Abstract
Owing to the serious natural ageing phenomenon of ancient wooden buildings, to better explore the effect of drying and wet ageing degree on the exothermic behaviour of wood combustion, firstly, wood treated with different degrees of dry and wet ageings was obtained by artificially accelerating the dry and wet ageing method. The pore characteristics, along with the capacity of the heat transport of wood treated and wood combustion heat flow with different dry and wet ageing degrees, were analysed by scanning electron microscopy, thermophysical property, and thermal analysis test. Finally, according to Friedman’s differential equivalent conversion method, the reaction process of the distribution of apparent activation energy of wood in various exothermic stages was computed and appraised, revealing the mechanism of the effect of the wood treated with dry and wet ageings on thermal reaction process. In the accelerated exothermic stage, the initial dry and wet ageing process (10 to 30 times) led to the opposite change in the trend of the apparent activation energy of wood and the fresh wood as a whole. The effect of the dry and wet ageings on the energy demand of the oxidation reaction at the end of prompt exothermic stage could decrease with the deepening of the ageing degree.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With economic development and social progress, the tourism business related to ancient buildings has been over developed, and the fire safety problem has become increasingly prominent. Fire is not only one of the main forms of ancient building disasters, but also the dominant factor inducing the complete destruction of ancient buildings. In recent years, thousands of such fire accidents have occurred worldwide [1], causing irreparable heavy losses. Because of the long existence of wooden ancient buildings, their wood has a serious ageing problem due to the harmful influence of natural factors. Among these factors, the water in the atmospheric environment has an intense influence on wood. The long time ageing phenomenon leads to a change of wood ignition and combustion mechanism. This can further affect the fire spread process.
To date, numerous scholars have conducted relevant research into the ageing characteristics of wood. Si et al. [2] studied the basic mechanical properties of wood fibre materials after the thermal oxidation ageing treatment was artificially accelerated. Liu et al. [3, 4] looked into the ageing behaviour of the surface of the wax by means of simulating natural ageing experiments. Irmouli et al. [5] discussed the interference of ultraviolet absorbents on the durability of oak and spruce through artificial accelerated ageing test. Boratynski et al. [6] simulated the natural climate to better study the ageing behaviour. Based upon the results, the compressive strength and density of wood decreased substantially during the ageing process. Liu et al. [7] into the ageing behaviour of wood through a variety of artificial accelerated ageing methods indicated that its ageing process was greatly affected by light and temperature. Liu et al. [8] probed the effects of sunlight and artificial light on wood ageing. Bao et al. [9] and Garcia et al. [10] explored the surface colour, luster, roughness, and wettability of wood. To that end, they accelerated the ageing conditions of the wood through ultraviolet radiation heat treatment. Matsuo et al. [11] compared the characteristics of colour of aged wood samples obtained from historical buildings with those heat-treated samples obtained from new wood at 90 to 180 ℃. Lovaglio et al. [12] delved into the hydrophobic effect and chemical alter nation of wood surface properties under the action of thermal treatment and alkyl ketene dimer before and after the artificial accelerated ageing test. Besides, Pei et al. [13] investigated the hygrothermal ageing behaviour of carbon fibre/bismaleimide (BMI) composites. Kránitz et al. [14] conducted different types of ageing experiments on wood. According to their study, we can have a better understanding of the alternations in properties after ageing. The conclusion drawn is that long-term ageing can possibly affect the burning characteristics of wood.
Bach et al. [15] compared the impact of dry and wet carbonisation on wood combustion kinetics. Sand et al. [16] experimented the pyrolysis process of dry and wet wood logs in a cylindrical heating chamber. To establish a drying simulation model, Salin et al., Cai et al. and Haberle et al. [17,18,19] studied the process of water migration and diffusion during the wood drying process. Thybring et al. [20] analysed the influence of the first drying on the wood cell. Osawa et al. [21] conducted water adsorption and drying tests on wood and evaluated the profile moisture content distribution of samples. As revealed by Kumar. [22] under the proper moisture content, the strain and moisture adsorption shrinkage coefficient of wood after the drying process can possibly reach a minimum. Bryś et al. [23] studied the water content change of pine during the holistic drying.
