Abstract
Tropomyosin and troponin have well known Ca2+-regulatory functions in the striated muscle sarcomere. In this review, we summarize experimental evidence that tropomyosin and troponin are localized, with as yet unidentified functional roles, in the striated muscle cell nucleus. We also apply bioinformatics approaches that predict localization of some tropomyosin and troponin to the nucleus, and that SUMOylation could be a covalent modification that modulates their nuclear localization and function. Further, we provide examples of cardiomyopathy mutations that alter the predicted likelihood of nuclear localization and SUMOylation of tropomyosin. These observations suggest novel mechanisms by which cardiomyopathy mutations in tropomyosin and troponin might alter not only cardiac contractility but also nuclear function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The eukaryotic nucleus, even during interphase, is a more highly dynamic and complex entity than might be supposed from its role as the organelle in which a cell’s DNA is stored, replicated and transcribed (Misteli and Spector 2011). Actin and myosin are found in the nucleus and are involved in key functions including transcription regulation and chromatin remodeling (Grummt 2006; Miralles and Visa 2006; de Lanerolle and Serebryannyy 2011). In addition, actin-binding proteins such as formins can also localize to the nucleus and promote polymerization of nuclear actin, an essential part of the pathway for serum response factor (Srf) dependent gene regulation (Baarlink et al. 2013). The finding that Ca2+-regulatory proteins tropomyosin and troponin are present not only in the myofilaments (Gordon et al. 2000; Parmacek and Solaro 2004; Tobacman 1996) but also in nuclei of mammalian striated muscle cells (Asumda and Chase 2012; Bergmann et al. 2011; Kajstura et al. 2010; Zhang et al. 2013b) suggests that the nucleoskeleton—the nucleus’ equivalent of the cytoskeleton—is structurally more complex, and may have more complex regulation, than previously recognized.
Evidence for nuclear localization of tropomyosin and troponin
Bergmann et al. (2009) demonstrated that isolated nuclei from adult human myocardium are recognized by antibodies against cTnI and cTnT. This property distinguished cardiomyocyte nuclei from nuclei of other cell types in the heart, but did not distinguish whether nucleus-associated cTnI and cTnT were located on the surface of, or within nuclei. Subsequent experiments showed immunofluorescence labeling with antibodies against cTnI of some (Kajstura et al. 2010) or nearly all (Bergmann et al. 2011) cardiomyocyte nuclei in sections of human myocardium. The observation that the extent of nuclear labeling in tissue sections depends on sample processing (Bergmann et al. 2011) indicates that nuclei of most if not all human cardiomyocytes contain cTnI (Laflamme and Murry 2011).
Nuclear localization of troponin and tropomyosin is not unique to cardiomyocytes from adult humans. Proteomic analysis of cardiac nuclei isolated from rodents identified the presence of cTnC, cTnI and several isoforms of Tm including cardiac αTm (Franklin et al. 2011). Fluorescent constructs of cTnT that contain the C-terminal portion of the molecule localize to nuclei of transfected skeletal muscle cells from mouse and C2C12 cells in culture (Zhang et al. 2013a, b). All three subunits of native cardiac troponin (cTnC, cTnI and cTnT) and cardiac αTm were identified by immunofluorescence in nuclei of rat neonatal ventricular cardiomyocytes in culture (Asumda and Chase 2012); confocal z-sections demonstrated that these Ca2+-regulatory proteins are located throughout the nucleus and are not just associated with the surface membrane. In addition, cTnC, cTnI, cTnT and αTm were also detected in both the cytoplasm and nuclei of rat bone marrow-derived mesenchymal stem cells between 3 and 5 days in culture media that induces cardiac differentiation (Asumda and Chase 2012). Taken together, these studies suggest that nuclear localization of Ca2+-regulatory proteins occurs rapidly (days), in parallel with appearance in myofilaments in the cytoplasm, and likely occurs early in development.
Tropomyosin and troponin have been found in nuclei in some non-muscle cells. Dingová et al. (2009) identified nuclear Tm by multiple techniques—including immunohistochemistry—in both HeLa cells and resting human lymphocytes. In this instance, the Tm may have been a non-muscle isoform although the study did not distinguish among Tm isoforms. Dingová et al. (2009) also examined nuclear localization of other actin-binding proteins in addition to Tm, but did not test for Tn because expression of Tn was unlikely in these cell types. Interestingly, TnI was detected by immunofluorescence in nuclei of ~15 % of cultured Drosophila S2 cells and also appeared to be in close association with chromosomes approaching metaphase in pre-cellular Drosophila embryos (Sahota et al. 2009). There was also a genetic association between nuclear division in pre-cellular Drosophila embryos and TnI or Tm. Furthermore, α-helical coiled-coil proteins with homology to Tm have been identified in nuclei of plant cells in Arabadopsis and carrot (Gardiner et al. 2011; Dittmer et al. 2007; Masuda et al. 1997). These limited data provide hints that nuclear Tm with or without Tn can be found in a variety of cell types, at some point in the cell cycle or during development, and thus could be evolutionarily widespread.
