Abstract
Following muscle injury, the damaged tissue and influx of inflammatory cells stimulate the secretion of growth factors and cytokines to initiate repair processes. This release of chemotactic signaling factors activates resident precursor cells and stimulates their mobilization and migration to the site of injury where terminal differentiation can occur. The three transforming growth factor-β (TGF-β) isoforms, and insulin-like growth factor-1 (IGF-1) are among the known regulatory factors released following muscle damage. We investigated the effect of recombinant active TGF-β1, -β2, -β3 and IGF-1 on C2C12 skeletal muscle satellite cell and P19 embryonal carcinoma cell terminal differentiation and migration. C2C12 myoblast fusion as well as P19 embryoid body formation and myogenic differentiation was assessed following 72 h TGF-β treatment (5 ng/ml), whereas the effect of the TGF-β isoforms on migration was determined following 7 h incubation. Our results showed that TGF-β decreases C2C12 myoblast fusion in an isoform-independent manner, whereas in the P19 cell lineage, results demonstrate that TGF-β1 specifically and significantly increased P19 embryoid body formation, but not expression of Connexin-43 or Myosin Heavy Chain. IGF-1 significantly increased migration compared to TGF-β isoforms, which, on their own, had no significant effect on the mobilization of either C2C12 or P19 cells. TGF-β isoforms decreased IGF-1-induced migration of both cell lineages. By distinguishing the factors involved in, and the molecular signals required for, myoblast recruitment during repair processes, strategies can be developed towards improved cell-mediated therapies for muscle injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The repair and regeneration of terminally differentiated adult skeletal myofibers is carried out by a small population of stem cells known as satellite cells (Jones et al. 2005; Le Grand and Rudnicki 2007). Under normal physiological conditions, satellite cells are present in a state of reversible growth arrest known as quiescence (Dhawan and Rando 2005). In response to disease or trauma, they are activated to form myoblasts, undergo proliferation, and migrate to the affected area where they subsequently differentiate into myocytes which fuse with each other, as well as with the existing myotubes (Cabane et al. 2003; Al-Shanti and Stewart 2008; Dhawan and Rando 2005). This regenerative process requires myoblast recognition, alignment, adhesion between cells, and finally fusion of the plasma membranes to re-arrange the cytoplasmic contents and form multinucleated, syncytial myotubes (Andres and Walsh 1996; Mitchell and Pavlath 2001; Nishiyama et al. 2004). However, under conditions of severe injury, an influx of inflammatory cells and interstitial fibroblasts will result in an excessively fibrotic environment which will prevent complete repair (Alexakis et al. 2007). Endogenous cardiac stem cells have also been identified, however they seem to play little role in physiological repair, as an ischemic event is normally followed by the deposition of dense scar tissue by macrophages and myofibroblasts, with little significant functional cardiomyocyte differentiation (Collins and Russell 2009; Niesler 2004; Boudoulas and Hatzopoulos 2009). Furthermore, although the transplantation of exogenous stem cells has shown promising results, consistent and improved cardiac repair has not been demonstrated in all studies, which could be as a result of the severe fibrotic conditions encountered (Qyang and Senyei 2009).
The TGF-β-superfamily of cytokines, which is up-regulated in response to injury and is known to be pro-fibrotic in many instances, plays a pivotal role in the regulation of tissue repair and regeneration (Niesler and Ferguson 2001). The isoforms TGF-β1, -β2 and -β3 inhibit differentiation of myoblasts (Lafyatis et al. 1991; Olson et al. 1986; Schabort et al. 2009), whereas they have been shown to induce both cardiac differentiation and angiogenesis of embryonic stem cells (Singla and Sun 2005). The effect of the TGF-β isoforms on muscle stem cell fusion has demonstrated inconsistent results, showing both inhibition of myoblast fusion in myogenic cell lines (Olson et al. 1986), as opposed to promotion of myoblast fusion in vivo (Filvaroff et al. 1994; Cusella-De Angelis et al. 1994; McLennan and Koishi 2002). The TGF-β isoforms have also been shown to induce multiple migratory responses depending on the cell type, concentration released, and other circulating factors (Bischoff 1997; Robertson et al. 1993). For example, in the presence of fibronectin, TGF-β, at a concentration of 1–10 ng/ml, optimally promotes the migration of satellite cells, whereas concentrations in excess of 25 ng/ml, results in a decrease in migration (Bischoff 1997).