Gündüz et al., Bhuiyan et al., Akguel et al., Yin et al., Jalaludin et al., and Ding et al. [24,25,26,27,28,29] analysed the various effects of thermal treatment on wood. They appraised the changes of physical parameters, such as dimensional stability, crystallinity, moisture absorption and micro mechanical properties. Also, to better scrutinise the interaction process of wood and water through the combination of experiment and theory, Zhang et al., Mantanis et al., Engelund et al., Callum et al., and Miyoshi et al. [30,31,32,33,34] assessed the adsorption behaviour, equilibrium moisture content, wood type, and other main influencing factors. Tsyganova et al. [35] looked into the clustering behaviour of moisture in the process of water adsorption in wood. They found that the cluster size could increase sharply with the increase of temperature under near saturated humidity. As suggested by Upadhyay [36], the equilibrium water content of soaked wood was higher than that of ordinary wood in terms of adsorption and desorption.
To date, the research on wood combustion characteristics has also attracted the attention of numerous scholars. In the investigations of Kaiyuan et al. [37] and Xu et al. [38], for example, they described and explained the phenomenon and reason of wood burning in detail through cone calorimetry test. Boiger [39] and Wadhwani et al. [40] probed the combustion model of wood. They acquired the kinetic mechanism function of wood pyrolysis. Jin [41] used a cone calorimeter to test the combustion performance of hard pine treated with phosphorus nitrogen mixed additives. Maake et al. and Wang et al. [42, 43] experimentally investigated the ageing degree, combustion dynamics and the alter rule of combustion of ancient wood. Song et al. [44] explored the high temperature and ignition behaviour of Hemlock impregnated with carbon nanomaterials.
To sum up, the predecessors have conducted numerous researches into the changes in wood properties and their combustion characteristics, but little attention has been given to the burning process of ageing wood. The same is the case for the thermal phenomenon of ageing wood. With this in mind, the focus of this study was on the exothermic behaviour of ageing wood. First, wood treated with various alternate dry and wet ageing (DAWA) degrees was obtained by the artificial acceleration of the alternate DAWA method. The assessment of the pore characteristics and heat transport capacity of wood with various DAWA degrees was made by scanning electron microscopy (SEM) and a thermophysical property test. Furthermore, the investigation into the development of wood combustion heat flow with the degree of DAWA was made by means of thermal analysis, and the effect of heating rate on the thermal energy release of ageing wood was examined. The characteristic exothermic stages of wood treated under various DAWA degrees were determined, and the effects of dry and wet ageing degrees on the exothermic characteristics of wood were clarified. Moreover, according to Friedman’s differential equivalent conversion method, the distribution of apparent activation energy of wood in various exothermic stages involved in the reaction process was reckoned and assessed. Finally, the mechanism of the effect of alternate DAWA on wood exothermic reaction was revealed as well.
Experimental methods
The artificially accelerated experiment of alternate DAWA
The method of alternate DAWA is a physical ageing process in which polymer molecules gradually return to equilibrium state under the alternate action of adsorption and desorption of water in wood. Therefore, this study heavily depends on the aforesaid standard to better carry out the artificially accelerated ageing simulation of wood with the consideration given to the two main aspects of water adsorption and desorption. Because the problem of the use of the ancient building woods over a long time span, the evolution degree of natural environmental behaviour suffered is more intense. Therefore, based upon the similar ageing process from which the existing ancient building wood suffered, this study determined the test method for the water adsorption and desorption of wood by referring to the criteria of extreme simulated environmental behaviour. First, the wood was soaked in water for almost 10 h before, then was placed in a drying oven heated to 100 ℃ for 6 h. This is commonly defined as an alternating DAWA. In this paper, the degree of the alternating DAWA was measured by the number of artificially accelerated alternate DAWAs. Thus, the greater the number of dry wet alternate ageings, the deeper the dry wet ageing. One sample every 10 times was alternately taken as the experimental sample in this study.
The proximate analysis results are recorded in Table 1. All indicators do not show monotonicity with the deepening of DAWA. In the early stage of DAWA, the moisture content of wood varied greatly, and when the ageing activity accelerated, the moisture content of wood varied slightly. The hydrophilic groups of wood decreased with the alternating DAWA, which resulted in complete loss of free adsorbed water. On the other hand, in the process of desorption, some polysaccharides of hemicellulose underwent splitting reaction. This caused the water adsorption point of wood to decrease, and so the permeability altered.
Test by field emission-scanning electron microscopy
In the course of this research, FEI Quanta 250 scanning electron microscope was employed to record the apparent morphology of the experimental samples. When compared with tungsten filament SEM, this SEM can better avoid the damage caused by radiation to the samples. Furthermore, this microscope has higher resolution, so it is relatively applicable to the observation of nanoscale pores. In this experiment, the sample was examined and tested with 1000 times magnification.
