Abstract
Forest bird species exhibit noticeable seasonal behavioral changes that might lead to contrasting effects of landscape pattern upon species abundance and performance. We assessed if the effect of patch and habitat attributes on the landscape use of thorn-tailed rayaditos (Aphrastura spinicauda), a forest bird in a relict patchy forest in northern Chile, varied temporally in association with changes in the behavior of individuals linked to breeding vs. non-breeding conditions. We also assessed the relationship between nest success and patch and habitat attributes, as nest success might be associated to the density rayaditos during the breeding season. We found that density of rayaditos was affected by patch size and functional connectivity but not by habitat structure and that the magnitude of the effect of patch size was greater during the non-breeding season, thus supporting the existence of a temporally variable effect of landscape pattern. Similarly, the nest success of rayaditos was positively affected by functional connectivity and negatively by structural connectivity. We hypothesize that these results emerged from the interaction among territorial behavior, resource limitation and predation risk. Despite the variable intensity of the effect of patch size upon density, however, this landscape attribute, in addition to connectivity, is essential for the persistence of rayaditos at this relict patchy forest landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of habitat fragmentation at landscape scales depend on the interaction between landscape attributes, such as amount, type, quality, composition, connectivity and dynamics of habitat patches (e.g., Andrén 1994; Villard et al. 1999; Chapa-Vargas and Robinson 2006) and species’ ecological and life-history traits (e.g., Tellería and Santos 1995; Keymer et al. 2000; Schrott et al. 2005). Most birds living in fragmented landscapes are particularly vulnerable to environmental factors acting at the local and landscape scales such as: (1) the reduction in habitat area, (2) the isolation of remnant patches and (3) the associated changes in habitat structure (Saunders et al. 1991; Lens et al. 2002; Manton et al. 2005) that might negatively impact bird abundance and reproductive success (e.g., Robinson et al. 1995). The effects of area and habitat structure on bird reproduction have been extensively documented in fragmentation studies. These effects, however, vary both in direction and intensity across species, because of the peculiarities of their ecological and life-history characteristics (e.g., McGarigal and McComb 1995; Schmiegelow and Mönkkönen 2002). Within species, the same species might respond differently to the same landscape structure at different stages of its life cycle (e.g., breeding vs. non-breeding) (Wiens 1976; Haila 1990; Pearson et al. 1996; Andrén et al. 1997). This dynamic effect of landscape structure is little appreciated, but might be of paramount importance for forest bird species. Consequently, our understanding of how habitat fragmentation influences population viability in fragmented landscapes, and the usefulness of the conservation guidelines that can be derived from fragmentation studies, can be hindered unless the dynamic effect of landscape structure is considered.
This study is aimed at testing the general hypothesis that the effects of patch area, connectivity and habitat structure on the Thorn-tailed rayadito (Aphrastura spinicauda), are time dependent and change accordingly to the life history of this species. Rayaditos are area-sensitive birds in the fragmented forest landscapes of Chile (Vergara et al. 2003) and their breeding abundance and nest site use is mainly determined by forest structure (Tomasevic and Estades 2006; Vergara and Simonetti 2004a). Nevertheless, this species varies seasonally in its territorial behavior, forming mixed-species flocks during the non-breeding season, while it is highly territorial during the breeding season (Vuilleumier 1967; Ippi and Trejo 2003). Recently, Tomasevic and Estades (2006) reported that the density of rayaditos during the breeding season was positively affected by variables at the nest site scale, such as nest box availability and forest structure, but during the non-breeding season the effect of these variables was reduced or null. The results of Tomasevic and Estades (2006) suggest that: 1. Habitat structure acts as an important cue in nest site selection behavior, as it could be related to either nesting success or a central-place foraging behavior (with birds returning to nests after foraging bouts, e.g., Alonso et al. 1994); 2. During the non-breeding season rayaditos move in response to the interaction between flocking behavior and landscape configuration (see Pulido and Díaz 1997; Rodríguez et al. 2001). This indicates that landscape use by rayaditos follows an important seasonal pattern, influenced by changes in the behavior of individuals.
Rayadito’s populations living in fragmented forests of northern Chile at Fray Jorge (Fig. 1), offer a good opportunity to testing for the dynamics effect of landscapes because of the extreme isolation of these populations, which minimizes the effect of regional dispersal upon patterns (Cornelius et al. 2000). In addition, rayaditos are locally abundant and have conspicuous behavior making them easy to sample by standard detection methods. Based on our hypothesis that patch area and connectivity effects on Rayadito populations depend on season, we made the following predictions:
-
1.
Since the dispersal behavior of rayaditos varies between breeding and non-breeding seasons, we expect that during the non-breeding season the effect of patch attributes (patch area and connectivity) on bird density will increase in response to the species’ flocking behavior, while the opposite is expected for the effect of habitat structure due to the loss of territorial behavior.