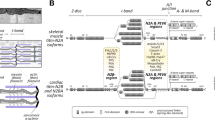
To further explore the potential for human cardiac tropomyosin and troponin subunits to localize in the cardiomyocyte nucleus, we used WoLF PSORT to predict subcellular localization based on primary sequence (Horton et al. 2007). Consistent with predictions by others for sTnT (Zhang et al. 2013a), cTnT and cTnI (Bergmann et al. 2009), features within the primary sequence for all four polypeptides indicate a significant likelihood that some protein would reside in the nucleus at least some of the time (Table 1). For reference, Table 1 also includes WoLF PSORT predictions for four transcription factors (Srf, Gata4, Nkx2.5, and Mef2a) that are exclusively localized to the nucleus, a fifth transcription factor (nuclear factor of activated T-cells, NFATC2) that translocates between the cytoplasm and nucleus in a phosphorylation-dependent manner,Footnote 1 and four metabolic enzymes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH; lactate dehydrogenase heart subunit, LDH-H; hexokinase type I, HK1; and muscle-type creatine kinase, M-CK) that are primarily cytoplasmic. The predicted likelihood of nuclear localization for cTnI was particularly high, and was only slightly lower than that for transcription factors that are always localized in the nucleus (Table 1). We confirmed the qualitative nature of predictions for Tm and Tn subunits obtained from WoLF PSORT using a second bioinformatics algorithm, CELLO 2.5 (Yu et al. 2006).
The experimentally observed distribution of Tm and Tn subunits within muscle cell nuclei is non-uniform using immunohistochemistry or expression of fluorescent constructs (Sahota et al. 2009; Bergmann et al. 2011; Asumda and Chase 2012; Zhang et al. 2013a, b). Biochemical fractionation suggests that cardiac αTm, cTnC and cTnI are part of a relatively small cohort of proteins that are ubiquitously present in all nuclear fractions of mouse cardiomyocytes examined by Franklin et al. (2011): the acid-soluble, chromatin-associated, and nucleoplasmic fractions. Based on co-localization studies, Zhang et al. (2013b) suggested that nuclear sTnT concentrates in nucleoli. It remains to be determined, however, whether the major function(s) of nuclear Tm and Tn occur in nucleoli, in another nuclear sub-domain such as PML bodies or nuclear dots (Shen et al. 2006; Strickfaden et al. 2012), or whether they function more broadly throughout nuclei.
Signals for nuclear localization of tropomyosin and troponin: possible role of SUMOylation
Tm and Tn can be covalently modified in the sarcomere—by phosphorylation, for example—and these modifications typically modulate their function (Schulz and Wieczorek 2013; Solaro and Kobayashi 2011). The phosphorylation state of some other proteins, such as the transcription factor NFAT, regulates their movement between the cytoplasm and nucleus. NFAT is predicted to reside in either the nucleus or cytoplasm, and translocate between them (Table 1). In cardiac myocytes, NFAT localization affects gene expression, and may thereby play a role in disease-related cardiac remodeling (Berry et al. 2011).
It is not known what signal(s) promote translocation of some Ca2+-regulatory proteins from the cytoplasm where they are synthesized into the nucleus, and what signal(s) their retention in the nucleus. Kajstura et al. (2010) proposed that cTnI localization in human cardiomyocytes was an artifact of aging, i.e., leakiness of nuclear pore complexes in senescent cells. This may not be correct, however, because the majority of cardiac nuclei are cTnI and/or cTnT positive in both children and adults (Bergmann et al. 2011).
The N-termini of human cTnI and cTnT are predicted to contain basic, evolutionarily conserved, nuclear localization signal (NLS) sequences (Bergmann et al. 2009), and additional portions of the sTnT sequence have been demonstrated to be important for nuclear localization (Zhang et al. 2013a, b). Bioinformatics analyses based on primary sequence indicate that human cardiac Tm and all three Tn subunits have a high likelihood of nuclear localization (Table 1). cTnI and cTnT might translocate into the nucleus independently, and perhaps even function independently in the nucleus. Alternatively, they might assemble along with cTnC in the perinuclear region (Reddy et al. 2005) and the ternary Tn complex would be actively transported into the nucleus as a single entity. The C-terminal domain of a fluorescent construct of mouse sTnT localizes to sub-nuclear regions, as does the full length, fluorescent sTnT (Zhang et al. 2013a, b); this provides support for the idea that the Tn complex is the relevant entity because the truncated construct should assemble with sTnI, and thus with sTnC, too. On the other hand, the C-terminal construct of sTnT also shows diffuse distribution in the nucleoplasm, while N-terminal and central domain constructs, as well as the fluorescent protein on its own, show diffuse distribution throughout the cytoplasm and nucleus. It is not clear whether cells handle all of the shorter constructs by the same mechanism(s) as full length sTnT, but all constructs exhibited similar patterns in non-muscle (NIH3T3 and COS7) cell lines (Zhang et al. 2013b) which presumably express little or no cTnI or cTnT. As argued by Zhang et al. (2013a), there is not likely to be a requirement for assembly of the Tn complex for nuclear localization when sTnT is overexpressed because of the presumed non-stoichiometric ratios of the three troponin subunits even in cultured muscle cell lines. Thus current evidence suggests that nuclear localization in muscle is possible because the protein is being synthesized and is therefore present in the cytoplasm, as also suggested by the data of Asumda and Chase (2012), and does not depend on muscle-specific transporters for nuclear localization.