In the current study, we utilize two cell lines, the skeletal muscle progenitor C2C12 cells, and the P19 embryonal carcinoma cells to determine the effect of the TGF-β isoforms on cellular migration as well as terminal myogenic differentiation. The murine C2C12 cell line, a diploid subclone of the C2 cell line, was originally selected for its ability to rapidly induce myogenic differentiation (Blau et al. 1985; Yaffe and Saxel 1977). The P19 cells, originally isolated from a teratocarcinoma in C3H/HC mice, can differentiate into a variety of cell types, including muscle in the presence of dimethylsulfoxide (Skerjanc 1999). Our results suggest that the TGF-β isoforms inhibit IGF-1-induced migration of both cell lines, whereas they only affect myogenesis of C2C12, but not P19 cells.
Materials and methods
Cell culture
C2C12 cells, a skeletal muscle stem cell line of murine origin (donated by the Cape Heart Centre, University of Cape Town), were maintained in growth medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM, Highveld Biological) supplemented with 10% FBS, 4% 2 mM l-glutamine and 1% PenStrep.
The P19 embryonal carcinoma cells (obtained from M.W. McBurney, University of Ottawa, Canada) were grown and maintained in culture medium consisting of Alpha MEM supplemented with 1.5 g/l NaHCO3 (Highveld Biological (Pty) Ltd, RSA), 7.5% newborn calf serum (Sigma), 2.5% fetal bovine serum (FBS, Gibco) and 1% PenStrep (Highveld Biological). Dimethyl sulfoxide (DMSO) was used to induce P19 muscle differentiation (Skerjanc 1999).
All cells were maintained in a humidified incubator at 37°C, 20% O2 and 5% CO2.
C2C12 cell fusion
To determine the effect of TGF-β isoforms on skeletal muscle cell fusion, C2C12 cells were plated in growth medium (day −1) onto glass coverslips in each well of six-well tissue culture-treated plates (Corning). On day 0, the medium was changed to mitogen-poor, differentiation-promoting medium (growth medium, but FBS replaced with 1% donor herd horse serum, Sigma) and the cells treated with TGF-β isoforms (5 ng/ml) every day for 72 h, which was compared to control conditions (cells receiving differentiation medium only). Thereafter, all cells were maintained in differentiation medium only which was changed every second day, and prepared for immunofluorescent staining on day 3, day 5 and day 7. At these time-points, coverslips were rinsed with PBS, fixed in an acetone:methanol solution (1:1), rinsed with PBS and blocked with 5% donkey serum (Jackson ImmunoResearch Laboratories, Inc.) for 20 min. Following this, immunostaining was performed using the rabbit polyclonal M-cadherin (1/50, H-71 Santa Cruz) as primary antibody and cells incubated for 4 h at 37°C. After washing the coverslips in PBS, cells were incubated for a further 45 min at room temperature with FITC-labeled donkey anti-rabbit IgG secondary antibody (1/500, Jackson ImmunoResearch Laboratories, Inc). Hoechst dye 33342 (1/200) was added during the last 10 min of this step for nuclear determination, after which the sections were again washed in PBS and mounted with Fluorescent Mounting Medium (S3023 DAKO). Sections were viewed under a fluorescence Nikon microscope (ECLIPSE E400) and photos taken with a digital camera using the 20× objective (Nikon DXM1200). To quantify cell fusion, M-cadherin images of the cells were merged with the Hoechst-stained image of the nuclei from the same area to determine bi-nuclear myoblast (two nuclei per cell) and myotube (three or more nuclei per cell) stages of differentiation. Total numbers of bi-nuclear myoblasts and myotubes were then counted. In addition, the total number of nuclei in these bi-nuclear myoblasts and myotubes were added and divided by the total number of nuclei in the same field of count to calculate the fusion index (%) (Nishiyama et al. 2004; Park and Chen 2005). A minimum of six photos were taken from different regions of each slide. The experiment was performed in triplicate.
