α-Melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev 29:581–602
Catania A, Gatti S, Colombo G, Lipton JM (2004) Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56:1–29
Colao A et al (2000) Increased prevalence of thyroid autoimmunity in patients successfully treated for Cushing’s disease. Clin Endocrinol 53:13–19
Cross AH et al (1995) Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 costimulation. J Clin Investig 95:2783
da Mota F, Murray C, Ezzat S (2011) Overt immune dysfunction after Cushing’s syndrome remission: a consecutive case series and review of the literature. J Clin Endocrinol Metab 96:E1670–E1674
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. In: Chemical biology. Springer, New York, pp 243–250
Egen JG, Kuhns MS, Allison JP (2002) CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 3:611–618
Fareau GG, Vassilopoulou-Sellin R (2007) Hypercortisolemia and infection. Infect Dis Clin 21:639–657
Fong L et al (2009) Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res 69:609–615
Fukazawa T et al (1999) CTLA-4 gene polymorphism may modulate disease in Japanese multiple sclerosis patients. J Neurol Sci 171:49–55
Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM (1993) Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci 90:6586–6590
Grohmann U et al (2002) CTLA-4–Ig regulates tryptophan catabolism in vivo. Nat Immunol 3:1097
Jahangiri A, Amani J, Halabian R (2017) In silico analyses of staphylococcal enterotoxin B as a DNA vaccine for cancer therapy. Int J Pept Res Ther 24(1):131–142. https://doi.org/10.1007/s10989-017-9595-3
Jahangiri A, Rasooli I, Owlia P, Fooladi AAI, Salimian J (2018a) Highly conserved exposed immunogenic peptides of Omp34 against Acinetobacter baumannii: an innovative approach. J Microbiol Methods 144:79–85
Jahangiri A, Rasooli I, Owlia P, Fooladi AAI, Salimian J (2018b) An integrative in silico approach to the structure of Omp33-36 in Acinetobacter baumannii. Comput Biol Chem 72:77–86
Kantarci OH et al (2003) CTLA4 is associated with susceptibility to multiple sclerosis. J Neuroimmunol 134:133–141
Kazemi Moghaddam E, Owlia P, Jahangiri A, Rasooli I, Rahbar MR, Aghajani M (2017a) Conserved OprF as a selective immunogen against Pseudomonas aeruginosa. Iran J Pathol 12:86–93
Kazemi Moghaddam E, Owlia P, Jahangiri A, Rasooli I, Rahbar MR, Aghajani M (2017b) Conserved OprF as a selective immunogen against Pseudomonas aeruginosa. Iran J Pathol 12:165–170
Khalili S, Rasaee M, Bamdad T (2017a) 3D structure of DKK1 indicates its involvement in both canonical and non-canonical Wnt pathways. Mol Biol 51:155–166
Khalili S, Rasaee MJ, Mousavi SL, Amani J, Jahangiri A, Borna H (2017b) In silico prediction and in vitro verification of a novel multi-epitope antigen for HBV detection. Mol Genet Microbiol Virol 32:230–240
Khalili S, Zakeri A, Hashemi ZS, Masoumikarimi M, Manesh MRR, Shariatifar N, Sani MJ (2017c) Structural analyses of the interactions between the thyme active ingredients and human serum albumin. Turk J Biochem 42(4):459–467
Khalili S, Jahangiri A, Hashemi ZS, Khalesi B, Mardsoltani M, Amani J (2017d) Structural pierce into molecular mechanism underlying Clostridium perfringens Epsilon toxin function. Toxicon 127:90–99
Khoury SJ, Akalin E, Chandraker A, Turka LA, Linsley PS, Sayegh MH, Hancock WW (1995) CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol 155:4521–4524
Kovalovsky D, Refojo D, Holsboer F, Arzt E (2000) Molecular mechanisms and Th1/Th2 pathways in corticosteroid regulation of cytokine production. J Neuroimmunol 109:23–29
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459–465
Krummel MF, Allison JP (1996) CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183:2533–2540
Krummel MF, Sullivan TJ, Allison JP (1996) Superantigen responses and co-stimulation: cD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo. Int Immunol 8:519–523
Kwon ED et al (2014) Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 15:700–712