-
2.
Since rayaditos are territorial during the breeding season, and considering that the most relevant aspect of the life history of this species during this season is concerned with reproductive performance, we expect that nest success will be affected mainly by habitat structure at the nest site scale rather than by patch attributes (area and connectivity).
Methods
Study site
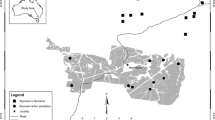
The study was conducted at the Fray Jorge National Park (lat 30°40′S, long 71°30′W), located 94 km south of Coquimbo, Chile (Fig. 1). Fray Jorge is a patchy relict forest enclosed by a xeric shrub matrix that is not used by rayaditos (Cornelius et al. 2000). Climate in Fray Jorge is Mediterranean-arid, with dry, hot summers and cool winters (López-Cortés and López 2004). Mean annual precipitation is 85 mm, falling mainly between May and September. The floristic composition of the Fray Jorge forest, however, closely resembles that of the Valdivian temperate rainforest currently distributed more than 1,200 km south of Fray Jorge (Hinojosa and Villagrán 1997). The forest is restricted mostly to sea-facing slopes on top of the coastal mountain range (600 m elevation); it has a total area of 95.6 ha and is composed of several fragments (n = 588) ranging between 0.001 to approximately 30 ha in area and 1 to 260 m in isolation (measured as nearest neighbor distance). Vegetation within patches includes trees as Aextoxicon punctatum, Drimys winteri and Myrceugenia correifolia; evergreen shrubs as Luma chequen and Raphithamnus spinosus and one prostrate species, Griselinia scandens (Gajardo 1993). Forest patches are distributed within a matrix of xeric vegetation (scrub matrix) that corresponds to the Coastal Stepparian Matorral of Coquimbo (Gajardo 1993). The scrub matrix in the mountain range extends into the lowland area (200 m elevation), with an associated increase in cover of dominant species such as Porlieria chilensis, Proustia pungens and Adesmia bedwellii (Gajardo 1993).
Bird density
We collected density data during the 2004 breeding season of rayaditos (September to December) and at the subsequent 2005 non-reproductive season (February to June) (Ippi and Trejo 2003). Density was measured using the variable circular-plot method (Ramsey and Scott 1979). Although the efficacy of the circular-plot method in estimating densities of birds may be lower when birds are forming flocks than during breeding (e.g., Bibby et al. 1992), this survey method was used in both seasons for the following reasons: First, rayaditos are conspicuous during the non-breeding season, frequently vocalizing conspicuous alarm calls, hence increasing bird detectability (see Hahn and Mattes 2000). Second, detectability of rayaditos in the non-breeding season using circular-plots is higher than with other methods such as mist-nets, and so, it is the recommended census method for this season (Estades et al. 2006). Third, seasonal variability in the effect of distance on bird detectability was minimized by fitting a detectability function for each season (see below).
To determine if the presence of nest boxes within patches increased bird density during the breeding season we compared censuses carried out in patches with and without nest boxes. To do this, we used a total of 97 points randomly distributed within 56 patches, of which 28 (50%) had nest boxes. This randomization procedure assured appropriate interspersion of nest box treatments among large and small patches (Hurlbert 1984). In patches with nest boxes, each point was centered at a nest box. The number of points per patch ranged from 1 to 17. Distance between points was always >150-m and each point was visited three times between 06:30 and 11:30 h. The maximum observation radius was 30 m, and each count lasted 8 min. At each point, the observer estimated the horizontal distances to the birds in 5-m intervals. To calculate bird density at each sampling station, we used a half-normal function that gives the probability of detecting a bird at different distances (Buckland et al. 1993). Points were visited three times during spring, the breeding season and three times during summer and fall, the non-breeding season.
Breeding data
We set 152 nest boxes made of wood in 28 randomly chosen forest patches ranging in size from 0.03 to 30 ha (mean ± SE = 1.83 ± 1.32 ha) during the breeding season. The nest boxes were 18 cm height, 18 cm wide, with an entrance hole diameter of 25 mm. Nest boxes were mounted on tree trunks [>30 diameters at breast height (DBH)] at 2.5–3.5 m above the ground with the entrance-hole facing N–E. The mean annual breeding density of rayaditos recorded during 10 years in six patches (8.2 pairs/ha, P. A Marquet and C. Cornelius, unpublished data) was used as a guide to fix the nest box density within patches. Thus, nest boxes were placed systematically forming a grid and separated from each other by a distance of 35 m. This spatial arrangement resulted in a density of eight boxes per hectare within each sample area in all patches. Furthermore, using the join-count statistic (Cliff and Ord 1981) we determined that the probability of a nest box being used during the breeding season was not influenced by the presence of neighbor individuals using nest boxes. We checked the nest boxes weekly between late September and late December. In each visit we measured: (1) the number of eggs and chicks; (2) presence of fledglings near the nest; (3) nest fate; (4) nest date and (5) direct evidence of nest predation, such as eggshells.