P19 embryoid body formation and differentiation
P19 cells were differentiated according to a modified version of the method of Skerjanc (McBurney 1993; Skerjanc 1999). Briefly, differentiation was initiated by plating 500,000 cells in 60 mm bacterial-grade dishes in the presence of 0.8% DMSO (Sigma) added to P19 culture medium (day 0). After 24 h (day 1), the media with aggregates were transferred to 100 mm bacterial-grade dishes and new media containing 0.8% DMSO added. Aggregates were maintained in 100 mm bacterial-grade dishes, but the medium changed and fresh medium containing 0.8% DMSO again added on day 2 and day 3. In addition, 5 ng/ml TGF-β1, -β2 or -β3 was added to the differentiating cell cultures on days 0, 1 and 2 (72 h treatment) and compared to control conditions (P19 culture medium containing only 0.8% DMSO). On day 4, maximum supernatant was removed and the aggregates transferred to 100 mm tissue culture-treated dishes. Differentiation of the cells continued in P19 culture medium which was changed every second day.
Once P19 cells are induced to differentiate, they form aggregates which progressively increase in size. After 4 days of differentiation, once these aggregates are re-plated into tissue culture-treated dishes, they adhere to the surface and grow further to form embryoid bodies.
Efficient differentiation of P19 cells depend on the prior formation of non-adhering embryoid bodies which resemble the inner cell mass of the embryo (Smith et al. 1987). Therefore, on day 6 of differentiation following incubation with TGF-β isoforms, aggregate numbers were established by counting the number of embryoid bodies in five fields of view, and determining an average number. The experiment was performed three times. All brightfield images were taken with an Olympus microscope and camera (Olympus CKX 31) at 20× magnification.
Cells were also harvested on days 12 and 17 for western blotting purposes by washing them with PBS, after which they were treated with lysis buffer, sonicated and whole cell-lysates stored at −20°C.
To assess terminal myogenic differentiation of P19 cells, protein expression levels of Connexin-43 and myosin heavy chain (MHC) were determined by standard immunoblotting procedures. Briefly, 50 μg whole cell lysates of each sample was prepared and loaded onto 5% polyacrylamide gels for electrophoretic separation. Following electrophoresis, proteins were transferred onto PVDF membranes (Immun-Blot 0.2 μm pore size, Bio-Rad; Immobilon-P, IPVH00010 Millipore). The PVDF membranes were then blocked by incubation with TBS-T containing 5% skimmed milk powder for 60 min at room temperature, after which they were incubated with the primary antibodies (1/8000 connexin-43, C-6219 Sigma; 1/100 MHC, A4-1025 Developmental Studies Hybridoma Bank) for 2–3 days at 4°C. Following electrophoresis, α-tubulin (1/100, B-7 Santa Cruz) was used to verify equal loading of protein samples. For protein detection, membranes were incubated for 60 min at room temperature with HRP-secondary antibody (DAKO) at 1/1000 dilution in TBS-T containing 5% skimmed milk powder. Antigen–antibody complexes were visualized using ECL-Plus according to the manufacturer’s instructions (Amersham Life Science Inc., Arlington Heights, IL). Protein expression levels were quantified using Simple PCI, version 4.0 (Compix Inc., Imaging Systems, USA) for densitometry. Each sample was evaluated in duplicate and all experiments repeated a minimum of three times.
Chemotactic migration assay
To test the effect of TGF-β on cell migration, both C2C12 and P19 cells were prepared. Cultured cells were trypsinised, washed with PBS, centrifuged and the pellet re-suspended in standard medium (negative control) consisting of DMEM containing 0.1% bovine serum albumin. Following preparation, 50,000 cells were plated into 8 μm pore size Falcon cell culture inserts (Becton–Dickinson Labware; 35-3182) and placed in the tissue culture-treated 12-well cell culture companion plates (Becton–Dickinson Labware; 35-3503). IGF-1 (positive control; standard medium supplemented with 10 ng/ml IGF-1) was selected as growth factor to induce chemotactic activity to which migration results of the TGF-β isoforms could be compared. TGF-β treatment conditions included (a) positive control medium supplemented with 0.5 ng/ml TGF-β1, -β2 or -β3; (b) positive control medium supplemented with 5 ng/ml TGF-β1, -β2 or -β3; and (c) 5 ng/ml TGF-β1, -β2 or -β3 only. After adding 2 ml of the treatment solution into each well of the companion plate, inserts were carefully placed inside the wells together with 50,000 cells suspended in 500 μl standard medium and cells allowed to migrate for 7 h in a humidified incubator at 37°C, 20% O2 and 5% CO2. After incubation, inserts were taken out of the companion plate and each insert carefully placed on top of a 100 μl drop of heated trypsin (37°C) and incubated for a further 10 min to allow maximum de-attachment of cells off the underside of the insert-membrane. The number of cells was then counted using a haemocytometer. Each treatment was performed in triplicate and the whole experiment was repeated a minimum of three times for each treatment condition.
