Abstract
Context
The importance of landscape complexity for biological control is well-known, but its functional roles are poorly understood.
Objectives
We evaluated the landscape capacity to provide floral resources for beneficial insects and its consequences for biological control in fields.
Methods
The gut contents of adult hoverflies sampled in 41 cereal fields were analysed to determine which plant species are exploited. The relative value of each habitat in providing adequate pollen resources was evaluated by vegetation survey. Then 15 cereal fields were selected along a gradient of landscape complexity, where the abundance of aphids, hoverfly larvae and aphid parasitism was monitored. The habitat’s proportions in landscape buffers surrounding these fields were used as landscape descriptors and to assess the potential level of pollen resources provision (LP index).
Results
Aphid abundance significantly decreased with an increase of the LP index mainly sustained by grassy strips and weeds in fields. However, hoverfly larvae abundance also decreased with the increasing LP index. The enhancement of the aphid parasitism rate with the LP index suggests that aphid parasitoids may benefit from the same floral resources as hoverflies. Their crop habitat specialism may give them a competitive advantage in fields where both aphid and floral resources are abundant.
Conclusions
Complex interaction networks involved in biological control may disrupt the expected direct effects of floral resource provisioning for a focal beneficial species. We highlighted fields and grassy strips as habitats provisioning floral resources for which the LP index could be very helpful to optimize agroecological management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely assumed that biological control of agricultural pests and pollination, two important ecosystem services provided to agriculture, are improved in presence of non-crop habitats in agricultural landscapes (e.g. Chaplin-Kramer et al. 2011; Holzschuh et al. 2012). Non-crop habitats provide seasonal refuges, nesting sites and various food resources to insect predators, parasitoids and pollinators, resulting in a spill-over of beneficial insects from these habitats into crop fields (Landis et al. 2000; Kremen et al. 2004; Tscharntke et al. 2005; Klein et al. 2007). A positive effect of non-crop habitats on beneficial insects has been demonstrated either globally through assessment of the relative area covered by the non-crop (e.g. Steffan-Dewenter et al. 2002; Bianchi et al. 2006; Holzschuh et al. 2012) or by focusing on one particular type of non-crop habitat such as woodlots and hedgerows (e.g. Rusch et al. 2012), grasslands (e.g. Ockinger and Smith 2007; Meyer et al. 2009), or grassy/floral strips (e.g. Gillespie et al. 2011; Ernoult et al. 2013). The influence of plant composition of these habitats has been particularly well studied with the aim of increasing beneficial insect population abundances (Altieri and Whitcomb 1979; Cowgill et al. 1993; White et al. 1995; Hickman and Wratten 1996; Patt et al. 1997; Rebek et al. 2005). The distances between crop fields and non-cultivated habitats have also been shown to affect the diversity of beneficial insects and the biocontrol service provided in crop fields (e.g. Garibaldi et al. 2011).
However, even if the abundance and diversity of beneficial populations is generally enhanced by landscape complexity, the influence of non-crop habitats on realised ecosystem services in terms of biological control appears more variable (Bianchi et al. 2006; Chaplin-Kramer et al. 2011; Tscharntke et al. 2016). On the one hand, while native vegetation in non-cultivated habitats has been shown to support predator reproduction across seasons, its influence on biological control in crop fields is unclear (Bianchi et al. 2013). On the other hand, crop fields appear to be important overwintering sites for some major beneficial species and confer a significant effect on biological control (Raymond et al. 2014). It would appear that some beneficial populations are strongly associated with crop habitat throughout their ecological cycles and consequently could be rather insensitive to the presence in the landscape of non-crop habitats. Our poor understanding of the ecological processes and functions involved means that the design of landscape management strategies for improving biological control service provision remains a central challenge. In this study we investigated the landscape functionality [i.e. landscape patterns in relation to their function (Forman and Godron 1986)] in terms of floral resource provisioning to beneficial insects and assessed how it affected the biological control of pests.
There is increasing evidence that the provision of floral resources in agricultural landscapes enhances the performance of parasitoids as part of conservation biological control (Tena et al. 2015; Jonsson et al. 2015). Adult parasitoids require both nectar and pollen as a source of energy (Tenhumberg et al. 2006) and a source of protein for reproduction (Rivero and Casas 1999). As floral resources, particularly nectar, are often scarce in agricultural ecosystems (Heimpel and Jervis 2005), the incorporation of targeted non-crop vegetation in agricultural landscapes can help support biological control (Landis et al. 2000).
Hoverflies (Diptera: Syrphidae) are another common and important group of beneficial insects in agro-ecosystems. They have been shown to provide significant pollination services to wild flowers and crops (Fontaine et al. 2006; Jauker and Wolters 2008). Moreover, some hoverfly species, aphidophagous at the larval stage, are among the most common natural enemies of aphids in crops (Tenhumberg and Poehling 1995; Schmidt et al. 2003; Brewer and Elliott 2004). Adult hoverflies feed on floral resources found in several types of habitats, with individuals moving from one habitat patch to another to find the resources they need to complete their entire life-cycle (Dunning et al. 1992). Hoverfly adults require floral resources for their high-energy flight, ovary maturation and egg production (Chambers 1988).
Studies on the use of flowers by aphidophagous hoverflies in intercropping flower strips or field margins have demonstrated a large spill-over of hoverflies from non-crop habitats to crop habitats (Cowgill et al. 1993; Gillespie et al. 2011). Explorative routine movements for daily resource-searching (pollen for feeding or aphids for egg laying) occur at small spatial scales, typically less than 200 m (Wratten et al. 2003). However, aphidophagous hoverflies exhibit long-distance dispersal behaviour as well (up to 1000 m) for movements associated with life-cycle stages and seasonality (Arrignon et al. 2007; Meyer et al. 2009). Hence, it has been found that landscape properties affect aphidophagous hoverfly diversity, abundance and the ecosystem services they provide, at both small and large spatial scales (Jauker et al. 2009; Meyer et al. 2009; Power and Stout 2011; Ernoult et al. 2013; Alignier et al. 2014).