In addition to NLS sequences, it is likely that other signals modulate nuclear localization of Ca2+-regulatory proteins in muscle. Elsewhere in this special issue, Schulz and Wieczorek (2013) report on effects of Tm phosphorylation at S238 in mouse and human hearts. One of their approaches was to generate Tg mice that have Tm containing mutation S238A, rendering that site non-phosphorylatable. Interestingly, although they do not address the issue of Tm localization in the myocyte nucleus, we find that mutation S238A is predicted to increase the likelihood that Tm would be found in the nucleus (note that the predictive algorithms only consider primary sequence and not phosphorylation state). Similar to what is observed with NFAT phosphorylation (Berry et al. 2011), it is possible that covalent modifications could alter the likelihood of transport into and/or retention in the nucleus, and that experimental modification of protein sequence may influence more aspects of protein structure and function than initially anticipated.
SUMOylation is an important modulator of cardiac function and gene regulation (Wang and Schwartz 2010). SUMOylation is known to be required for nuclear localization of β-actin (Hofmann et al. 2009). Nuclear actin in the non-muscle HeLa and COS-7 cell lines is SUMOylated with isoforms SUMO2 and SUMO3 (Hofmann et al. 2009), which can lead to polymodification. Little is known about SUMOylation of Ca2+-regulatory proteins in striated muscle, although several lines of evidence, in addition to the presumptive commonality of SUMOylation of nuclear actin, suggest that the possibility should be investigated.
Small ubiquitin-like modifier proteins are small, ubiquitin-related modifier polypeptides of ~10 kDa that can be reversibly added to specific Lys residues of proteins (Johnson 2004; Geiss-Friedlander and Melchior 2007; Herrmann et al. 2007). SUMOylation modulates the function of a number of diverse proteins in addition to β-actin. Modulated functions include nuclear transport and localization of proteins (SUMOylated proteins in the nucleus are concentrated in PML bodies; Shen et al. 2006), thereby regulating key processes in cells including transcription and genome integrity.
Nuclear localization of TnI in Drosophila S2 cells requires SUMOylation of a Lys residue near the C-terminus (Sahota et al. 2009). The Lys residue that is SUMOylated in Drosophila TnI resides within a consensus sequence for SUMOylation as predicted using SUMOsp 2.0 (Ren et al. 2009). It aligns with residues within,Footnote 2 or immediately adjacent toFootnote 3 a cluster of three Lys residues near the C-terminus of human cTnI (the equivalent regions of fast and slow isoforms of human sTnI have only two adjacent Lys residues). None of the Lys residues at the C-termini of human TnI isoforms correspond to predicted consensus sequences for SUMOylation, but the most C-terminal of the three Lys residues in cTnI is predicted to have significant likelihood as a non-consensus sequence target for SUMOylation.
Human αTm is associated with the SUMO1 isoform in SUMOylated proteins from HEK293 cells, identified through immunoprecipitation (Manza et al. 2004). While this experiment does not distinguish SUMOylated proteins from unmodified proteins which are bound tightly to other SUMOylated proteins such as nuclear actin, human αTm does contain several predicted SUMOylation sites (Table 2) as recognized in the primary sequence using SUMOsp 2.0 (Ren et al. 2009). SUMOsp 2.0 also predicts several likely SUMOylation sites in human cardiac troponin subunits, a few comprised of consensus sequences and most of non-consensus sequences (not shown). Manza et al. (2004) did not provide specific information on whether αTm, SUMOylated or not, is localized in the nucleus because cytoplasmic and nuclear fractions were combined. Nuclear localization of αTm was anticipated in the study of Manza et al. (2004) because immunohistochemistry showed that the majority of SUMO, independent of isoform, was localized in the nucleus, with the most intense staining in nuclear bodies. SUMOylation may not be the sole mechanism controlling nuclear localization—and likely function because we do not know the influence of SUMOylation on structure, assembly and functional interactions—of Ca2+-regulatory proteins, but it seems worthwhile exploring the possibility that it has such a role in striated muscle.
Possible roles for nuclear tropomyosin and troponin: muscle physiology, aging and pathophysiology
Based on decades of knowledge about their function in the myofilaments of striated muscle’s cytoplasmic compartment, the most likely role for Tn and Tm in the nucleus—assuming that their presence in the nucleus is not simply an artifact of large amounts being synthesized in the cytoplasmic (myofilament) compartment—is to confer Ca2+-regulation to processes that involve nuclear actin. Although very little is specifically known about striated muscle cells, nuclear actin can exist in both monomeric and polymeric forms (Baarlink et al. 2013; McDonald et al. 2006). Many key functions for nuclear actin have been identified, including regulation of transcription and chromatin remodeling (de Lanerolle and Cole 2002; Grummt 2006; Visa and Percipalle 2010; de Lanerolle and Serebryannyy 2011). The clearest evidence for function of nuclear Tn and Tm comes from developmental studies in Drosophila where TnI may be associated with chromosomes, and TnI and Tm are both required for nuclear division (Sahota et al. 2009). There is, however, no direct evidence for (or against) association of nuclear Tn, Tm and actin in this or other studies. Co-localization, or more precisely molecular interactions between nuclear Tn subunits, Tm and actin in cells is an important area for future investigations. Both in vivo and in vitro studies are needed to provide information about whether presumptive SUMOylation or other modifications influence assembly and function of nuclear actin, Tm and Tn.