Statistical analysis
Statistical evaluations were made by one-way analysis of variance (ANOVA) and Bonferroni’s multiple comparison test using STATISTICA. Fisher’s multiple comparison test for post-hoc analysis was used to evaluate migration data. A student’s t-test was used to determine significance in P19 embryoid body numbers between groups. Significant differences were taken at p < 0.05. Data are expressed as mean ± SEM.
Results
TGF-β decreases fusion of differentiating C2C12 myoblasts
Myoblast fusion is required for the completion of terminal differentiation of skeletal muscle cells, resulting in the formation of post-mitotic, multinucleated myotubes (Andres and Walsh 1996). To determine the effect of TGF-β isoforms on skeletal myoblast fusion, we assessed the total number of bi-nuclear myoblasts plus myotubes (containing three nuclei or more) in differentiating C2C12 cells. A fusion index (%), which takes into account the total nuclear count (and can therefore be influenced by stimuli affecting proliferation as well as differentiation), was also determined. C2C12 cells were treated with TGF-β isoforms for 72 h and analyzed on days 3, 5 and 7 (Fig. 1a).
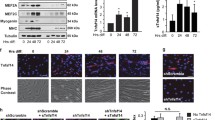
TGF-β isoforms decrease fusion of C2C12 myoblasts. a Representative images (20× magnification) of control (i) and TGF-β1-treated (ii) C2C12 cells at day 7 following 72 h incubation with TGF-β1 (arrow m-cadherin, arrowhead Hoechst-stained nuclei). b Total bi-nuclear myoblast plus myotube numbers were significantly decreased when compared to control conditions on days 3, 5 and 7 following 72 h incubation with TGF-β1, -β2 and -β3 (5 ng/ml; # p < 0.01). c The fusion index was also significantly decreased at all time-points analysed when compared to control conditions following treatment with TGF-β isoforms (# p < 0.01). Data are expressed as mean ± SEM; n = 3
As expected, an increase in fusion was observed under control conditions from day 3 to day 7 (Fig. 1b; p < 0.05). C2C12 myoblasts were seen to increase their fusion index with time and indices ranged from 2.3 ± 0.4% at day 3 to 6.6 ± 0.9% at day 7 (Fig. 1c; p < 0.05). Compared to control conditions, all TGF-β isoforms significantly reduced the total bi-nuclear myoblast plus myotube numbers following 72 h incubation at all time-points analysed (Fig. 1b; p < 0.01). The fusion index was also significantly 6-9-fold reduced following TGF-β incubation at all time-points analyzed (Fig. 1c; p < 0.01). The fusion index showed limited change or recovery over time in TGF-β-treated conditions, ranging from 0.3 ± 0.1% to 0.5 ± 0.2% at day 3, and only increasing maximally to 0.9 ± 0.2% at day 7 (Fig. 1c). No isoform-specific effects were evident, although TGF-β2 showed the greatest inhibitory effect at day 5 and day 7 (Fig. 1b, c).
TGF-β1 increases embryoid body formation in differentiating P19 cells
Quantification of embryoid body formation was carried out to assess progress towards terminal differentiation of P19 embryonal carcinoma cells. In response to 72 h incubation with TGF-β isoforms, TGF-β1 significantly increased (p < 0.05) the number of embryoid bodies formed at day 6 compared to control conditions (77% increase, Fig. 2a). Although TGF-β2 increased embryoid body formation, this was to a lesser extent (31% increase, Fig. 2a), and not significant. TGF-β3 had no effect on embryoid body formation. Interestingly, TGF-β1 not only increased the number, but also the size of embryoid bodies generated, when compared with TGF β3 and control (Fig. 2b).