Until now, studies of pollen feeding by hoverflies have focussed on (1) analysing gut contents to determine visited plant species (Cowgill et al. 1993; Hickman et al. 1995), (2) testing consumption of various introduced flowering plants with the aim of making recommendations for the composition of the flower strips adjacent to crop fields (e.g. Hogg et al. 2011), and (3) using pollen as markers in studies of hoverfly dispersion from a controlled pollen source (e.g. Rader et al. 2011). Despite the significant amount of available information on pollen feeding by hoverflies, the floral sources actually exploited in agricultural landscapes are still unknown, with woodlands, hedgerows, grasslands, grassy strips or even weeds in crop fields being potential sources of pollen. By combining analyses of the gut content of adult hoverflies sampled in crop fields with a large vegetation survey of main non-crop and crop habitats, and an assessment of the proportion of these habitats covering the landscape surrounding crop fields in which aphid and larval hoverfly abundances have been monitored, we addressed two questions. What is the relative importance of non-crop and crop habitats as pollen sources for hoverflies? Does landscape functionality in terms of floral food provision to hoverflies influence aphid biological control in crop fields? We also consider the parasitoid community, through the aphid parasitism rate in monitored crop fields, in order to explore other effects of floral food supply.
Methods
Study area
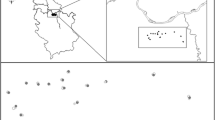
The study took place in Brittany, a region of western France, in the Ille-et-Vilaine Department (Fig. 1). The study area is dominated by mixed dairy farming and is part of the Long Term Ecological Research (LTER) site “Zone Atelier Armorique” integrated in the Long-term Biodiversity, Ecosystem and Awareness Research Network (Alter-net). The area comprises a gradient of agricultural land-use intensity. The southern part is characterized by a dense hedgerow network and a large percentage of areas covered by grasslands and fodder crops. Farming systems are mainly oriented toward dairy production. The northern part of the study area is a more open landscape resulting from land re-allotment with agriculture mainly oriented toward mixed dairy-cattle and crop production and approximately 1/3 of the area covered by grasslands and fodder crops. As a result, there is a pronounced gradient from South to North with a decrease in the surface covered by grasslands and woody habitats and an increase in the surfaces devoted to annual crops associated with an increase in mean field size.
Map of the study area localizing (1) vegetation surveys, (2) aphidophagous hoverfly sampling for gut content analyses, (3) crop fields monitored for aphid biological control. Vegetation surveys were conducted in 2007 (Ernoult et al. 2013) and 2011 (Duflot et al. 2015) in a total of five habitats representing the main habitats of the area
Dependent variables: monitoring of aphid, hoverfly and mummy abundance in focal cereal crop fields
In 2011, the respective abundance of aphids, aphid mummies and hoverflies (eggs, larvae and pupae) was recorded for 15 winter cereal fields in the “Zone Atelier Armorique” (Fig. 1). These fields were selected along a gradient of landscape complexity (Appendix 1 of supplemetary material). From the second week in April to the first week in July, the number of aphids, hoverflies and mummies were counted every 15 days on 50 wheat stalks randomly chosen in three 1 m2 plots located at more than 20 m from the edge of the field. For each field, we calculated the total abundance of aphids, the total abundance of hoverflies (sum of all the eggs, larvae and pupae of hoverflies counted in the three samples over all dates of sampling) and the aphid parasitism rate (sum of all the mummies counted in the three samples over all dates of sampling/all live aphids and aphid mummies).
Land use maps of the “Zone Atelier Armorique” were drawn, based on direct field observations using ArcMap software, ArcGis for Desktop 10, version ArcInfo advanced. Land use polygons were digitalized and attributed, based on georeferenced numeric orthophotographs BDOrtho© (IGN, 2010). Landscape descriptors were calculated in five circular buffers of different sizes (with respective radii of 100, 250, 500, 750 and 1000 m) centered on the centroid of each monitored field using ArcToolBox scripts. The proportion of area covered by the six main non-crop and crop habitats (woodlands, meadows, grassy strips, hedgerows, winter cereal crops and other crops), and Shannon’s diversity index were calculated for the five buffer sizes. The percentage of non-crop habitats around the monitored fields varied from 2% at the buffer size of 100 m for the lowest value to 74% at the buffer size of 250 m for the highest value (Appendix 1 of supplemetary material).
Reference data
Adult aphidophagous hoverflies (Dipterous, Syrphidae) were caught in cereal fields (winter cereal and maize) using yellow water traps. Three trap bowls (40 × 30 cm) were placed 20 m apart from each other in 19 winter cereal fields (at 50 m from the edge) for 12 weeks from April to July 2008 (Fig. 1). This period corresponds to the peak of flowering. From April to July 2009, 11 winter cereal fields and 11 maize fields were monitored, following the same procedure. The traps were filled with water to which drops of liquid detergent were added and were emptied weekly. Adult hoverflies were identified to species level, when possible, according to van Veen (2004). They were stored individually in Eppendorf tubes at −20 °C, until their dissection. The guts were removed and opened on a slide to expose the pollen. After extraction of lipids using diethyl ether, the diverticulum was placed on a slide with glycerin jelly containing basic fuchsin to stain the pollen (Hyde and Adams 1958). Pollen was examined under an optic microscope at 400× magnification and determined to family or species level by comparison with the INRA—Le Magneraud’s pollen collection (Aupinel et al. 2001).