Habitat and patch data
Habitat structure was measured in 0.04-ha plots around the nest boxes, including understory and canopy cover (%), tree density (tree/ha), diameters at breast height (DBH, cm), canopy height (m), tree richness (number of species), density of snags and den trees (number/ha) (James and Shugart 1970). We measured tree density for >5 cm DBH trees. We measured canopy cover with a densitometer in each cardinal direction, canopy height was measured using a clinometer, and ground cover was measured using 1 m2 circular quadrats (Vergara and Simonetti 2004b). Standing dead trees were considered as snags and decayed trees with visible hollows as dens (>40 cm DBH) (Mannan et al. 1994). All these habitat variables could affect the abundance of rayaditos (Vergara 2006).
We selected and quantified patch size and connectivity from 56 patches using orthophotographs (1:10,000) taken in 1993, that were digitized as polygons in Arcview 3.2 (ESRI, Redland, CA, USA). Because forest fragments were in a protected area, we expected minimal change in their size from 1993 to 2004. Patch position and their boundaries were checked in the field with a Global Positioning System with differential correction. Patch distances were estimated as the shortest distance from edge-to-edge of patches for each focal patch. We also measured distance from edges as the distance from each sampling plot to the nearest forest edge. Functional connectivity was estimated following Hanski’s (1994) formulation and structural connectivity through the Krackhardt index (Krackhardt 1994). The former is an estimate of functional connectivity because it takes into account distances to all potential source patches and their sizes based on the patch movement ability of individuals (Moilanen and Nieminen 2002). Because we lack prior information on the number of individuals per patch, we assumed that abundance on each patch was directly proportional to patch area (Verboom et al. 2001). Hence, connectivity can be expressed as:
where S i (ha) is the functional connectivity of patch i, which is the sum of all contributions of neighboring patches j (total patches = k) in terms of their dispersal probabilities, which are dependent on distance to focal patch i (d ij , m), weighted by their area (A j , ha). The contribution of a patch at distance d declines exponentially with the species-specific parameter α, such that 1/α represents the mean dispersal distance of species for a particular season (Moilanen and Nieminen 2002). Parameter α was estimated using data of between-patch movements of rayaditos gathered from 65 playback trials made during the breeding season and 35 during the non-breeding season (Vergara 2006). We fitted a negative exponential distribution to between-patch movement data using a Binomial regression with a complementary log–log link function and log(distance) as an offset term. This regression analysis allowed obtaining the cumulative probability function (Martuzzi and Elliott 1998), according to the following formulae:
where v ij is the cumulative probability of between-patch movements up to a distance d ij [Pr(x ≤ dij)]. We calculated v ij as the cumulative proportion of trials with rayaditos crossing from the focal patch i to the neighbor patches j. The 95 confidence intervals of the regression coefficients of the above model indicated that the mean dispersal distance (1/α) of the breeding and non-breeding seasons were not different (1/α ± CI: 38.3 ± 4.9 and 34.6 ± 5.4 m, respectively). In both seasons, coefficients were significant at p < 0.05.
Krackhardt index (structural connectivity) is an estimate derived from graph theory, which assesses the contribution of each patch to the connectivity of the whole patch network (Krackhardt 1994). To estimate the structural connectivity we first used inter-patch distances to build a minimum spanning tree (MST), which corresponds to the shortest path connecting all patches in a network (e.g., Urban and Keitt 2001). Once the MST is calculated, we can estimate the contribution of each patch to the connectivity of the MST as the total number of paths that go through it in order to connect two other patches in the MST. This measure is based on the total number of patch pairs joined through each focal patch (n i ) and the total number of possible pairs in the patch network (n t ). The structural connectivity of patch i (D i ) is estimated as:
where \( {\hbox{ }}C_{n,j}^2 \) and \( {\hbox{ }}C_k^2 \) are the number of combinations of n and k patches taken two at a time, respectively, n, j represents the number of patches in the jth patch subset joined to the patch network through patch i and k is the total number of patches in the network. Thus, D i estimates the probability two patches are connected by a particular patch. Functional and structural connectivity estimates were calculated using the R statistical package (http://www.R-project.org).