Thermal property experiments
Our test was mainly based upon the thermal diffusion coefficient model proposed by Tanaka [45], Mendez et al. [46], and Majka et al. [47]. The thermal conductivity was tested with a laser flash device. The tableting device was used to press the sample for its use, and the thickness of the sample was 1 mm. As planned, the test temperature range was 30–210 °C and a temperature collection point posed set every 20 ℃. The atmosphere was air, the flow rate was kept constant at 100 mL min−1, and the heating rate was 1 ℃ min−1.
Differential scanning calorimetry (DSC) experiment
DSC was employed to conduct the experiment. First, atmospheric air was used in the experiment. The flow rate was maintained at 100 mL min−1. The sample dosage was 10 mg, and the temperature test range was 30–600 °C. Finally, the heating rate was 10, 20, 30, and 40 ℃ min−1, individually.
Results and discussion
Appearance features of ageing wood
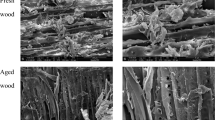
Figures 1 and 2 depict the appearance of fresh wood (FW) and wood under 10, 20, 30, 40, 50, and 60 times of DAWA. The pore boundary of both the FW and the DAWA wood was “messy”. In addition, the pore distribution was irregular, and the sizes were distinct. The pore structure was irregular, as they were all shaped honeycomb shape. The local structure of the ageing wood collapsed to varying degrees. The surface was torn to a large extent. Moreover, the thickness of the secondary wall of the cell wall became prominently thinner, and almost all of the intercellular layers were separated from the secondary wall.
Under the influence of environmental temperature changes, the wood expanded and shrank. The porous microstructure of the DAWA wood was more obvious than that of the FW. When the ageing degree of the dry and wet alternation varied greatly, water adsorption and desorption constantly occurred. Meanwhile, the mechanical absorption creep stress of wood cell walls continued to increase, thereby leading to cracks in the surface of the cell wall. In the process of water absorption, instantaneous water as well as instantaneous hydrogen bonds were yielded in the wood cell wall. This destroyed the equilibrium state of wood polymer and so generated temporary local stress in the cell wall. During desorption, some polymer molecules tended to gather close together after the adsorption hydrolysis and adsorption left the cell wall adsorption point. On the other hand, as water molecules left the cell wall, they tended to form holes in the cell wall. This not only enabled a certain amount of free volume to be generated but also allowed the structure of the cell wall layer a chance to relax. In addition, pine is a type of softwood because it cracks and deforms readily, so the mechanical adsorption creep stress can possibly have a substantial impact. Weak cracks appeared when it was ageing 20 times, and they expanded laterally when it was ageing 30 times. Moreover, the internal space increased substantially for 40 times, and the crack depth increased significantly when 60 times. This was accompanied by numerous weak cracks.
Analysis of the thermal transport characteristics of DAWA wood
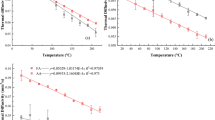
Thermal conductivity was used to characterise the effect of DAWA on the transport of wood heat. In general, the change in the trend of the thermal conductivity of DAWA wood was similar to that of the FW, because it showed a downward trend, as illustrated in Fig. 3. Clearly, there is a critical point for both the FW and the DAWA wood. This was utterly due to the continuous emissions of water vapour and volatile gases from wood tissue after the critical point is exceeded because the transfer of its energy mainly takes place in the gas phase.
As presented in Figs. 4 and 5, with the continuous increase in the cycle times of water adsorption and desorption in the wood, the cell pores mostly depended on the joint action of the elastic stress and creep stress, and the thermal transport capacity of the wood presents an “S” type change law when ageing was accelerated. At 40 times, the mechanical creep adsorption stress of wood reaches the ultimate and could be the strength under experimental conditions. The connection between the thermal conductivity of the wood and the degree of the DAWA at several temperatures was described by the sine damping function. Meanwhile, the coefficient of the fitting function is provided in Table 2.