The number of sampled adult hoverflies was low, ranging from 1 to 10 individuals (often 2 or 3) per field, with only 1–2 pollen species in the individual guts. Consequently, the samples were merged in one group for a further analysis (see Jacobs index (D) calculation description). The group comprised all the hoverflies caught across the “Zone Atelier Armorique” (Fig. 1), distributed over an area covering 57 km2 according to Al Hassan et al. (2013). In this area, large-scale landscape descriptors were calculated using ArcToolBox scripts for the proportions of the area covered by the main non-crop and crop habitats (woodlands, grasslands, grassy strips, hedgerows, winter cereal crops and other crops).
Previous studies have been conducted on vegetation in the studied area (for more details see Ernoult et al. 2013; Duflot et al. 2015). We used their data corresponding to surveys in five habitat types, representing the main habitats of the area: conventionally managed winter cereal crops (N = 40), hedgerows (N = 40), woodlands (N = 40), grasslands (N = 80) and grassy strips (N = 54, Fig. 1). We used the presence/absence data for each plant species (a total of 278 species) in the sample replicates to estimate its relative pollen availability for hoverflies in each of the five habitats. For example, a plant found in 10 hedgerows among the 40 surveyed hedgerows obtained a pollen availability index equal to 0.25 for this habitat. The presence/absence data were chosen as the pollen-vegetation relationship is non-linear because of the high variability in pollen productivity between species (Broström et al. 2008); this depends on many factors such as the number of flowers per inflorescence, pollen produced per flowers, but also climatic conditions etc. (e.g. Wroblewska and Stawiarz 2012). Hereafter, only the plant species effectively supplying pollen to aphidophagous hoverflies, i.e., found in the gut content analyses, were considered in the subsequent analyses.
Indices calculation
Gut content analyses provided the list of pollens consumed by aphidophagous hoverflies and an estimate of their consumption rate (CR). From the vegetation surveys in the main crop and non-crop habitats, we obtained an estimate of the availability of each pollen plant source in each habitat (PA). Finally, from the landscape habitat composition analyses, we calculated the proportion covered by each main crop and non-crop habitat (HP) (1) in the reference area of hoverfly sampling for gut content analyses at hand (2) for each buffer size around the 15 cereal fields monitored for biological control.
All data were obtained between 2007 and 2011 in the same area. Yearly climatic conditions were equivalent during this period. As there were no major changes in agricultural practices during this period, flora diversity sampled in 2007 and 2011 in a large set of conventional crop fields and non-crop habitats appeared to be a relevant reference for hoverfly gut contents sampled in 2008.
Selectivity occurs when a feeder consumes available and co-occurring edible resources at different rates (Jacobs 1974). Jacobs’s selection index allows comparing for a particular species its consumption frequency to its availability in the landscape. A positive Jacobs’s selection index value indicates a preference (here called “over-selected” species) while a negative value indicates a lower consumption than expected from the resource abundance (here called “under-selected” species). We applied Jacobs’s selection index D to the hoverfly selection preferences for pollen species i (Table 1), using:
where CRi is the consumption rate for pollen of the species i (i = 1–40), PAij is the availability of pollen species i in habitat j (j = 1–5) and HPj is the proportion of the referenced area covered by habitat j.
We explored the similarity between the set of plant species for which the pollen was consumed by the hoverflies and the set of plant species found in each habitat j by the Sørensen index (Sørensen 1948), calculated as:
where Aj is the number of species common to the two sets, Bj is the total number of species in the set of plant species for which the pollen is consumed by the hoverflies, and Cj is the total number of species found in the habitat j.
We calculated the value of each habitat j for the provision of pollen resources to hoverflies (Hj) by:
where CRi is the consumption rate for pollen of the species i (i = 1–40) and PAij is the availability of pollen species i in habitat j (j = 1–5).
Three different Hj indices were calculated for each habitat j, by considering alternatively (1) all plant species identified in the gut content analyses (HjAll ); (2) only the “over-selected” plant species (\({\text{H}}_{\text{j + }} \, = \,\sum {{\text{i}}\left( {{\text{PA}}_{\text{ij}} \times {\text{CR}}_{\text{i}} | {\text{D}}_{\text{i}} \, > \,0} \right)}\)), i.e. species exhibiting a positive Jacobs index (Di); and (3) only the “under-selected” plant species \(\left( {{\text{H}}_{{{\text{j}} - }} \, = \,\sum {{\text{i}}\left( {{\text{PA}}_{\text{ij}} \times {\text{CR}}_{\text{i}} | {\text{D}}_{\text{i}} \, < \,0} \right)} } \right)\), i.e. species exhibiting a negative Jacobs index.
We developed an index of landscape potential for pollen provisioning for aphidophagous hoverflies (LP index) taking into account landscape composition, by averaging habitat values as Hj, weighted according to their proportion in the landscape buffer:
where Hj is the value of habitat j in pollen provisioning for hoverflies and HBj is the proportion of the area considered, covered by habitat j. HBj values were calculated for the five buffer sizes with circular buffers centred on the centroids of each cereal field monitored for biological control.
We once again calculated three LP indices considering alternatively (1) all plant species identified in the gut content analyses (LP All ); (2) only the “over-selected” plant species (LP+) and (3) only the “under-selected” plant species (LP−), which is mainly related to the presence of weeds in the crops (cf. Results part). We also calculated the global contribution of each crop habitat j to the value of the LP index by means of the equation, LP index/j = Hj × HBj/LP index.
Statistical analyses
A log-transformation of the abundance data and an arcsine square root transformation of the parasitism rate and hoverfly/aphid ratio were applied to normalize them.