Data transformation
Prior to these analyses we assessed if there were significant correlations between and within variable sets (patch attributes and habitat variables, Table 1), which if found, might affect model coefficients due to multicollinearity (Saville and Wood 1991). In order to control for possible effects of these correlations, we used the residuals of a principal components regression (PCR) applied to habitat variables to derive new orthogonal (uncorrelated) variables (i.e., corrected variables) (Jolliffe 2002). PCR was implemented in the following sequence: (1) we carried out a principal components analysis (PCA) based on patch size and connectivity (structural and topological), (2) we estimated the scores of the first factor, which accounted for 96% of the variance, (3) we did a regression between each habitat variable and the PCA scores (Jolliffe 2002) and (4) we took the residuals of this regression, which would correspond to the corrected variable. However, the habitat variables corrected by patch attributes can still suffer from collinearity due to correlations with the other habitat variables. To control for these effects, variables were further corrected by the effect of the other habitat variables following the same procedure described above (i.e., by regressing them on the scores of a PCA of the other variables). Finally, area and patch connectivity were also corrected by their reciprocal effect by replacing the original values of the variable area by the residuals of a regression analysis of patch area on connectivity; this new variable was called corrected patch area. We normalized all habitat variables by a logarithmic transformation, with the exception of DBH and canopy cover which were normalized by an arcsine transformation (Kolmogorov–Smirnov test p > 0.05 in all cases).
Statistical analysis of bird density
Bird density per patch (bird/ha) was calculated as the mean density of all point counts in a patch. Density per point was estimated as the average density at a point including all visits during a season. Density estimator was normalized by a log(1 + density) transformation. Density in each patch was spatially independent from another patches such that we did not detect spatial autocorrelation in density among patches [average Moran’s I-values (package spdep, R 2.1.1) were not significant for all distance classes]. We evaluated the additive effect of habitat and patch variables on bird density by means of a stepwise GLM-Repeated Measures Analysis with Gaussian error (Underwood 1997). The independent predictors were selected using a backward stepwise procedure based on Akaike’s information criterion (AIC). The presence of nest boxes was included as a fixed factor. Presence of nest boxes was considered as a measure of nest site availability in forest fragments (Tomasevic and Estades 2006). We controlled for the positive effect of occupied nest boxes on density by including nest box occupancy at the patch scale as a random factor. We assumed that the addition of nest boxes increased nest site availability, since rayaditos, as other forest birds, prefer nest boxes over natural cavities (Vergara et al. 2003; Tomasevic and Estades 2006). We determined whether the relationship between density with patch and habitat variables was consistent over the two seasons by estimating interactions between patch variables and season.
Statistical analysis of nest success
To examine the additive effect of habitat structure and patch attributes on the nest success of rayaditos, we used logistic-exposure models in which each interval between visits to a nest is treated as one independent observation in the analysis (Shaffer 2004). All habitat variables measured in this study were used as independent variables, including the edge distance. Re-nesting attempts were omitted from the analysis because they occurred in too few cases to allowing for a statistical assessment.
A set of four candidate models were developed based on combinations of the variables that could explain variation in daily nest success. (1) Patch (patch area and connectivity); (2) habitat (including seven habitat variables); (3) distance from edge; (4) time-specific effects including the nesting stage (egg laying, incubation and nestling), the nest initiation date (Julian) and the age (in days). We assessed the fit of the global model (with all variables included) using Pearson’s χ 2 test for binomial data, with data values for each predictor variable replaced with integer values (e.g., Pulkstenis and Robinson 2002). In addition, we built a null or constant-survival model (intercept only) to determine if it received more support from the data than a model including any of the remaining variables. Models were selected by judging the degree of support as measured by AICc (corrected for small sample size; Burnham and Anderson 2002). Models were ranked by comparing their ΔAICc and Akaike weights, which provide a measure of the strength of evidence for each model (Burnham and Anderson 2002). Models with ΔAICc <2 are considered to have substantial support, while and Akaike weights (w i ) indicate the probability that the model is the best among the whole set of candidate models (Burnham and Anderson 2002). We computed a weighted average of the estimated coefficients based on model uncertainty, i.e., parameters of each model (i) are weighted by the Akaike weight (w i ), and averaged across models, thus reducing bias and increasing precision (Burnham and Anderson 2002). The strength of each model parameter was interpreted by using both odd ratios estimated from model-averaged regression coefficients and the proportion of change in odd ratios (Hosmer and Lemeshow 2000). A variable cannot be considered a useful predictor in the logistic model when its odds ratio is not different from 1.0 (Hosmer and Lemeshow 2000). Thus, we did not interpret odd ratios whose confidence intervals did include 1.0.
Logistic-exposure models were implemented by numeric minimization of a binomial log-likelihood function using a stochastic global optimization method (Nocedal and Wright 1999). Standard errors of parameters were calculated by estimating the inverse sample information matrix. We used estimates of daily survival rate, raised to the number of days in the nesting cycle (33 days, P. M. Vergara, personal observation) to estimate nest success.