Whatever the function of nuclear Tn and Tm, it is presumably specific to striated muscle, at least in vertebrates. The question of striated muscle specificity is raised by observations that nuclear actin has been identified in a wide variety of cell types, and that some isoforms of Tm (Dingová et al. 2009) and of both Tn and Tm (Sahota et al. 2009) are present in nuclei of some non-muscle cells, but not others (Asumda and Chase 2012). There is a trivial explanation that may explain nuclear localization of specific Tn and Tm isoforms in vertebrate muscle, and tropomyosin in non-muscle: if these proteins are expressed in a cell, then some will translocate into the nucleus and be retained there. The data of Zhang et al. (2013a, b) provide the clearest evidence to support this idea; fluorescent constructs of sTnT expressed in cultured NIH3T3 and COS7 cells exhibited similar nuclear localization patterns as in skeletal muscle cells. The situation may be somewhat more complex in arthropods, with nuclear Tn and Tm being relevant early in development of the organism (Sahota et al. 2009); this remains to be examined in Drosophila muscle tissue.
Troponin presumably confers Ca2+-dependence to some aspect of nuclear function. Like many cell types, cardiac myocytes exhibit Ca2+ transients not only in the cytoplasm but also in the nucleoplasm. Ca2+ transients in both cultured, neonatal and adult rat heart cells exhibited substantial differences between the nucleus and the cytoplasm, including mechanism of regulation (Wu and Bers 2006; Minamikawa et al. 1995), although the lumen of the sarcoplasmic reticulum and nuclear envelope are effectively connected as a single Ca2+ store (Wu and Bers 2006). Perinuclear Ca2+ also appears to be significant for cardiac myocyte signaling (Escobar et al. 2011; Wu et al. 2006).
Nuclei in many if not all cell types act as integrators of, and thus respond to both chemical and mechanical signals, and this would be particularly important to regulate in cardiomyocytes. Ca2+-dependent processes in the nucleus could regulate aspects of nuclear function, or could stabilize nuclear function in the presence of continuously varying Ca2+ and mechanical forces. It is currently unknown if Ca2+ influences any aspect of nuclear mechanics in striated muscle cells. One suggestion that may be relevant is the necessity for control of dynamic organization of chromatin within the long-lived interphase nuclei of slowly dividing, adult human cardiomyocytes (Bergmann et al. 2011, 2009; Laflamme and Murry 2011). It is generally relevant that, in non-muscle cells, nuclear Ca2+ has been shown to regulate expression of specific genes, cell growth, and cell proliferation (Resende et al. 2013; Andrade et al. 2011).
We note the potential for enhanced actomyosin cycling in the nucleus due to the presence of dATPFootnote 4 and troponin. dATP has been demonstrated to enhance kinetics and mechanical function of skeletal muscle (Regnier and Homsher 1998; Regnier et al. 1998a, b; Clemmens and Regnier 2004; Racca et al. 2013), and has even greater effects in cardiac muscle (Schoffstall and Chase 2008; Schoffstall et al. 2006b; Regnier et al. 2000; Korte et al. 2011; Nowakowski et al. 2013). Both skeletal and cardiac troponins also enhance kinetics of actomyosin function (Regnier et al. 1996; Homsher et al. 1996; Gordon et al. 1997, 1998; Homsher et al. 2000; Schoffstall et al. 2006a; Brunet et al. 2012), possibly due to a direct influence of Tn on myosin (Schoffstall et al. 2011). What is not yet known is whether kinetics of various myosin isoforms that are found in the nucleus (Pestic-Dragovich et al. 2000; de Lanerolle and Cole 2002; de Lanerolle and Serebryannyy 2011) are differentially affected when dATP is at least partially substituted for ATP.
Aging
Zhang et al. (2013b) suggest that nuclear TnT may play a role in age-related muscle dysfunction, the underlying mechanism of which has previously eluded investigation (Phillips et al. 1993). Manza et al. (2004) found that αTm was no longer found with SUMOylated proteins in HEK293 cells treated with the lipid oxidation-derived electrophile 4-hydroxynonenal (HNE), a specific product of lipid oxidation associated with more general oxidative stress that may contribute to aging (Mandavia et al. 2012). Although the suggestion that cTnI in cardiomyocyte nuclei is related to senescence (Kajstura et al. 2010) is most likely not correct (Bergmann et al. 2011; Laflamme and Murry 2011; Asumda and Chase 2012), presence or absence of nuclear β-actin in non-muscle (epithelial) cells is an important determinant of whether the cells are quiescent (Spencer et al. 2011). Presumably at least some Tm and Tn associate with actin when all are simultaneously present in the nucleus, and thus changes in stoichiometry could be associated with aging, which in turn could alter mechanical and also Ca2+ signal transmission from the myofilaments to transcription.
Inherited cardiomyopathies
It is well established that cardiac mechanics are altered by cardiomyopathy associated mutations in myofilament proteins (Marston 2011; Tardiff 2011; Watkins et al. 2011; Willott et al. 2010; Ahmad et al. 2005; Gomes and Potter 2004; Parmacek and Solaro 2004). Disease-related mutations in tropomyosin that have been examined affect flexural rigidity and structural stability (Loong et al. 2012a, b; Li et al. 2012; Ly and Lehrer 2012; Wang et al. 2011; Kremneva et al. 2004). These structural and mechanical changes at the molecular level likely explain functional changes observed such as altered Ca2+ sensitivity of thin filament and sarcomere function (Mathur et al. 2011; Bai et al. 2011; Chang et al. 2005; Prabhakar et al. 2003, 2001; Karibe et al. 2001; Michele et al. 1999; Golitsina et al. 1999; Bing et al. 1997).