Kyi C, Postow MA (2014) Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 588:368–376
Lee JY et al (2016) Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat Commun 7:13354
Lenschow DJ, Walunas TL, Bluestone JA (1996) CD28/B7 system of T cell costimulation. Annu Rev Immunol 14:233–258
Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R (1994) Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1:793–801
Manzotti CN, Tipping H, Perry LC, Mead KI, Blair PJ, Zheng Y, Sansom DM (2002) Inhibition of human T cell proliferation by CTLA-4 utilizes CD80 and requires CD25 + regulatory T cells. Eur J Immunol 32:2888–2896
McCoy KD, Le Gros G (1999) The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol 77:1–10
Metzler WJ et al (1997) Solution structure of human CTLA-4 and delineation of a CD80/CD86 binding site conserved in CD28. Nat Struct Biol 4:527–531
Mocellin S, Nitti D (2013) CTLA-4 blockade and the renaissance of cancer immunotherapy. Biochimica et Biophysica Acta (BBA) 1836:187–196
Mohammadpour H, Khalili S, Hashemi ZS (2015) Kremen is beyond a subsidiary co-receptor of Wnt signaling: an in silico validation. Turk J Biol 39:501–510
Mohammadpour H, Pourfathollah AA, Zarif MN, Khalili S (2016) Key role of Dkk3 protein in inhibition of cancer cell proliferation: an in silico identification. J Theor Biol 393:98–104
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276
Payandeh Z, Rajabibazl M, Mortazavi Y, Rahimpour A, Taromchi AH, Dastmalchi S (2019) Affinity maturation and characterization of the ofatumumab monoclonal antibody. J Cell Biochem 120:940–950
Pender MP, Greer JM (2007) Immunology of multiple sclerosis. Curr Allergy Asthma Rep 7:285–292
Pivonello R, De Martino MC, De Leo M, Tauchmanovà L, Faggiano A, Lombardi G, Colao A (2007) Cushing’s syndrome: aftermath of the cure. Arquivos Brasileiros de Endocrinol Metab 51:1381–1391
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982
Rahbar MR et al (2019) Trimeric autotransporter adhesins in Acinetobacter baumannii, coincidental evolution at work. Infect Genet Evol 71:116–127
Schwartz RH (1992) Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 71:1065–1068
Selby M, Engelhardt J, Quigley M, Henning K, Chen T, Srinivasan M (2013) Korman A (2013) Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 1(1):32–42. https://doi.org/10.1158/2326-6066
Stamper CC et al (2001) Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 410:608–611
Suvannang N, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V (2011) Molecular docking of aromatase inhibitors. Molecules 16(5):3597–3617
Takasu N, Ohara N, Yamada T, Komiya I (1993) Development of autoimmune thyroid dysfunction after bilateral adrenalectomy in a patient with Carney’s complex and after removal of ACTH-producing pituitary adenoma in a patient with Cushing’s disease. J Endocrinol Investig 16:697–702
Topalian SL, Sharpe AH (2014) Balance and imbalance in the immune system: life on the edge. Immunity 41:682–684
Torino F, Barnabei A, De Vecchis L, Salvatori R, Corsello SM (2012) Hypophysitis induced by monoclonal antibodies to cytotoxic T lymphocyte antigen 4: challenges from a new cause of a rare disease. Oncologist 17:525–535
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Ueda H et al (2003) Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423:506
Wolchok JD et al (2013) Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 1291:1–13
Wykes MN, Lewin SR (2017) Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 18(2):91
Acknowledgements
The authors wish to thank Tarbiat Modares University for supporting the conduct of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramezani, A., Zakeri, A., Mard-Soltani, M. et al. Structure Based Screening for Inhibitory Therapeutics of CTLA-4 Unveiled New Insights About Biology of ACTH. Int J Pept Res Ther 26, 849–859 (2020). https://doi.org/10.1007/s10989-019-09891-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09891-7