Results
Bird density
Density of rayaditos was affected by patch attributes rather than by habitat structure and these effects varied seasonally. Bird density per patch ranged from 0 to 4.30 ind/ha during breeding and 0–5.75 ind/ha during the non-breeding season. Out of the 56 surveyed patches, individual presence was recorded in 39 (69%) during the breeding season and in 23 (41.1%) in the non-breeding season. Density of rayaditos was not different between seasons (1.02 ± 0.15 vs. 0.91 ± 0.21) and it was not affected by presence of nest boxes. Additionally, no habitat variables were associated with bird density. Patch size and functional connectivity positively affected the density of rayaditos in both seasons (R 2 = 0.74 and 0.78, for the best stepwise models of the breeding and non-breeding seasons, respectively, Table 2). Although functional connectivity affected density in both seasons, there was a marginally significant interaction between season and functional connectivity (Table 2, Fig. 2). In contrast, the magnitude of the effect of patch size on density of rayaditos changed between seasons resulting in a significant interaction (Table 2). The slope of the relationship between density and patch area increased about 1.8 times from the breeding to the non-breeding season (Table 2, Fig. 2).
Relationship between density of Thorn-tailed Rayaditos and (a ) patch area corrected by connectivity (see text for variable transformation) (b) functional connectivity for breeding (empty squares, dashed line) and non-breeding seasons (filled squares, continuous line). We used back-transformed variable to help interpretation of the results. Linear regression analysis shows that patch area explains 24 and 38% of the variance in density (R 2), while this for functional connectivity varies between 61 and 54%, for breeding and non-breeding, respectively
Nest success
A total of 48 (32%) nesting attempts out of the 152 nest boxes were observed in 12 patches (43%). Additionally, in 20 boxes rayaditos built nests that were not used during the period. The total number of intervals between successive visits to nests was 265.
The best-supported exposure-logistic models indicated that the nest success of rayaditos was affected by patch attributes instead of habitat structure (Table 3). Goodness-of-fit tests indicated that the global model fit the observed data (X 2 = 230.4, p = 0.820). We found no support for the effects of habitat structure, temporal effect and distance from edge (ΔAICc > 8) but found strong support for an effect of patch attributes (Table 3). With the exception of both functional and structural connectivity, all confidence intervals of odd ratios estimated from model-averaged model parameters overlapped 1.0 (Table 4). Model-averaged odd ratios indicated an increase in the odds of nest success with both an increase in functional connectivity and a decrease in structural connectivity, respectively (Table 4). This indicates that for every increase of 1 log (ha) in functional connectivity the daily survival rate increased by 45% and for every increase of one unit of structural connectivity the daily survival rate decreased by 89% (Fig. 3). For functional connectivity, the greatest increases in daily nest survival occurred for patches with low connectivity values (Fig. 3), Nonetheless, daily nest survival decreased in a linear way with increasing structural connectivity (Fig. 3). Using the patch model (Table 3), the predicted nest success (averaged on all patches) was 60.2% (range 0–91%). We found nest predation evidence in 7 nests out of 13 failed nests (53%), including two nests where nestlings disappeared prematurely. Nest failure for the remaining six nests was apparently caused by nest abandonment.
Discussion
Bird density
In agreement with our first prediction, density of rayaditos at Fray Jorge is primarily influenced by patch attributes, but the magnitude of these effects is depended on season. During the non-breeding season the effect of area on bird density increased, with an accumulation of individuals in the largest patches and a corresponding decrease in the density observed in small patches as compared to the pattern observed during the breeding season.
We hypothesize that the observed differences in the effect of patch size on density can be explained by the interplay among territorial behavior, resource availability and abiotic conditions. Rayaditos are territorial during the breeding season and this could limit the number of individuals in a patch because nesting individuals either evict intruders from their territories or show facultative central-place foraging (e.g., Andersson 1981). Large patches may provide suitable sites for rayaditos, and territoriality might force some individuals to nest in small patches. On the contrary, the disappearances of territories during the non-breeding season, when rayaditos move into family groups, allows for an increase in density in the large patches. Large and connected patches may be either more easily to be reached by individuals and/or concentrate more resources. Field data indicate that the abundance of insects increase with patch size (Barbosa and Marquet 2002; P. A. Marquet, unpublished) which suggests that rayadito density is positively correlated to resource availability. Food limitation in small patches might be especially important during the dry non-breeding season (austral summer), because the abundance and richness of invertebrate in patches is lower (Barbosa and Marquet 2002). In addition to food limitation, rayaditos may avoid using small patches during the non-breeding season as a way to reduce thermal stress, since small patches are more similar in temperature to the conditions prevailing in the xeric matrix habitat (Barbosa and Marquet 2002; Del-Val et al. 2006).