Mutations of cardiac tropomyosin that are associated with hypertrophic cardiomyopathy typically increase Ca2+ sensitivity of function, while mutations associated with dilated cardiomyopathy typically decrease Ca2+ sensitivity. Similar changes are typically observed for cardiomyopathy mutations in troponin subunits and other myofilament proteins (Brunet et al. 2012; Bai et al. 2011; Mathur et al. 2011; Wang et al. 2011; Marston 2011; Tardiff 2011; Willott et al. 2010; Landstrom et al. 2008; Gafurov et al. 2004; Gomes and Potter 2004; Köhler et al. 2003). While the molecular changes help us to understand changes in cardiac mechanical function, it has been more challenging to understand how they could influence nuclear functions such as transcription, e.g., to produce more muscle proteins in the hypertrophic phenotype (Watkins et al. 2011; Tardiff 2011). Altered Ca2+ binding and Ca2+ sensitivity of sarcomere function could indirectly influence various signaling pathways that modulate gene expression (Kataoka et al. 2007), particularly via changes in Ca2+ signaling (Wu et al. 2006).
There may be additional, direct influences of mutations in Tm and Tn on nuclear function. Altered Ca2+ sensitivity for Tm and Tn mutants in myofilaments would also affect functions of these mutant proteins in the nucleus. Furthermore, some disease-related mutations in Tm and Tn alter the predicted likelihood of nuclear localization and/or the predicted likelihood of SUMOylation. Table 3 shows an example of a hypertrophic cardiomyopathy mutation in αTm, I172T (Van Driest et al. 2003), that increases the predicted likelihood of localization toward the nucleus. Table 4 shows four examples of cardiomyopathy-associated mutations in αTm (Tardiff 2011) that alter predicted likelihood of SUMOylation. Two mutations, one associated with hypertrophic cardiomyopathy (E40K) and one associated with dilated cardiomyopathy (E192K) introduce Lys residues that are new, potential sites of SUMOylation; both of these mutations are part of consensus sequences for SUMOylation and are predicted to have a high likelihood of being SUMOylated. A third mutation (E62Q), one that is associated with hypertrophic cardiomyopathy, increases the predicted likelihood that a nearby Lys residue (K65) could be SUMOylated (Table 4). A fourth mutation (K70T), also associated with hypertrophic cardiomyopathy, markedly reduces the likelihood that αTm could be SUMOylated (Table 4) because it removes a Lys residue that is part of a non-consensus SUMOylation site (Table 2). We furthermore note that there is, in general, a relation of predicted SUMOylation sites and hot spots for mutations in a variety of proteins (Park et al. 2011).
Thus, in addition to altering sarcomere function, cardiomyopathy mutations and/or covalent modifications of tropomyosin and troponin may alter nuclear structure and function through their influence on the localization, retention and function of tropomyosin and troponin in myocyte nuclei.
Notes
WoLF PSORT does not explicitly account for phosphorylation state or other covalent modifications of proteins in predictions of subcellular localization.
Protein sequences aligned with EMBL-EBI Clustal Omega Multiple Sequence Alignment using default parameters.
Protein sequences aligned with NCBI Cobalt Multiple Alignment Tool using default parameters (Papadopoulos and Agarwala 2007).
2′-Deoxyadenosine-5′-triphosphate (dATP) is the nucleotide that is incorporated into DNA and is missing a 2′ oxygen compared with adenosine-5′-triphosphate (ATP), which is the nucleotide that is incorporated into RNA and is the conventional substrate for actomyosin along with many other energy-requiring processes in cells.
Abbreviations
- cTn:
-
Cardiac troponin complex
- cTnC:
-
Cardiac troponin C
- cTnI:
-
Cardiac troponin I
- cTnT:
-
Cardiac troponin T
- dATP:
-
2′-Deoxyadenosine 5′-triphosphate
- PML bodies:
-
Nuclear promyelocytic leukemia protein bodies (nuclear dots)
- sTn:
-
Skeletal troponin complex
- sTnC:
-
Skeletal troponin C
- sTnI:
-
Skeletal troponin I
- sTnT:
-
Skeletal troponin T
- SUMO:
-
Small ubiquitin-like modifier protein
- SUMO1:
-
SUMO isoform 1
- SUMO2:
-
SUMO isoform 2
- SUMO3:
-
SUMO isoform 3
- Tm:
-
Tropomyosin
- Tn:
-
Troponin complex
References
Ahmad F, Seidman JG, Seidman CE (2005) The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet 6:185–216
Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM, Robert M, Nathanson MH, Leite F (2011) Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol 55(3):626–635
Asumda FZ, Chase PB (2012) Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation 83(3):106–115
Baarlink C, Wang H, Grosse R (2013) Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340(6134):864–867
Bai F, Weis A, Takeda AK, Chase PB, Kawai M (2011) Enhanced active cross-bridges during diastole: molecular pathogenesis of tropomyosin’s HCM mutations. Biophys J 100(4):1014–1023
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102