As depicted in Fig. 5, the damping of the heat transport gradually increases with the degree of ageing. This was because the cell wall had a certain degree of toughness. On the other hand, the creep strain during the mechanical adsorption was a type of permanent strain. This type of strain existed in the wood during the alternation of dry and wet, and so it tended to become stable after reaching the maximum value with the deepening of ageing. The pore deformation under the influence of two types of stress demonstrated periodicity and damping, respectively. This is similar to mechanical vibration. The vibration system is subject to medium resistance, and which can make the amplitude gradually attenuate with time, and so a damped vibration can be observed [48, 49]. Because both the cell wall and the pore of wood were deformed by the damping stress during the alternation of DAWA, and the molecular diffusion process was directly affected by the pore shape; the heat transport process was also changed with the pore deformation. Although the wood cell wall underwent the stress limit and irreversible plastic deformation during 40 ageing cycles, its damaged cell wall slowly returned to the equilibrium state with a certain mechanical energy. Accordingly, the wood cell experienced local stress and creep effect again in the further adsorption–desorption cycle. Therefore, with the deepening of DAWA, the overall thermal conductivity illustrated an upward trend at 50 and 60 times.
Analysis of heat flow evolution characteristics
The wood heat flow curve with various DAWA degrees is exhibited in Fig. 6. This section mainly dealt with the investigate of the effect law of the evolution of wood heat flow through the two aspects of the heat flow characteristic peak as well as the continuous temperature range of different stages.
By referring to the division of wood ignition and combustion stages by predecessors, this study divided the DSC curve change in the trend of wood combustion process into four stages: dehydration, volatile precipitation, volatile combustion, and carbon oxidation [50, 51].
Effect of heating rate
During the data analysis process, it was found that there was a decreasing stage in the heat flow curve under the condition of 40 °C min−1, as shown in Fig. 7. Therefore, the derivative of the curve was obtained, and the temperature range of the wood heat flow curve under various ageing degrees that continued to decline is shown in Table 3. It can be found that the initial temperature drop point was basically after the complete evaporation of water, indicating that the reduction in heat flow was mainly caused by the process of wood pyrolysis and volatilisation analysis. The DAWA process had a significant influence on the volatilisation process of wood pyrolysis gas. In addition, the temperature point at which the heat flux of dry- and wet-aged wood begins to decrease was smaller than that of new pine wood, while the temperature point at which the heat flux of dry- and wet-aged wood decreased became relatively small, indicating that the effect of DAWA on the thermal reaction process of wood was limited and continuous at 40 °C min−1.
From the perspective of sustained temperature, the initial DAWA process narrowed the temperature range of heat flow decrease, while at 40 and 50 cycles, its temperature range increased, indicating that although the effect of DAWA was irreversible. As the degree of DAWA deepens, the range of temperature fluctuations that continued to decrease in heat flow was fixed. Based upon experimental data, it was speculated that the cycle of fluctuations was between 20 and 30 cycles, raising questions. Why were there reciprocating fluctuations? The authors believe that this is related to the pore size and thermal reaction process inside the wood. The DAWA process promoted the expansion of wood pores, enhancing energy transport. Secondly, the impact of the thermal reaction process was mainly the consumption and generation of the internal micro-structure of wood.
It can be seen that the reason for the short duration of temperature in the early stages of DAWA was due to the small loss of tissue structure inside the wood, which would not affect the progress of the thermal reaction process of wood. When the degree of DAWA was deep, the continuous drying and moisture absorption process led to the continuous consumption of active tissues that could participate in the reaction inside the wood, resulting in the absence of functional groups that could participate in the reaction during the thermal reaction process, thus increasing the continuous temperature range. As a result, there was a reciprocating phenomenon where the continuous temperature range decreased and then increased.
From the analysis of basic physical properties, the alternation of DAWA could ameliorate the change in the strength of the pore thermal conduction of the wood. Furthermore, plastic creep could occur in the cell pores at the initial stage of wood absorption–desorption cycle, and the free volume tended to increase. This could promote energy transport. The creep deformation reached the limit after the stress in the pores reached the limit, and internal cracking occurred. This further caused not only the molecular movement to be blocked, but also the heat flow transmission to be slowed. Therefore, we can see that the temperature range of the heat flow decrease during the initial dry and wet alternate ageing (10–30 times) was diminished, while the temperature range is increased again after 40 and 50 times. Second, the influence of the thermal reaction was also related to the consumption of the group structure on the wood in the adsorption–desorption cycle. It can be observed that the loss of the tissue structure in the wood at the initial stage was small, which would not affect the advancement of the wood thermal reaction. However, when the DAWA degree was deep, the continuous adsorption–desorption process could lead to the continuous consumption of active tissue. This causes a reduction in the participation of the groups in the reaction to occur, and so the continuous temperature range was increased. Thus, after the continuous temperature range decreases first, it can possibly increase, so reciprocation can appear.