The positive effect of patch size and connectivity may be related to suitability of habitat matrix. At the Fray Jorge forest the scrub matrix is not used by rayaditos for foraging or perching, suggesting that the effect of patch area and connectivity on density is stronger than in other fragmented native forests. In areas where the forest is surrounded by a matrix of plantations, rayaditos nest and forage in the matrix (Vergara and Simonetti 2004a; Tomasevic and Estades 2006). However, at Fray Jorge the scrub matrix does not act as an absolute barrier to movement, since data of between-patch movements of rayaditos gathered from playback experiments indicate that individuals can move more than 100 m through the matrix (Vergara 2006). This suggests that the observed pattern of landscape use by rayaditos is determined by movement decisions based on patch attributes (Vergara 2006).
Structural connectivity lacks any explanatory value for density probably because it does not consider patch size or habitat quality. In contrast, the functional connectivity is biologically more realistic because it includes the interaction between patch area and inter-patch distance. This result suggests that movements of rayaditos in Fray Jorge depend on these two patch features, and that they do not occupy patches based solely on the inter-patch movement distance as measured by the structural connectivity. Further, given that functional connectivity is an estimate of the habitat area found within the neighborhood of patches, it suggests that movements and densities of rayaditos are related to the amount of suitable habitat.
Nest success
In disagreement with our second prediction, nest success of rayaditos was not affected by habitat variables. Nevertheless, patch attributes were the main factors determining nest success of rayaditos, probably because of their effect on predation risk and resource availability (Lens et al. 2002). Contrarily, habitat features, which could have affected nest success, by reducing negative effects of wind damage as well as accessibility for nest predators like birds (e.g., Bowman and Woolfenden 2002), were not important in explaining nest success and hence breeding density.
Functional connectivity was the most important patch attribute in affecting (positively) nest success and breeding density. In contrast, structural connectivity negatively affected nest success but had no effect on breeding density. This negative effect on nest success could be related to biotic or abiotic factors other than those associated to functional connectivity. Possibly, increasing the connectivity for some nest predators that move across patches by minimizing inter-patch distances. The positive effect of functional connectivity on nest success may be associated to the total amount of resource available for individuals and predation risk (Hinsley 2000; Norris and Stutchbury 2001). Previous studies of nesting birds have not found a significant effect of patch isolation on nest success, although isolated patches tend to be unoccupied or to have lower bird density values (e.g., Matthysen and Adriaensen 1998; Cooper et al. 2002). Nevertheless, other studies have also shown that the breeding success of birds occupying large home ranges is positively associated with the amount of available forest area (Kurki et al. 2000). It is expected that birds living in fragmented landscapes occupy territories comprising more than one patch (Hinsley 2000) and this might be especially likely for rayaditos in Fray Jorge where patches are closer than 20 m from each other, a distance smaller than the mean dispersal distances estimated from our movement data. Thus, larger functional connectivity values may imply both a lower nest predation risk by nest predators associated to the matrix habitat and more available forest area for nesting individuals, reducing the search costs associated to either the risk of mortality or the encounter rate of low-quality habitats. Consequently, since connected patches are suitable sites for nesting rayaditos it is probable that this particular patch attribute acts as a cue in attracting birds during the breeding seasons and hence increasing the density of rayaditos at the connected patches.
Contrary to expectations, our results did not evidence a positive effect of distance from edge and patch size on nesting success, which is usually observed (e.g., Angelstam 1986; Keyser et al. 1998). Negative edge effects on nesting birds could be critical in other human modified habitats, such as the Maulino forest at central Chile (Vergara 2005). Although our results indicated that nest predation was an important source of nest failure, our data were insufficient to test for an association between functional connectivity and nest predation. Probably, nest predators associated to the shrub matrix are more abundant around clusters of small patches, which have small values of connectivity (see Verboom et al. 2001). We conclude that both patch size and connectivity were important landscape attributes that are essential for the persistence of rayaditos at Fray Jorge.