Bergmann O, Zdunek S, Alkass K, Druid H, Bernard S, Frisén J (2011) Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res 317(2):188–194
Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, Rothermel BA, Hill JA (2011) Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res 109(4):407–417
Bing W, Redwood CS, Purcell IF, Esposito G, Watkins H, Marston SB (1997) Effects of two hypertrophic cardiomyopathy mutations in α-tropomyosin, Asp175Asn and Glu180Gly, on Ca2+ regulation of thin filament motility. Biochem Biophys Res Commun 236:760–764
Brunet NM, Mihajlović G, Aledealat K, Wang F, Xiong P, von Molnár S, Chase PB (2012) Micromechanical thermal assays of Ca2+-regulated thin-filament function and modulation by hypertrophic cardiomyopathy mutants of human cardiac troponin. J Biomed Biotechnol 2012:657523
Chang AN, Harada K, Ackerman MJ, Potter JD (2005) Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in α-tropomyosin. J Biol Chem 280(40):34343–34349
Clemmens EW, Regnier M (2004) Skeletal regulatory proteins enhance thin filament sliding speed and force by skeletal HMM. J Muscle Res Cell Motil 25(7):515–525
de Lanerolle P, Cole AB (2002) Cytoskeletal proteins and gene regulation: form, function, and signal transduction in the nucleus. Sci STKE (139):pe30
de Lanerolle P, Serebryannyy L (2011) Nuclear actin and myosins: life without filaments. Nat Cell Biol 13(11):1282–1288
Dingová H, Fukalová J, Maninová M, Philimonenko VV, Hozák P (2009) Ultrastructural localization of actin and actin-binding proteins in the nucleus. Histochem Cell Biol 131(3):425–434
Dittmer TA, Stacey NJ, Sugimoto-Shirasu K, Richards EJ (2007) LITTLE NUCLEI genes affecting nuclear morphology in Arabidopsis thaliana. Plant Cell 19(9):2793–2803
Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C (2011) Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol 50(3):451–459
Franklin S, Zhang MJ, Chen H, Paulsson AK, Mitchell-Jordan SA, Li Y, Ping P, Vondriska TM (2011) Specialized compartments of cardiac nuclei exhibit distinct proteomic anatomy. Mol Cell Proteomics 10(1):M110.000703
Gafurov B, Fredricksen S, Cai A, Brenner B, Chase PB, Chalovich JM (2004) The Δ14 mutant of troponin T enhances ATPase activity and alters the cooperative binding of S1-ADP to regulated actin. Biochemistry 43(48):15276–15285
Gardiner J, Overall R, Marc J (2011) Putative Arabidopsis homologues of metazoan coiled-coil cytoskeletal proteins. Cell Biol Int 35(8):767–774
Geiss-Friedlander R, Melchior F (2007) Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8(12):947–956
Golitsina N, An Y, Greenfield NJ, Thierfelder L, Iizuka K, Seidman JG, Seidman CE, Lehrer SS, Hitchcock-DeGregori SE (1999) Effects of two familial hypertrophic cardiomyopathy-causing mutations on α-tropomyosin structure and function. Biochemistry 38(12):3850
Gomes AV, Potter JD (2004) Molecular and cellular aspects of troponin cardiomyopathies. Ann NY Acad Sci 1015:214–224
Gordon AM, LaMadrid M, Chen Y, Luo Z, Chase PB (1997) Calcium regulation of skeletal muscle thin filament motility in vitro. Biophys J 72:1295–1307
Gordon AM, Chen Y, Liang B, LaMadrid M, Luo Z, Chase PB (1998) Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol 453:187–197
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Grummt I (2006) Actin and myosin as transcription factors. Curr Opin Genet Dev 16(2):191–196
Herrmann J, Lerman LO, Lerman A (2007) Ubiquitin and ubiquitin-like proteins in protein regulation. Circ Res 100(9):1276–1291
Hofmann WA, Arduini A, Nicol SM, Camacho CJ, Lessard JL, Fuller-Pace FV, de Lanerolle P (2009) SUMOylation of nuclear actin. J Cell Biol 186(2):193–200
Homsher E, Kim B, Bobkova A, Tobacman LS (1996) Calcium regulation of thin filament movement in an in vitro motility assay. Biophys J 70:1881–1892
Homsher E, Lee DM, Morris C, Pavlov D, Tobacman LS (2000) Regulation of force and unloaded sliding speed in single thin filaments: effects of regulatory proteins and calcium. J Physiol 524(Pt 1):233–243
Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35(Web Server issue):W585–W587
Johnson ES (2004) Protein modification by SUMO. Annu Rev Biochem 73:355–382
Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P (2010) Cardiomyogenesis in the adult human heart. Circ Res 107(2):305–315
Karibe A, Tobacman LS, Strand J, Butters C, Back N, Bachinski LL, Arai AE, Ortiz A, Roberts R, Homsher E, Fananapazir L (2001) Hypertrophic cardiomyopathy caused by a novel α-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation 103(1):65–71
Kataoka A, Hemmer C, Chase PB (2007) Computational simulation of hypertrophic cardiomyopathy mutations in troponin I: influence of increased myocyte calcium sensitivity on isometric force, ATPase and [Ca2+]i. J Biomech 40(9):2044–2052
Köhler J, Chen Y, Brenner B, Gordon AM, Kraft T, Martyn DA, Regnier M, Rivera AJ, Wang C-K, Chase PB (2003) Familial hypertrophic cardiomyopathy mutations in troponin I (K183Δ, G203S, K206Q) enhance filament sliding. Physiol Genomics 14(2):117–128
Korte FS, Dai J, Buckley K, Feest ER, Adamek N, Geeves MA, Murry CE, Regnier M (2011) Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. J Mol Cell Cardiol 51(6):894–901