First, predator–prey dynamics could imply that an initial increase of hoverflies may result in a later decrease due to lower aphid abundances. Consequently, few female hoverflies may lay their eggs when there are not many aphids. To deal with this time factor, we analysed (1) how later abundance of aphids was related to initial abundance of hoverflies and (2) how initial abundance of hoverflies was related to initial abundance of aphids. The first three sessions of insect sampling and the last three sessions were pooled to respectively form the initial and the later abundances. The relationship between parasitism rate and larval hoverfly abundance was analysed for the whole season to explore the possibility of a competitive relationship.
We performed random resampling by bootstrap method in the data of pollen consumption rate, using 1000 bootstrap samples to calculate the confidence intervals (2.5 and 97.5%) of D for each plant species. We then calculated H+ for each habitat, including (1) all unconfident under-selected and over-selected species and the confident over-selected species (max), and (2) only the confident over-selected species (min, Fig. 3). The minimum and maximum H− values were also calculated.
We performed standard Multiple Linear Models (LM) for each buffer size to investigate the effects of LP indices (LP All , LP+ and LP−) on (1) larval hoverfly abundance (2) aphid abundance, (3) hoverfly/aphid ratio, and (4) aphid parasitism rate in the 15 cereal fields monitored. We compared this approach with the one based on landscape composition, using the proportional area covered by each of the six habitats (woodlands, meadows, grassy strips, hedgerows, winter cereal crops and other crops) for each buffer size as factors in the LM. Landscape variables were centred on their mean and scaled according to their standard deviation for each buffer size. In each model focusing on one buffer size, the insect abundances, their ratio or the parasitism rate were related to the LP index or the set of habitat proportions in simple or additive models respectively. The set of best-fitting models was selected based on Akaike’s information criterion, corrected for small sample sizes (AICc, Burnham and Anderson 2002), and using the limit of two AICc units of the model with the lowest AICc. Estimates of model parameters are reported for these models. To complement these analyses, correlations between the three LP indices and the Shannon diversity index of landscape for each buffer size were calculated.
Statistical analyses were carried out using R 3.1.3 (R Development Core Team 2015) with the lme4, lmerTest, pgirmess and MuMIn libraries and the dredge function (Version 1.5.9).
Results
We caught 112 adult hoverflies using yellow water traps in crops belonging to 13 taxa. Most of them were successfully identified at species level. The entire sample comprised 31 individuals of Eupeodes corollae, 29 of Melanostoma mellinum, 21 of Episyrphus balteatus, 11 of Spaerophoria scripta, 6 of Syrphus ribesii, 4 of Melanostoma sp., 3 of Platycherus sp., 2 of Syrphus sp., 1 of Eupeodes latifasciatus, 1 of Scaeva pyrastri, 1 of Pipezella sp., 1 of Platycheirus albimanus, and 1 of Platycherus granditarsus. Twenty-five per cent of the insects had an empty gut. A maximum of 2 pollen types were found per individual, belonging to one of a total of 40 plant species (see list in Fig. 2). Pollen consumption rates varied from 0.01 to 0.16 (mean = 0.03, s.e. = 0.03). Pollens of six plant species were found only once in the gut of insects and belonged to plants that had not been recorded in the vegetation survey (Aegopodium podagria, Bryonia dioica, Hemerocallis sp., Syringa sp., Trifolium campestre and Valeriana sp.).
Availability of pollen species in each habitat, based on their presence rate found in the vegetation survey (PA). Pollen species are classified according to their consumption rate (CR) by aphidophagous hoverfly, into one of four categories: ‘Highly consumed’ when consumption rates are >10% of meals, ‘Often consumed’ when consumption rates are between 5 and 10% of meals, ‘More rarely consumed’ when consumption rates are between 2.5 and 5% of meals, and ‘Opportunistically consumed’ when pollen species were found only once in the set of meals
In monitored cereal crop fields, we counted 143–6866 aphids, 3–37 hoverflies at pre-imaginal stages and 10–157 mummies during the season, giving a mean count of 1695 aphids, 14 hoverflies and 64 mummies per field.
A particular high consumption rate (CR) by aphidophagous hoverflies was found for two pollen species (0.13 and 0.14 of the records respectively for both Achillea millefolium and Lotus corniculatus). These two plant species were not abundant in any habitat and were found mainly in grassy strips, but also in grasslands and winter cereal crops (Fig. 2). Both species had a positive Jacobs index (D) close to 1 (Fig. 3). In contrast, the most frequent plant species in habitats, Rubus sp., had a negative Jacobs index. Most of the plant species whose pollen was often consumed (ca. 6% of the records) or occasionally consumed (ca. 3% of the records) by hoverflies were present in all habitats. However, some of these species were found in only one habitat, such as Anthriscus sylvestris in grassy strips or Geranium molle and Trifolium pratense in grasslands (Fig. 2).
Selectivity of pollen sources by aphidophagous hoverflies (Jacobs index D). An index value >1 indicates a higher relative abundance of the pollen species in hoverfly gut compared to the vegetation survey (consumption preference), whereas a value <1 indicates a negative selection for the pollen species. Species with * present a confident positive or negative D, following the calculation of the confidence intervals (2.5 and 97.5%) with bootstrap resampling in pollen species data
The Jacobs index (D) presented confident results for five over-selected pollen species and 8 under-selected pollen species (Fig. 3). The relative value of each habitat in pollen resources provision to hoverflies (H) appeared to differ from its expected value, based on the similarity between the set of plant species consumed by hoverflies and the set of plant species found in each habitat (Sorensen index S, Table 2). Grassy strips had the highest Hj All , followed by grasslands, hedgerows and crop fields. Woodlands had the lowest HjAll (= 1.14). When considering only plant species having a positive Jacobs index, grassy strips still had the highest Hj+ followed by hedgerows, grasslands, crop fields and woodlands. When considering only plant resources having a negative Jacobs index, crop fields had the highest Hj−, followed by grassland, grassy strips, hedgerows and woodlands.