TGF-β1 increases P19 embryonal carcinoma embryoid body formation. a Embryoid body number at day 6 following 72 h incubation with TGF-β isoforms (5 ng/ml). TGF-β1 significantly increased embryoid body formation in differentiating P19 embryonal carcinoma cells relative to control conditions (*p < 0.05). b Typical images (at 5× magnification) of P19 embryoid bodies at day 6 in control-, TGF-β1-, and TGF-β3-treated P19 cells
TGF-β isoforms have no significant effect on myogenic differentiation of P19 cells
To assess the effect of TGF-β isoforms on terminal differentiation (day 12 and day 17), P19 embryonal carcinoma cells were induced to differentiate and treated with the three TGF-β isoforms as before. The expression levels of myocyte-specific proteins, Connexin-43 and MHC, were used to assess terminal differentiation. No significant differences between control- and TGF-β-treated cells were found (Fig. 3a, b; n = 8; data not shown for day 17). Therefore, the increase in embryoid body formation in response to TGF-β1 did not translate into a change in Connexin-43 or MHC protein expression levels.
IGF-1, but not TGF-β, induces migration of P19 and C2C12 cells
To study the chemotactic behaviour of C2C12 and P19 cells in response to TGF-β isoforms, an in vitro migration assay was performed. Cells were exposed to both IGF-1 and TGF-β isoforms as well as a combination of these growth factors. Pilot dose finding studies indicated that, when added at a concentration between 0.1 and 10 ng/ml, TGF-β1 was not able to affect basal migration of C2C12 cells (data not shown). It was therefore not surprising when a detailed study of each TGF-β isoforms (5 ng/ml) revealed that, on their own, TGF-β’s are not able to significantly induce migration of either C2C12 or P19 cells in the absence of IGF-1 stimulation (Fig. 4a, b). IGF-1 (10 ng/ml) significantly stimulated both C2C12 and P19 cell migration, although P19 cells demonstrated limited migration when compared to the C2C12 counterparts (Fig. 4a). IGF-1-stimulated C2C12 myoblasts demonstrated an approximate 5-fold increase in cell migration number over control. Specifically, 34.4 ± 5.6% of treated cells migrated, compared to 7.1 ± 1.8% determined under control conditions (Fig. 4a; p < 0.01). IGF-1-stimulated P19 cells demonstrated an 8-fold increase in migration, however absolute levels only reached 6.4 ± 2.0% of the total amount seeded compared to 0.74 ± 0.74% under control conditions (Fig. 4b; p < 0.01).
IGF-1 induces C2C12 myoblast and P19 embryonal carcinoma cell migration. a C2C12 myoblasts and b P19 embryonal carcinoma cells were treated with either IGF-1 (10 ng/ml), TGF-β1, -β2 or -β3 (5 ng/ml). IGF-1 significantly stimulated migration of C2C12 myoblasts when compared to control- and TGF-β-treated conditions (# p < 0.01; a). IGF-1 significantly stimulated migration of P19 cells only when compared to control (# p < 0.01; b) conditions. Data are expressed as mean ± SEM; n = 9
TGF-β reduces IGF-1-induced migration of C2C12 and P19 cells
To study the response of IGF-1-stimulated migrating C2C12 and P19 cells to TGF-β isoforms, cells were exposed to a lower (0.5 ng/ml) and a higher (5 ng/ml) concentration of TGF-β isoforms. The addition of TGF-β isoforms at both concentrations reduced the number of IGF-1-induced migrating C2C12 cells, with significant effects seen in response to the lower concentration of TGF-β2 (p < 0.05) and TGF-β3 (p < 0.01; Fig. 5a). Similar to C2C12 cells, the addition of TGF-β isoforms reduced migration of IGF-1-induced P19 cells, although only the lower concentration of TGF-β1 and -β3 was significant (Fig. 5b; p < 0.05).