Kremneva E, Boussouf S, Nikolaeva O, Maytum R, Geeves MA, Levitsky DI (2004) Effects of two familial hypertrophic cardiomyopathy mutations in α-tropomyosin, Asp175Asn and Glu180Gly, on the thermal unfolding of actin-bound tropomyosin. Biophys J 87(6):3922–3933
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473(7347):326–335
Landstrom AP, Parvatiyar MS, Pinto JR, Marquardt ML, Bos JM, Tester DJ, Ommen SR, Potter JD, Ackerman MJ (2008) Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J Mol Cell Cardiol 45(2):281–288
Li XE, Suphamungmee W, Janco M, Geeves MA, Marston SB, Fischer S, Lehman W (2012) The flexibility of two tropomyosin mutants, D175N and E180G, that cause hypertrophic cardiomyopathy. Biochem Biophys Res Commun 424:493–496
Loong CKP, Zhou H-X, Chase PB (2012a) Familial hypertrophic cardiomyopathy related E180G mutation increases flexibility of human cardiac α-tropomyosin. FEBS Lett 586(19):3503–3507
Loong CKP, Zhou H-X, Chase PB (2012b) Persistence length of human cardiac α-tropomyosin measured by single molecule direct probe microscopy. PLoS One 7(6):e39676
Ly S, Lehrer SS (2012) Long-range effects of familial hypertrophic cardiomyopathy mutations E180G and D175N on the properties of tropomyosin. Biochemistry 51(32):6413–6420
Mandavia CH, Pulakat L, DeMarco V, Sowers JR (2012) Over-nutrition and metabolic cardiomyopathy. Metabolism 61(9):1205–1210
Manza LL, Codreanu SG, Stamer SL, Smith DL, Wells KS, Roberts RL, Liebler DC (2004) Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem Res Toxicol 17(12):1706–1715
Marston SB (2011) How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl Res 4(3):245–255
Masuda K, Xu Z-J, Takahashi S, Ito A, Ono M, Nomura K, Inoue M (1997) Peripheral framework of carrot cell nucleus contains a novel protein predicted to exhibit a long α-helical domain. Exp Cell Res 232(1):173–181
Mathur MC, Chase PB, Chalovich JM (2011) Several cardiomyopathy causing mutations on tropomyosin either destabilize the active state of actomyosin or alter the binding properties of tropomyosin. Biochem Biophys Res Commun 406(1):74–78
McDonald D, Carrero G, Andrin C, de Vries G, Hendzel MJ (2006) Nucleoplasmic β-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J Cell Biol 172(4):541–552
Michele DE, Albayya FP, Metzger JM (1999) Direct, convergent hypersensitivity of calcium-activated force generation produced by hypertrophic cardiomyopathy mutant α-tropomyosins in adult cardiac myocytes. Nat Med 5(12):1413–1417
Minamikawa T, Takahashi A, Fujita S (1995) Differences in features of calcium transients between the nucleus and the cytosol in cultured heart muscle cells: analyzed by confocal microscopy. Cell Calcium 17(3):167–176
Miralles F, Visa N (2006) Actin in transcription and transcription regulation. Curr Opin Cell Biol 18(3):261–266
Misteli T, Spector DL (eds) (2011) The nucleus. Cold Spring Harbor Laboratory Press, Woodbury
Nowakowski SG, Kolwicz SC, Korte FS, Luo Z, Robinson-Hamm JN, Page JL, Brozovich F, Weiss RS, Tian R, Murry CE, Regnier M (2013) Transgenic overexpression of ribonucleotide reductase improves cardiac performance. Proc Natl Acad Sci USA 110(15):6187–6192
Papadopoulos JS, Agarwala R (2007) COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics 23(9):1073–1079
Park S, Yang J-S, Shin Y-E, Park J, Jang SK, Kim S (2011) Protein localization as a principal feature of the etiology and comorbidity of genetic diseases. Mol Syst Biol 7:494
Parmacek MS, Solaro RJ (2004) Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis 47(3):159–176
Pestic-Dragovich L, Stojiljkovic L, Philimonenko AA, Nowak G, Ke Y, Settlage RE, Shabanowitz J, Hunt DF, Hozak P, de Lanerolle P (2000) A myosin I isoform in the nucleus. Science 290(5490):337–341
Phillips SK, Wiseman RW, Woledge RC, Kushmerick MJ (1993) Neither changes in phosphorus metabolite levels nor myosin isoforms can explain the weakness in aged mouse muscle. J Physiol 463:157–167
Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, Wieczorek DF (2001) A familial hypertrophic cardiomyopathy α-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol 33(10):1815–1828
Prabhakar R, Petrashevskaya N, Schwartz A, Aronow B, Boivin GP, Molkentin JD, Wieczorek DF (2003) A mouse model of familial hypertrophic cardiomyopathy caused by a α-tropomyosin mutation. Mol Cell Biochem 251(1–2):33–42
Racca AW, Beck AE, Rao VS, Flint GV, Lundy SD, Born DE, Bamshad MJ, Regnier M (2013) Contractility and kinetics of human fetal and human adult skeletal muscle. J Physiol 591(Pt 12):3049–3061
Reddy KK, Oitomen FM, Patel GP, Bag J (2005) Perinuclear localization of slow troponin C m RNA in muscle cells is controlled by a cis-element located at its 3′ untranslated region. RNA 11(3):294–307
Regnier M, Homsher E (1998) The effect of ATP analogs on post hydrolytic and force development steps in skinned skeletal muscle fibers. Biophys J 74:3059–3071
Regnier M, Martyn DA, Chase PB (1996) Calmidazolium alters Ca2+ regulation of tension redevelopment rate in skinned skeletal muscle. Biophys J 71:2786–2794