The range of values of the three LP indices (All, (+) and (−), =landscape potential to offer pollen resources for aphidophagous hoverflies) according to buffer size is summarized in Appendix 2 of supplemetary material. The mean contribution of crop habitat to the value of the LP index was high, ranging for example for the LP− from 56% (±13) for the buffer size of 1000 m to 86% (±11) for the buffer size of 100 m. No significant correlation was observed between any LP index value and the contribution of any habitat to this LP index value.
Correlations between all indices for the same buffer size are shown in Appendix 3 of supplemetary material. LP− at 500 m was significantly correlated with LP All at 500 m (r = 0.95, P < 0.001).
Correlations between the three LP indices and the Shannon diversity index were very variable and negative. The LP− shared from 21% (at the buffer size of 100 m) to 62% (at the buffer size of 1000 m) common information with the Shannon diversity index (Appendix 4 of supplemetary material).
When considering the time factor and predator–prey dynamics, a non-significant relationship was recorded between late aphid abundance and early larval hoverfly abundance in cereal fields (t = −0.37, P = 0.72, R2 = 0.01). No relationship was recorded between initial larval hoverfly abundance and initial aphid abundance (t = −1.77, P = 0.10, R2 = 0.13). No relationship was recorded between larval hoverfly abundance and aphid parasitism rate in cereal fields for the whole season (t = −1.40, P = 0.19, R2 = 0.06).
A model comparison based on AICc identified the LP− (for a buffer size of 500 m) as the variable best fitting larval hoverfly and aphid abundances and aphid parasitism rate in cereal crop fields, with the lowest AICc and the highest adjusted R2 (Table 3). Both aphid and larval hoverfly abundances were significantly lower with higher LP− (t = −3.18, R2 = 0.44, P = 0.01 and t = −3.21, r2 = 0.45, P = 0.01 respectively, Fig. 4), while the aphid parasitism rate was significantly enhanced with the LP− (t = 2.42, R2 = 0.26, P = 0.03). A positive effect of LP All was recorded for the aphid parasitism rate at buffer sizes of 500 m, but with a marginal trend toward significance. No effect of any LP index was recorded on the hoverfly/aphid ratio in cereal fields.
Regression between aphidophagous hoverfly abundance (a), aphid abundance, (b) or aphid parasitism rate, (c) in crop fields and the LP index (i.e. landscape potential for offering pollen resources for aphidophagous hoverflies) for buffer size of 500 m around the crop fields. The LP index considered takes into account pollen species with a negative Jacobs index (D). Coefficient of determination (R2) of the linear regressions and its level of significance are presented
Using habitat proportions as landscape descriptors, a model comparison systematically identified the % of grassy strip in the landscape as a significantly positive or negative variable fitting the insect abundances, the hoverflies/aphids ratio or the parasitism rate in cereal crop fields (Table 4). A significant effect of the % of winter cereal crop was also recorded on larval hoverfly abundance for a buffer size of 500 m (t = −4.64, P = 0.0006), while significant effects of the % of grassland and woodland were observed on the aphid abundance for a buffer size of 500 m (t = 4.15, P = 0.002 and t = 3.77, P = 0.03 respectively). A significant effect of the % of woodland was recorded on parasitism rate for a buffer size of 1000 m (t = −3.52, P = 0.004). The adjusted R2 of the models were high, from 0.43 (P = 0.005) for the model explaining the aphid parasitism rate to 0.79 (P = 0.0001) for the one explaining aphid abundance.
Discussion
By combining data on pollen found in the gut of aphidophagous hoverflies, with the availability of the corresponding plants in different habitat types and then by correlating indices of suitability of different habitats in landscapes with natural enemies and prey abundances, we explored the value of different landscapes for biological control of cereal aphids. As our approach is novel, some limits have to be discussed. First, the relative pollen availability for hoverflies was estimated based on the presence-absence of plant species in habitats. Estimating pollen productivity remains a great scientific challenge, notably in archeological palynology, because of the non-linear relationship between pollen and vegetation abundances, which is related to the high variability in pollen productivity between plant species (Broström et al. 2008). Then, presence-absence data appears at present to enable the least-biased estimates of the relative pollen availability in habitat. The developing data bases of the life-history traits of plants, such as the LEDA-Traitbase concerning the Northwest European flora (Kleyer et al. 2008) or the TRY Database (Kattge et al. 2011), offer promising perspectives for precisely estimating pollen productivity when this trait is available. Second, we have based the floral preferences of hoverflies on consumed pollen, but nectar may also be an important floral resource for selecting flowers as clearly demonstrated recently by Van Rijn and Wäckers (2016). The two sets of plant species used do not match, but it is interesting to note that the most consumed two pollen species in our study, Achillea millefolium and Lotus corniculatus, reveal respectively accessible nectar and non-accessible nectar according to Van Rijn and Wäckers (2016). The combination of plant species presenting a high rate of pollen consumption by beneficial insects and easily accessible nectar offers promising perspectives for preserving or introducing a plant community in and around crop fields favouring these insects.