References
Alonso JA, Alonso JC, Carrascal LM, Muñoz-Pulido R (1994) Flock size and foraging decisions in central place foraging white storks, Ciconia ciconia. Behavior 129:279–292
Andersson M (1981) Central place foraging in the Whinchat, Saxicola Rubetra. Ecology 62:538–544
Andrén H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Andrén H, Delin A, Seiler A (1997) Population responses to landscape changes depends on specialization to different landscape elements. Oikos 80:193–196
Angelstam P (1986) Predation on ground-nesting birds’ nests in relation to predator densities and habitat edge. Oikos 47:365–373
Barbosa O, Marquet PA (2002) Effects of forest fragmentation on the beetle assemblage at the relict forests of Fray Jorge, Chile. Oecologia 132:296–306
Bibby CJ, Burgess ND, Hill DA (1992) Bird census techniques. Academic, London
Bowman R, Woolfenden GE (2002) Nest site selection by Florida Scrub-jays in natural and human-modified habitats. Wilson Bull 114:128–135
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993) Distance sampling. Estimating abundance of biological populations. Chapman & Hall, London
Burnham KP, Anderson DR (2002) Model selection and inference. Springer, New York
Chapa-Vargas L, Robinson SK (2006) Nesting success of a songbird in a complex floodplain forest landscape in Illinois, USA: local fragmentation vs. vegetation structure. Landscape Ecol 21:525–537
Cliff AD, Ord JK (1981) Spatial processes, models and applications. Pion Limited, London
Cooper CB, Walters JR, Ford H (2002) Effects of remnant size and connectivity on the response of Brown Treecreepers to habitat fragmentation. Emu 102:249–256
Cornelius C, Cofré H, Marquet PA (2000) The effects of habitat fragmentation on bird species in a relict temperate forest in semiarid Chile. Conserv Biol 14:1–11
Del-Val E, Armesto JJ, Barbosa O, Christie DA, Gutiérrez AG, Jones CG, Marquet PA, Weathers KC (2006) Rain forest islands in the Chilean semiarid region: fog-dependency, ecosystem persistence and tree regeneration. Ecosystems 9:598–608
Estades CF, Escobar MAH, Tomasevic JA, Vukasovic MA, Páez M (2006) Mist-nets versus point counts in the estimation of forest bird abundances in South-Central Chile. Ornitol Neotrop 17:203–212
Gajardo R (1993) La vegetación natural de Chile. Editorial Universitaria, Santiago
Haila Y (1990) Toward an ecological definition of an island: a northwest European perspective. J Biogeogr 17:561–568
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63:151–162
Hinojosa LF, Villagrán C (1997) Historia de los bosques del sur de Sudamérica I: antecedentes paleobotánicos, geológicos y climáticos del Terciario del cono sur de América. Rev Chil Hist Nat 70:225–239
Hinsley SA (2000) The costs of multiple patch use by birds. Landscape Ecol 15:765–775
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Ippi S, Trejo A (2003) Dinámica y estructura de bandadas mixtas de aves en un bosque de lenga (Nothofagus pumilio) del Noroeste de la Patagonia argentina. Ornitol Neotrop 14:353–362
James FC, Shugart HH (1970) A quantitative method of habitat description. Aud Field Notes 24:727–736
Jolliffe IT (2002) Principal component analysis, 2nd edn. Springer, New York
Keymer JE, Marquet PA, Velasco-Hernández JX, Levin SA (2000) Extinction thresholds and metapopulation persistence in dynamic landscapes. Am Nat 156:478–494
Keyser AJ, Hill GE, Soehren EC (1998) Effects of forest fragment size, nest density, and proximity to edge on the risk of predation to ground-nesting passerine birds. Conserv Biol 12:986–994
Krackhardt D (1994) Graph theoretical dimensions of informal organizations. In: Carley KM, Prietula MJ (eds) Computational organization theory. Lawrence Erlbaum and Associates, Hillsdale, pp 89–111
Kurki S, Nikula A, Helle P, Linden H (2000) Landscape fragmentation and forest composition effects. Ecology 81:1985–1997
Lens L, Van Dongen S, Norris K, Githiru M, Matthysen E (2002) Avian persistence in fragmented rainforest. Science 298:1236–1238
López-Cortés F, López D (2004) Antecedentes bioclimáticos del Parque Nacional Bosque Fray Jorge. In: Squeo FA, Gutiérrez JR, Hernández IR (eds) Historia Natural del Parque Nacional Bosque Fray Jorge. Ediciones Universidad de La Serena, La Serena, pp 45–60
Mannan RW, Conner RN, Marcot B, Peek JM (1994) Managing forestlands for wildlife. In: Bookhout TA (eds) Research and management techniques for wildlife and habitats, 5th edn. The Wildlife Society, Bethesda, pp 689–721
Manton MG, Angelstam P, Mikusiński G (2005) Modelling habitat suitability for deciduous forest focal species—a sensitivity analysis using different satellite land cover data. Landscape Ecol 20:827–839
Martuzzi M, Elliott P (1998) Estimating the incidence rate ratio in cross-sectional studies using a simple alternative to logistic regression. Ann Epidemiol 8:52–55
Matthysen E, Adriaensen F (1998) Forest size and isolation have no effect on reproductive success of Eurasian Nuthatches (Sitta europaea). Auk 115:955–963
McGarigal K, McComb WC (1995) Relationships between landscape structure and breeding birds in the Oregon Coast Range. Ecol Monogr 65:235–260