TGF-β decreases IGF-1-induced migration of C2C12 and P19 cells. a C2C12 myoblasts, and b P19 embryonal carcinoma cells were treated with IGF-1 (10 ng/ml) in the presence or absence of TGF-β1, -β2 and -β3 at (i) low (0.5 ng/ml) and (ii) high (5 ng/ml) concentrations. TGF-β2 (*p < 0.05) and TGF-β3 (# p < 0.01) treatment at the low dosage resulted in significantly lower C2C12 cell migration when compared to IGF-1-induced cell migration (a). For P19 cells, only TGF-β1 and -β3 treatment at the low dosage resulted in significantly lower (*p < 0.05) cell migration when compared to IGF-1-induced conditions (b). Data are expressed as a % of the IGF-1 cell migration number, mean ± SEM; n = 9
Discussion
The chemotactic response of progenitor cells during regeneration is regulated by overlapping gradients of several effector molecules produced at the site of injury, both from the damaged tissue, as well as from monocytes, macrophages and fibroblasts which are attracted to the site of injury following the inflammatory response (Robertson et al. 1993; Niesler and Ferguson 2001). Following muscle damage, the ability of progenitor cells to migrate to the area of injury is an essential process for completion of terminal differentiation and repair. The fibrotic environment which pervades the repair site following a severe injury can hamper this process (Alexakis et al. 2007; Boudoulas and Hatzopoulos 2009). By understanding the affect of the post-injury pro-fibrotic environment on progenitor cell migration and terminal differentiation, subsequent regeneration, whether by local (resident) cells or from a transplanted population, could be modulated. TGF-β exerts multiple effects on myogenesis and has shown to induce and inhibit cell migration, growth and development depending on the cell type (Bischoff 1997; Kottler et al. 2005; Schabort et al. 2009). The present study was undertaken to determine the extent of terminal differentiation of skeletal muscle progenitor cells (represented by fusion indices) and P19 embryonal carcinoma cells (represented by MHC and connexin-43 expression) in vitro following treatment with TGF-β isoforms. In addition, the in vitro chemotactic ability of migrating and non-migrating C2C12 and P19 cells towards TGF-β was assessed.
In the skeletal muscle cell line, results following treatment with TGF-β isoforms showed a significantly lower total number of bi-nucleated myoblasts and myotubes, as well as fusion index, at all time-points analyzed. No recovery was evident by day 7 suggesting that an increased presence of TGF-β isoforms during the initial stages of myoblast development has long-term negative effects on fusion and therefore terminal differentiation. Furthermore, no significant isoform-specific effects were observed. The decreased fusion could be due to the increased proliferation seen in response to TGF-β as it is known that the processes of proliferation and differentiation are mutually exclusive (Schabort et al. 2009). Alternatively, TGF-β isoforms could influence the regulatory proteins signaling progression to fusion. In this respect, Andres and Walsh (1996) have suggested that the induction of MHC expression precedes cell fusion. Results from our previous study showed that total MHC expression in C2C12 cells was significantly reduced following TGF-β treatment, suggesting a possible mechanism for the decrease in cell fusion observed (Schabort et al. 2009). Taken into consideration its positive effect on proliferation, these results suggest that, by regulating the timing of myoblast fusion and terminal commitment to myogenesis, TGF-β isoforms could ensure that adequate progenitor numbers are first generated before irreversible commitment is induced (Mejia-Luna and Avila 2004; Olson et al. 1986). TGF-β isoforms could also reduce fusion by decreasing the Ca2+-influx through T-type channels required for myotube formation. TGF-β1 has shown to down-regulate the number of these channels in the plasma membrane, resulting in a subsequent decrease in myotube formation (Avila et al. 2006).
In contrast to the C2C12 cells. TGF-β isoforms had no significant effect on terminal differentiation of P19 embryonal carcinoma cells, despite an initial increase in number and size of embryoid body formation. The increase in embryoid body formation and size may reflect the pro-proliferative nature of TGF-β, thereby resulting in more P19 cells which can aggregate in response to the differentiation cue. However, the lack of effect on differentiation is evident by the similar levels of connexin-43 and myosin heavy chain in control and treated samples. Enhanced embryoid body formation and subsequent cardiac differentiation following treatment with TGF-β2 has previously been demonstrated using embryonic stem cells (Singla and Sun 2005). In a recent study using P19CL6 cells, early induction of TGF-β1, but not of TGF-β2 or TGF-β3, expression was observed at the mRNA and protein levels during cardiomyocyte differentiation (Lim et al. 2007). Furthermore, neutralization of TGF-β1 inhibited the expression of the cardiac transcription factor Nkx2.5 in these cells (Lim et al. 2007). Although our results are in contrast to those from Singla and Sun (2005), they do agree to a certain extent with those of Lim et al. (2007). This discrepancy could be due to the use of different cell lines, or the culture agent used to induce differentiation. Both embryonic stem cells and the P19CL6 cells are known to differentiate more efficiently into cardiomyocytes than P19 cells (Habara-Ohkubo 1996). In addition, in the above mentioned study, leukaemia inhibitory factor was used to induce embryonic stem cell differentiation, whereas in our study, P19 embryonal carcinoma cells were induced to differentiate with DMSO. The increase in embryoid body formation could reflect an increase in proliferation, as previously shown in C2C12 cells (Schabort et al. 2009). The increased embryoid body formation did not, however, translate into an effect on differentiation, as Connexin-43 and MHC expression at day 12 and 17 of differentiation were not significantly different when comparing TGF-β treated cells to control conditions. This is in agreement with the study by Lim et al., where protein expression of MHC on day 12 did not differ between control and TGF-β1 treatment (Lim et al. 2007). It is possible that the cells may have had time to return to their normal differentiation response at this late time-point after the TGF-β stimulus was removed.