Our original approach enables significant insight into the mechanisms underlying previously well-studied relationships between landscape composition, habitat quality and indicators of pest control (e.g. Parry et al. 2015; Schellhorn et al. 2015). Non-cultivated habitats clearly play unequal roles in floral food supply to hoverflies, despite hosting the same common plant species. Grassy strips appeared to provide the highest quantity of pollen resources to hoverflies. This habitat hosted both over-selected plant species and under-selected plant species. We also showed that crop fields significantly provided pollen resources consumed by adult hoverflies. In contrast, woodlands made the lowest contribution to pollen provisioning. However their role as overwintering habitats for adult hoverflies could nevertheless mean that they are important landscape elements for maintaining biological control (Arrignon et al. 2007).
To evaluate landscape functionality in providing floral resources to hoverflies, we calculated an original index taking into account the landscape composition (the LP index) by combining hoverfly’s pollen preferences, the plant frequency in each habitat and the proportion of each habitat in the landscape. A comparison of the LP index with the classical Shannon diversity index attested its originality as a landscape descriptor. Their negative relationship may be due to the important contribution of crop fields in floral food supply. Indeed, a high crop proportion in landscape is generally associated with a low Shannon diversity index (e.g. Fahrig et al. 2011). The LP index brings new insights on the results of analyses using habitat proportions, which mix the different ecological roles that these habitats may play in pest biological control, such as resource supply, overwintering sites, reproduction sites, refuges or corridors for a set of natural enemies (Bianchi et al. 2006). Even if many natural enemies could be important in suppressing aphids, by considering the landscape through the prism of floral food supply for one taxonomic group (the aphidophagous Syrphidae), we obtained significant results that explicitly link aphid control with the level of the floral resources used by hoverflies and that are available in and around the crop field. The results obtained with these two approaches are strongly concordant and complement one another; they highlight the important role of floral resource supply for natural enemies and its consequences on biological control. They notably point out the role of grassy strips in floral provision for natural enemies.
The landscape potential for pollen provisioning based on over-selected plant species (LP+) was not correlated to aphid and hoverfly abundance in fields. These plants are more abundant in non-crop habitats and may attract hoverflies away from a field (Landis et al. 2000). In contrast, the LP− index, whose value is influenced by the presence of weed plants in crop fields, was significantly and negatively correlated to aphid abundances in fields, with the highest correlation for a buffer size of 500 m. However, an unexpected negative correlation was observed between this LP index and the abundance of predatory hoverfly at larval stages, while the aphid parasitism rate was positively correlated with this LP index. First, results on aphid and hoverfly abundance could in part be due to fewer hoverfly eggs being laid where there are fewer aphids present, but no relationship has been found between initial aphid and hoverfly abundances. Even if the ratio of hoverflies to aphids is positively related to the LP− index, suggesting that there are more hoverflies among aphid colonies, the results were not significant. Second, another question to be considered is how much the LP index is specific to hoverflies. A comparison with the list of pollen consumed by another group of natural enemies of pests in the same pseudo-climatic region, Chrysoperla species (Neuroptera: Chrysopidae, Villenave-Chasset et al. 2006), showed that only two species were shared. But aphid parasitoids, which also consume floral resources, appeared to benefit from the same floral resources as hoverflies in the landscape. These floral resources concerned only the under-selected plant species, i.e., mainly weeds in crop fields. Our results are strongly consistent with those of Tylianakis et al. (2004) who demonstrated that floral resources significantly increase the aphid parasitism rate in cereal fields when these floral resources are very close (< 14 m). Moreover, as pest aphid parasitoids are highly specialised to crop habitat (Derocles et al. 2014), and according to the resource concentration hypothesis (Root 1973), they may be favoured by a high proportion of crop field area in the landscape in which both aphid and floral resources are abundant. The presence of mummies among aphid colonies limits hoverfly egg-laying (Almohamad et al. 2008). Consequently, even if hoverflies are more abundant in the landscape owing to the pollen resources offered by the different habitats, competition with parasitoids limits them in the crop fields.
Altogether, the present results suggest that crop fields form habitats that have been largely under-estimated in their capacity to sustain pest control services, as recently hypothesised by Tscharntke et al. (2016). Our study illustrates how crop fields, even when conventionally managed as in our case, could play a prominent ecological role in agricultural landscapes, being more than just sink habitats of beneficial populations from surrounding non-crop habitats. Practically, our results call for combining (1) the management of grassy strips through the choice of species composition at initial sowing or the tuning of the mowing dates in order to enhance strips’ function of providing floral resources to hoverflies, and (2) the development of weed-control practices balancing in an optimal way the negative effect of weeds caused by competition with crops and their positive effects as beneficial insect supports. The original LP index proposed in this study may contribute to the design and assessment of such agricultural management strategies at a landscape scale in order to significantly improve biological control for a sustainable crop production.