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83:1131–1145
Nocedal J, Wright SJ (1999) Numerical optimization. Springer, New York
Norris DR, Stutchbury BJM (2001) Extraterritorial movements of a forest songbird in a fragmented landscape. Conserv Biol 15:729–736
Pearson SM, Turner MG, Gardner RH, O’Neill RV (1996) An organism-based perspective of habitat fragmentation. In: Szaro RC, Johnston DW (eds) Biodiversity in managed landscapes. Oxford University Press, New York, pp 77–95
Pulido FJ, Díaz M (1997) Linking individual foraging behavior and population spatial distribution in patchy environments: a field example with Mediterranean blue tits. Oecologia 111:434–442
Pulkstenis E, Robinson T (2002) Two goodness-of-fit tests for logistic regression with continuous covariates. Stat Med 21:79–93
Ramsey FL, Scott JM (1979) Estimating population densities from variable circular plot surveys. In: Cormack RM, Patil GP, Robson DS (eds) Sampling biological populations. Statistical ecology series, vol 5. International Co-operative Publishing House, Fairland, pp 155–181
Robinson SK, Thompson FR, Donovan TM, Whitehead DR, Faaborg J (1995) Regional forest fragmentation and the nesting success of migratory birds. Science 267:1987–1990
Rodríguez A, Andrén H, Jansson G (2001) Habitat-mediated predation risk and decision making of small birds at forest edges. Oikos 95:383–396
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Saville D, Wood GR (1991) Statistical methods: the geometric approach. Springer, New York
Schmiegelow FKA, Mönkkönen M (2002) Habitat loss and fragmentation in dynamic landscapes: avian perspectives from the boreal forest. Ecol Appl 12:375–389
Schrott GR, With KA, King ATW (2005) On the importance of landscape history for assessing extinction risk. Ecol Appl 15:493–506
Shaffer TL (2004) A unified approach to analyzing nest success. Auk 121:526–540
Tellería JL, Santos T (1995) Effects of forest fragmentation on a guild of wintering passerines: the role of habitat selection. Biol Conserv 71:61–67
Tomasevic JA, Estades CF (2006) Stand attributes and the abundance of secondary cavity-nesting birds in southern beech (Nothofagus) forests in South-Central Chile. Ornitol Neotrop 17:1–14
Underwood AJ (1997) Experiments in ecology. Cambridge University Press, Cambridge
Urban D, Keitt T (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82:1205–1218
Verboom J, Foppen R, Chardon P, Opdam P, Luttikhuizen P (2001) Introducing the key patch approach for habitat networks with persistent populations: an example for marshland birds. Biol Conserv 100:89–101
Vergara PM (2005) Predation on bird nests in forest edges of a national reserve of the coastal range of the Maule Region. In: Smith C, Armesto JJ, Valdovinos C (eds) Biodiversidad y Ecología de los bosques de la Cordillera de la Costa de Chile. Editorial Universitaria, Chile, pp 554–560
Vergara PM (2006) Estructura espacial de la población de rayaditos (Aphrastura spinicauda) del bosque fragmentado de Fray Jorge. Doctoral Thesis, Facultad de Ciencias Biologicas, Pontificia Universidad Católica de Chile
Vergara PM, Simonetti JA (2004a) Avian responses to fragmentation of the Maulino in central Chile. Oryx 38:383–388
Vergara PM, Simonetti JA (2004b) Does nest-site cover reduce nest predation for rhinocryptids? J Field Ornithol 75:188–191
Vergara PM, Marquet P, Zeballos H (2003) Nesting of the Thorn-tailed Rayadito (Aphrastura Spinicauda) in the metropolitan region of Central Chile. Abstracts of VI Neotropical Ornithological Congress, Chile, pp 153
Villard MA, Trzcinski MK, Merriam G (1999) Fragmentation effects on forest birds: relative influence of woodland cover and configuration on landscape occupancy. Conserv Biol 13:774–783
Vuilleumier F (1967) Mixed species flocks in Patagonian forest, with remarks on interspecies flock formation. Condor 64:400–404
Wiens JA (1976) Population responses to patchy environments. Annu Rev Ecol Syst 7:81–120
Acknowledgments
This study was financed by AT-4040007 CONICYT fellowship, FONDAP-FONDECYT 1501-0001 and ICM (P05-002). We thank to Corporación Nacional Forestal (CONAF) for granting us access to the Fray Jorge National Park for our study. We also thank to J. M. Torres-Hidalgo and E. Ruiz for their help and assistance. We also thank S. Abades for suggestions and assistance with the R programming language and T.O. Crist for his comments and suggestions that greatly improved the manuscript. Part of this work was conducted while PM was a Sabbatical Fellow at the National Center for Ecological Analysis and Synthesis, a Center funded by NSF (Grant #DEB-0072909), the University of California, and the Santa Barbara campus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vergara, P.M., Marquet, P.A. On the seasonal effect of landscape structure on a bird species: the thorn-tailed rayadito in a relict forest in northern Chile. Landscape Ecol 22, 1059–1071 (2007). https://doi.org/10.1007/s10980-007-9091-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-007-9091-9