The P19 cells demonstrated and overall lower tendency to migrate when compared to the C2C12 cells. However, IGF-1 was able to induce a significant and similar fold-increase in migration in both cell lines. An even higher response in the cell lines may be achievable if different growth factors were used to stimulate migration. A differentiated mesodermal line, also derived from P19 cells, has previously been shown to have a high chemotactic response to PDGF supporting this premise (Liapi et al. 1990). TGF-β isoforms significantly decreased the observed IGF-1-induced migration in both cell types. Given that TGF-β has also been shown to be pro-proliferative (Schabort et al. 2009), one may expect to see an inhibitory effect on migration. Furthermore, TGF-β has been shown to inhibit IGF-1-dependent differentiation by decreasing the transcription and translation of insulin-like growth factor binding protein 5 (IGFBP-5), and thereby decreasing the bioavailability of the growth factor (Rousse et al. 2001). A similar mechanism could be mediating the decrease in IGF-1-induced migration by TGF-β observed in our study. Interestingly, the effect of TGF-β1 has been shown to differ dramatically in a dose-dependent manner, with femtomolar concentrations promoting and picomolar concentrations suppressing the humoral immune system (McKarns et al. 2003). This bifunctional effect of TGF-β was suggested to be facilitated, at least in part, by Smad3. An analysis of this signalling protein in our cells in response to low and high TGF-β doses would be of interest and could shed light on the mechanism behind the dose dependent effect seen.
Taken together, our results indicate that the TGF-β isoforms decrease IGF-1-induced migration of both C2C12 and P19 embryonal carcinoma cells, but have no effect on non-migrating cells in vitro. In this regard, the largest significant effects were seen in response to TGF-β2 and TGF-β3 in C2C12 cells, and TGF-β1 and TGF-β3 in P19 cells, potentially suggesting an isoform-specific effect. Furthermore, TGF-β decreased C2C12 myoblast fusion in an isoform-independent manner, while TGF-β1 specifically and significantly increased P19 embryoid body formation. These results underscore the importance of evaluating progenitor cell migration and fusion in the context of the cellular and cytokine environment.