References
Al Hassan D, Georgelin E, Delattre T, Burel F, Plantegenest M, Kindlmann P, Butet A (2013) Does the presence of grassy strips and landscape grain affect the spatial distribution of aphids and their carabid predators? Agric For Entomol 15:24–33
Alignier A, Raymond L, Deconchat M, Menozzi P, Monteil C, Sarthou JP, Vialatte A, Ouin A (2014) Landscape influence on the abundance of aphids, mummies and aphidophagous hoverflies in winter wheat fields varies over the time. Biol Control 77:76–82
Almohamad R, Verheggen FJ, Francis F, Hance T, Haubruge E (2008) Discrimination of parasitized aphids by a hoverfly predator: effect on larval performance, foraging and oviposition behavior. Entomol Exp Appl 128:73–80
Altieri MA, Whitcomb WH (1979) The potential use of weeds in the manipulation of beneficial insects. HortScience 14:12–18
Arrignon F, Deconchat M, Sarthou JP, Balent G, Monteil C (2007) Modelling the overwintering strategy of a beneficial insect in a heterogeneous landscape using a multi-agent system. Ecol Model 205:423–436
Aupinel P, Genissel A, Tasei JN, Poncet J, Gomond S (2001) Collection of spring pollens by Bombus terrestris queens. Assessment of attractiveness and nutritive value of pollen diets. Acta Horticult 561:101–105
Bianchi FJ, Booij CJH, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc Biol Sci Ser B 273:1715–1727
Bianchi FJ, Schellhorn NA, Cunningham SA (2013) Habitat functionality for the ecosystem service of pest control: reproduction and feeding sites of pests and natural enemies. Agric For Entomol 15:12–23
Brewer MJ, Elliott NC (2004) Biological control of cereal aphids in North America and mediating effects of host plant and habitat manipulations. Annu Rev Entomol 49:219–242
Broström A, Nielsen AB, Gaillard MJ, Hjelle K, Mazier F, Binneey H, Bunting J, Fyfe R, Meltsov V, Poska A (2008) Pollen productivity estimates of key European plant taxa for quantitative reconstruction of past vegetation: a review. Veg Hist Archaeobot 17:461–478
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, Berlin
Chambers RJ (1988) Syrphidae. In: Minks AK, Harrewijn P (eds) World crop pests: aphids, their biology. Natural Enemies and Control Elsevier, Amsterdam
Chaplin-Kramer R, O’Rourke ME, Blitzer EJ, Kremen C (2011) A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol Lett 14:922–932
Cowgill SE, Wratten SD, Sotherton NW (1993) The selective use of floral resources by the hoverfly Episyrphus balteatus (Diptera: Syrphidae) on farmland. Ann Appl Biol 122:223–231
Derocles SA, Le Ralec A, Besson MM, Maret M, Walton A, Evans DM, Plantegenest M (2014) Molecular analysis reveals high compartmentalization in aphid-primary parasitoid networks and low parasitoid sharing between crop and noncrop habitats. Mol Ecol 23:3900–3911
Duflot R, Aviron S, Ernoult A, Fahrig L, Burel F (2015) Reconsidering the role of ‘semi-natural habitat’ in agricultural landscape biodiversity: a case study. Ecol Res 30:75–83
Dunning JB, Danielson BJ, Pulli HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Ernoult A, Vialatte A, Butet A, Michel N, Rantier Y, Jambon O, Burel F (2013) Grassy strips in their landscape context, their role as new habitat for biodiversity. Agric Ecosyst Environ 166:15–27
Fahrig L, Baudry J, Brotons L, Burel F, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Fontaine C, Dajoz I, Meriguet J, Loreau M (2006) Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol 4:129–135
Forman RTT, Godron M (1986) Landscape ecology. Wiley, New York, p 619
Garibaldi LA, Steffan-Dewenter I, Kremen C, Morales JM, Bommarco R, Cunningham SA (2011) Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol Lett 14:1062–1072
Gillespie M, Wratten S, Sedcole R, Colfer R (2011) Manipulating floral resources dispersion for hoverflies (Diptera: Syrphidae) in a California lettuce agro-ecosystem. Biol Control 59:215–220
Heimpel GE, Jervis MA (2005) Does floral nectar improve biological control by parasitoids? In: Wäckers F, vanRijn P, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, pp 267–304
Hickman JM, Wratten SD (1996) Use of Phacelia tanacetifolia strips to enhance biological control of aphids by hoverfly larvae in cereal fields. J Econ Entomol 89:832–840
Hickman JM, Lovei GL, Wratten SD (1995) Pollen feeding by adults of the hover fly Melanostoma fasciatum (Diptera: Syrphidae). N Z J Zool 22:387–392
Hogg BN, Bugg RL, Daane KM (2011) Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol Control 56:76–84
Holzschuh A, Dudenhoffer JH, Tscharntke T (2012) Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Biol Conserv 153:101–107
Hyde HA, Adams KF (1958) An atlas of airborne pollen grains. Macmillan, London, p 112
Jacobs J (1974) Quantitative measurement of food selection: a modification of the forage ratio and Ivlev’s electivity index. Oecologia 14:413–417
Jauker F, Diekötter T, Schwarzbach F, Wolters V (2009) Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landscape Ecol 24:547–555
Jauker F, Wolters V (2008) Hoverflies are efficient pollinators of oilseedrape. Oecologia 156:819–823
Jonsson M, Straub CS, Didham RK, Buckley HL, Case BS, Hale RJ, Gratton C, Wratten SD (2015) Experimental evidence that the effectiveness of conservation biological control depends on landscape complexity. J Appl Ecol 52:1274–1282
Kattge J, Diaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, Van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanoves F, Cavender-Bares J, Chambers JQ, Chapin Iii FS, Chave J, Coomes D, Cornwell WK, Craine JM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernández-Méndez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kühn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusià J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Müller SC, Nadrowski K, Naeem S, Niinemets Ü, Nöllert S, Nueske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordonez J, Overbeck G, Ozinga WA, Patiño S, Paula S, Pausas JG, Peñuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, Poschlod P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negret B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana J-F, Swaine E, Swenson N, Thompson K, Thornton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C (2011) TRY—a global database of plant traits. Glob Change Biol 17:2905–2935
Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc Biol Sci Ser B 274:303–313
Kleyer M, Bekker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, Poschlod P, Van Groenendael JM, Klimeš L, Klimešová J, Klotz SRGM, Rusch GM, Hermy M, Adriaens D, Boedeltje D, Bossuyt B, Dannemann A, Endels P, Götzenberger L, Hodgson JG, Jackel AK, Kühn I, Kunzmann D, Ozinga WA, Römermann C, Stadler M, Schlegelmilch J, Steendam HJ, Tackenberg O, Wilmann B, Cornelissen JHC, Eriksson O, Garnier E, Peco B (2008) The LEDA Traitbase: a database of life-history traits of the Northwest European flora. J Ecol 96:1266–1274
Kremen C, Williams NM, Bugg RL, Fay JP, Thorp RW (2004) The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecol Lett 7:1109–1119
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201
Meyer B, Jauker F, Steffan-Dewenter I (2009) Contrasting resource-dependent responses of hoverfly richness and density to landscape structure. Basic Appl Ecol 10:178–186
Ockinger E, Smith HG (2007) Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J Appl Ecol 44:50–59
Parry H, Macfadyen S, Hopkinson JE, Bianchi FJJA, Zalucki MP, Bourne A, Schellhorn NA (2015) Plant composition modulates arthropod pest and predator abundance: evidence for culling exotics and planting natives. Basic Appl Ecol 16:531–543
Patt JM, Hamilton GC, Lashomb JH (1997) Impact of strip-insectary intercropping with flowers on conservation biological control of the Colorado potato beetle. Adv Hortic Sci 11:175–181
Power EF, Stout JC (2011) Organic dairy farming: impacts on insect-flower interaction networks and pollination. J Appl Ecol 48:561–569
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rader R, Edwards W, Westcott DA, Cunningham SA, Howlett BG (2011) Pollen transport differs among bees and flies in a human-modified landscape. Divers Distrib 17:519–529
Raymond L, Plantegenest M, Gauffre B, Sarthou JP, Vialatte A (2014) Immature hoverflies overwinter in cultivated fields and may significantly control aphid populations in autumn. Agric Ecosyst Environ 185:99–105
Rebek EJ, Sadof CS, Hanks LM (2005) Manipulating the abundance of natural enemies in ornamental landscapes with floral resource plants. Biol Control 33:203–216
Rivero A, Casas J (1999) Incorporating physiology into parasitoid behavioral ecology: the allocation of nutritional resources. Res Popul Ecol 41:39–45
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol Monogr 43:95–124
Rusch A, Valantin-Morison M, Roger-Estrade J, Sarthou JP (2012) Local and landscape determinants of pollen beetle abundance in overwintering habitats. Agric For Entomol 14:37–47
Schellhorn N, Gagic V, Bommarco R (2015) Time will tell: resource continuity bolster ecosystem services. Trends Ecol Evol 30:524–530
Schmidt MH, Lauer A, Purtauf T, Thies C, Schaefer M, Tscharntke T (2003) Relative importance of predators and parasitoids for cereal aphid control. Proc R Soc London B 270:1905–1909
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biologiske Skrifter/Kongelige Danske Videnskabernes Selskab 5:1–34
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape structure on three pollinator guilds. Ecology 83:1421–1432
Tena A, Pekas A, Cano D, Wackers FL, Urbaneja A (2015) Sugar provisioning maximizes the biocontrol service of parasitoids. J Appl Ecol 52:795–804
Tenhumberg B, Poehling HM (1995) Syrphids as natural enemies of cereal aphids in Germany: aspects of their biology and efficacy in different years and regions. Agric Ecosyst Environ 52:39–43
Tenhumberg B, Siekmann G, Keller MA (2006) Optimal time allocation in parasitic wasps searching for hosts and food. Oikos 113:121–131
Tscharntke T, Karp DS, Chaplin-Kramer R, Batary P, DeClerck F, Gratton C, Hunt L, Ives A, Jonsson M, Larsen A, Martin EA, Martinez-Salinas A, Meehan TD, O’Rourke M, Poveda K, Rosenheim JA, Rusch A, Schellhorn N, Wanger TC, Wratten S, Zhang W (2016) When natural habitat fails to enhance biological control—five hypotheses. Biol Conserv 204:449–458
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity: ecosystem service management. Ecol Lett 8:857–874
Tylianakis JM, Didham RK, Wratten SD (2004) Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85:658–666
Van Rijn PCJ, Wäckers FL (2016) Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J Appl Ecol 53:925–933
Van Veen MP (2004) Hoverflies of Northwest Europe, identification keys to the Syrphidae. KNNV publishing, Utrecht, p 254
Villenave-Chasset J, Deutsch B, Lodé T, Rat-Morris E (2006) Pollen choice by the Chrysoperla species (Neuroptera: Chrysopidae) occurring in the crop environment of western France. Eur J Entomol 103:771–777
White AJ, Wratten SD, Berry NA, Weigmann U (1995) Habitat manipulation to enhance biological control of Brassica pests by hoverflies (Diptera: Syrphidae). J Econ Entomol 88:1171–1176
Wratten SD, Bowie MH, Hickman JM, Evans AM, Sedcole JR, Tylianakis JM (2003) Field boundaries as barriers to movement of hoverflies (Diptera: Syrphidae) in cultivated land. Oecologia 134:605–611
Wroblewska A, Stawiarz E (2012) Flowering of two Arctium L. species and their significance as a source of pollen for visiting insects. J Apic Res 51:78–84
Acknowledgements
The Authors would like to thank Eric Petit for his advice on statistical analyses, Andrew Hamilton for revising the English in the manuscript, and Tony Couanon, Julia Saulais, Ludmilla Martin and the local Landscaphid staff for their help in insect sampling. The study was supported by funding from « Programmes Interdisciplinaires du CNRS 2008 » for the project named ParaBEn and the ANR Systerra program (French National Research agency, ANR-09-STRA-05).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vialatte, A., Tsafack, N., Hassan, D.A. et al. Landscape potential for pollen provisioning for beneficial insects favours biological control in crop fields. Landscape Ecol 32, 465–480 (2017). https://doi.org/10.1007/s10980-016-0481-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-016-0481-8