Regnier M, Lee DM, Homsher E (1998a) ATP analogs and muscle contraction: mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophys J 74:3044–3058
Regnier M, Martyn DA, Chase PB (1998b) Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys J 74:2005–2015
Regnier M, Rivera AJ, Chen Y, Chase PB (2000) 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res 86(12):1211–1217
Ren J, Gao X, Jin C, Zhu M, Wang X, Shaw A, Wen L, Yao X, Xue Y (2009) Systematic study of protein sumoylation: development of a site-specific predictor of SUMOsp 2.0. Proteomics 9(12):3409–3412
Resende RR, Andrade LM, Oliveira AG, Guimarães ES, Guatimosim S, Leite MF (2013) Nucleoplasmic calcium signaling and cell proliferation: calcium signaling in the nucleus. Cell Commun Signal 11(1):14
Sahota VK, Grau BF, Mansilla A, Ferrús A (2009) Troponin I and Tropomyosin regulate chromosomal stability and cell polarity. J Cell Sci 122(Pt 15):2623–2631
Schoffstall B, Chase PB (2008) Increased intracellular [dATP] enhances cardiac contraction in embryonic chick cardiomyocytes. J Cell Biochem 104(6):2217–2227
Schoffstall B, Brunet NM, Wang F, Williams S, Barnes AT, Miller VF, Compton LA, McFadden LA, Taylor DW, Dhanarajan R, Seavy M, Chase PB (2006a) Ca2+-sensitivity of regulated cardiac thin filament sliding does not depend on myosin isoform. J Physiol 577(Pt 3):935–944
Schoffstall B, Clark A, Chase PB (2006b) Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophys J 91(6):2216–2226
Schoffstall B, LaBarbera VA, Brunet NM, Gavino BJ, Herring L, Heshmati S, Kraft BH, Inchausti V, Meyer NL, Moonoo D, Takeda AK, Chase PB (2011) Interaction between troponin and myosin enhances contractile activity of myosin in cardiac muscle. DNA Cell Biol 30(9):653–659
Schulz EM, Wieczorek DF (2013) Tropomyosin de-phosphorylation in the heart: what are the consequences? J Muscle Res Cell Motil. doi:10.1007/s10974-013-9348-7
Shen TH, Lin H-K, Scaglioni PP, Yung TM, Pandolfi PP (2006) The mechanisms of PML-nuclear body formation. Mol Cell 24(3):331–339
Solaro RJ, Kobayashi T (2011) Protein phosphorylation and signal transduction in cardiac thin filaments. J Biol Chem 286(12):9935–9940
Spencer VA, Costes S, Inman JL, Xu R, Chen J, Hendzel MJ, Bissell MJ (2011) Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci 124(Pt 1):123–132
Strickfaden H, Cremer T, Rippe K (2012) Higher order chromatin organization and dynamics. In: Rippe K (ed) Genome organization and function in the cell nucleus. Wiley-VCH, Weinheim, pp 417–447
Tardiff JC (2011) Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res 108(6):765–782
Tobacman LS (1996) Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol 58:447–481
Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ (2003) Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation 108(4):445–451
Visa N, Percipalle P (2010) Nuclear functions of actin. Cold Spring Harb Perspect Biol 2(4):a000620
Wang J, Schwartz RJ (2010) Sumoylation and regulation of cardiac gene expression. Circ Res 107(1):19–29
Wang F, Brunet NM, Grubich JR, Bienkiewicz E, Asbury TM, Compton LA, Mihajlović G, Miller VF, Chase PB (2011) Facilitated cross-bridge interactions with thin filaments by familial hypertrophic cardiomyopathy mutations in α-tropomyosin. J Biomed Biotechnol 2011:435271
Watkins H, Ashrafian H, Redwood C (2011) Inherited cardiomyopathies. N Engl J Med 364(17):1643–1656
Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD (2010) Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol 48(5):882–892
Wu X, Bers DM (2006) Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res 99(3):283–291
Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM (2006) Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116(3):675–682
Yu C-S, Chen Y-C, Lu C-H, Hwang J-K (2006) Prediction of protein subcellular localization. Proteins 64(3):643–651
Zhang T, Birbrair A, Delbono O (2013a) Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis. Cytoskeleton 70(3):134–147
Zhang T, Birbrair A, Wang ZM, Taylor J, Messi ML, Delbono O (2013b) Troponin T nuclear localization and its role in aging skeletal muscle. Age (Dordr) 35(2):353–370
Acknowledgments
The authors are grateful to many colleagues at The Florida State University, and colleagues and collaborators from elsewhere, for thoughtful discussions. We especially thank Dr. Thomas C.S. Keller III, Dr. Campion K.P. Loong, Dr. Myriam A. Badr and Faizal Z. Asumda for insightful discussions, and Matthew S. Shachner for assistance with initial bioinformatics analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Submitted as a review for the special issue on ‘Tropomyosin: form and function,’ S. Marston and M, Gautel, eds.
Rights and permissions
About this article
Cite this article
Chase, P.B., Szczypinski, M.P. & Soto, E.P. Nuclear tropomyosin and troponin in striated muscle: new roles in a new locale?. J Muscle Res Cell Motil 34, 275–284 (2013). https://doi.org/10.1007/s10974-013-9356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-013-9356-7