References
Alexakis C, Partridge T et al (2007) Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol 293:C661–C669
Al-Shanti N, Stewart CE (2008) PD98059 enhances C2 myoblast differentiation through p38 MAPK activation: a novel role for PD98059. J Endocrinol 198:243–252
Andres V, Walsh K (1996) Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol 132:657–666
Avila T, Andrade A et al (2006) Transforming growth factor-beta1 and bone morphogenetic protein-2 downregulate CaV3.1 channel expression in mouse C2C12 myoblasts. J Cell Physiol 209:448–456
Bischoff R (1997) Chemotaxis of skeletal muscle satellite cells. Dev Dyn 208:505–515
Blau HM, Pavlath GK et al (1985) Plasticity of the differentiated state. Science 230:758–766
Boudoulas KD, Hatzopoulos AK (2009) Cardiac repair and regeneration: the Rubik’s cube of cell therapy for heart disease. Dis Model Mech 2:344–358
Cabane C, Englaro W et al (2003) Regulation of C2C12 myogenic terminal differentiation by MKK3/p38 alpha pathway. Am J Physiol Cell Physiol 284:C658–C666
Collins JM, Russell B (2009) Stem cell therapy for cardiac repair. J Cardiovasc Nurs 24:93–97
Cusella-De Angelis MG, Molinari S et al (1994) Differential response of embryonic and fetal myoblasts to TGF beta: a possible regulatory mechanism of skeletal muscle histogenesis. Development 120:925–933
Dhawan J, Rando TA (2005) Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15:666–673
Filvaroff EH, Ebner R et al (1994) Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-beta receptor. Development 120:1085–1095
Habara-Ohkubo A (1996) Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct Funct 21:101–110
Jones NC, Tyner KJ et al (2005) The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol 169:105–116
Kottler UB, Junemann AG et al (2005) Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon’s capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res 80:121–134
Lafyatis R, Lechleider R et al (1991) Secretion and transcriptional regulation of transforming growth factor-beta 3 during myogenesis. Mol Cell Biol 11:3795–3803
Le Grand F, Rudnicki M (2007) Satellite and stem cells in muscle growth and repair. Development 134:3953–3957
Liapi C, Raynaud F et al (1990) High chemotactic response to platelet-derived growth factor of a teratocarcinoma differentiated mesodermal cell line. In Vitro Cell Dev Biol 26:388–392
Lim JY, Kim WH et al (2007) Involvement of TGF-beta1 signaling in cardiomyocyte differentiation from P19CL6 cells. Mol Cells 24:431–436
McBurney MW (1993) P19 embryonal carcinoma cells. Int J Dev Biol 37:135–140
McKarns SC, Letterio JJ, Kaminski NE (2003) Concentration-dependent bifunctional effect of TGF-beta 1 on immunoglobulin production: a role for Smad3 in IgA production in vitro. Int Immunopharmacol 3:1761–1774
McLennan IS, Koishi K (2002) The transforming growth factor-betas: multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol 46:559–567
Mejia-Luna L, Avila G (2004) Ca2+ channel regulation by transforming growth factor-beta 1 and bone morphogenetic protein-2 in developing mice myotubes. J Physiol 559:41–54
Mitchell PO, Pavlath GK (2001) A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol Cell Physiol 281:C1706–C1715
Niesler CU (2004) Old dogmas and new hearts: a role for adult stem cells in cardiac repair? Cardiovasc J S Afr 15:184–189 discussion 189
Niesler CU, Ferguson MWJ (2001) TGF-beta superfamily cytokines in wound healing. In: SN B, Basel WS (eds) TGF-ß and related cytokines in inflammation. Birkhauser Verlag AG, Basel, pp 173–198
Nishiyama T, Kii I et al (2004) Inactivation of Rho/ROCK signaling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J Biol Chem 279:47311–47319
Olson EN, Sternberg E et al (1986) Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol 103:1799–1805
Park IH, Chen J (2005) Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J Biol Chem 280:32009–32017
Qyang Y, Senyei G (2009) Regeneration of a heart cell. Yale J Biol Med 82:117–119
Robertson TA, Maley MA et al (1993) The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 207:321–331
Rousse S, Lallmand F et al (2001) Transforming growth factor-b inhibition of insulin-like growth factor-binding protein-5 synthesis in skeletal muscle cells involves a c-Jun N-terminal kinase-dependent pathway. J Cell Biol 276:46961–46967
Schabort EJ, van der Merwe M et al (2009) TGF-beta’s delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp Cell Res 315:373–384
Singla DK, Sun B (2005) Transforming growth factor-beta2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem Biophys Res Commun 332:135–141
Skerjanc IS (1999) Cardiac and skeletal muscle development in P19 embryonal carcinoma cells. Trends Cardiovasc Med 9:139–143
Smith SC, Reuhl KR et al (1987) The role of aggregation in embryonal carcinoma cell differentiation. J Cell Physiol 131:74–84
Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725–727
Acknowledgments
The work was supported by the South African National Research Foundation, South African Medical Research Council, the University of Stellenbosch and the University of KwaZulu-Natal. The A4-1025 Myosin Heavy Chain antibody was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schabort, E.J., van der Merwe, M. & Niesler, C.U. TGF-β isoforms inhibit IGF-1-induced migration and regulate terminal differentiation in a cell-specific manner. J Muscle Res Cell Motil 31, 359–367 (2011). https://doi.org/10.1007/s10974-011-9241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-011-9241-1