Evolution of heat flow characteristic peak with the degree of DAWA
The first peak of the heat flow curve represents the volatile combustion stage, which is mastered by the thermal decomposition of hemicellulose, cellulose, and some lignins. As depicted in Fig. 8, governed by the influence of the pore thermal diffusion property, with the deepening of DAWA, the change of the peak temperature in the trend of the test wood at different heating rates was basically consistent. The maximum temperature is observed at 20 times because the wood undergoes dehydration. At 20 times, the thermal transport capacity of pine was in a low state, and the reaction process lasts for a long time. Meanwhile, the temperature also increased. The second extreme value appears at 50 times because after the creep limit of the cell wall, internal cracking occurs and the thermal conduction capacity of the wood is curtailed.
Because of the change in the pore thermal transfer characteristics caused by the alternate of DAWA, the sensitivity of the wood to the thermal environment was altered. As the heating rate increased, the peak temperature of the wood with various DAWA degrees gradually changed. Thus, this peak temperature changed from the temperature less than that of the FW to the temperature 0 more than that of the FW. At the low heating rate, the peak temperature of both DAWA wood is weaker than that of the FW, and the peak temperature is advanced. When the heating rate is too fast, the DAWA effect acted as a factor through which the occurrence of the peak temperature could be forestalled. Simply stated, the sensitivity of the DAWA effect was lessened, and the tardy phenomenon occurred. Therefore this increased the time required for burnout. Clearly, the aforementioned can be mainly due to the influence of DAWA on volatile gas of wood pyrolysis. On the one hand, when the heating rate became low, the entire thermal reaction process can last for a long time. However, ageing could enhance the strength of the thermal transport, so the reaction rate increased. Moreover, the generated pyrolysis gas was faster, and the peak heat flow was reached earlier. Meanwhile, because of the continuous loss and consumption of the chemical structure, it was easy to participate in the reaction during the dry wet alternate ageing, and the threshold value of the pyrolysis gas generated in the thermal reaction was large. But this requires more energy and cannot fully reacted in a short time. Accordingly, this causes its peak temperature to become higher than that of FW when the temperature rise rate was fast.
As can be seen from Fig. 9, under diversified heating rates, the characteristics of volatile combustion peaks are inconsistent with the deepening of DAWA. Only observed at 30 ℃ min−1, the peak value of different DAWA wood was higher than that of the FW. Because of the hysteresis of the pore thermal transfer, 10 and 20 ℃ min−1 were similar, and both increased or decreased monotonically with the deepening of ageing degree. The test result derived from 30 ℃ min−1 was consistent with that obtained from 40 ℃ min−1, and it basically fluctuated horizontally with the deepening of the DAWA. Therefore, the fluctuation amplitude of the peak strength could alter wood porosity, which would slowly weaken.
The second exothermic peak mainly resulted from the exothermic reaction of carbon oxidation. As clearly alternation in Fig. 10, under the multifarious heating rates, with the deepening of the DAWA, the change in the trend of the wood charcoal combustion peak temperature was basically consistent, but the fluctuation amplitude was quite different. Apparently, except for the heating condition of 20 ℃ min−1, the peak temperature of the wood under various DAWA degrees was lower than that of the FW under other heating rates. The peak temperature of DAWA wood is lower than that of the FW at the early stage of ageing due to the substantial reduction in the moisture content. In spite of this, with the deepening of the DAWA, the peak temperature gradually reaches that of the FW. At various heating rates, the peak value of the wood curve appears at 50 times of the DAWA. The DAWA could enhance the thermal transport capacity of the wood in the carbon oxygen thermal reaction stage, as shown in Fig. 11. Although the change in the trend of the wood at various heating rates was inconsistent, the difference between the peak value of wood and FW gradually increases with the deepening of DAWA. In addition, it was consistent with the change in the trend of peak temperature; that is, the change trend of heat flow peak at multifarious heating rates is consistent at 40–60 times.
Influence of DAWA degree on continuous temperature range in different stages
A further comparative analysis of the continuous temperature range in different reaction stages was made (Fig. 12). It should be noted here that although affected by the heating rate, the stage division under the conditions of 10 and 20 ℃ min−1 was divided according to the previous analysis. The temperature point at the end of water evaporation at 30 and 40 ℃ min−1 was selected, while the temperature point with the growth rate of heat flow of 0. Meanwhile, the selection principle of other stages was consistent with 10 ℃ min−1. Under the condition of 10 ℃ min−1, the end temperature points of the volatile matter in the separated stage of the DAWA wood were relatively close. At the same heating rate, the temperature of the end thermal energy release of the DAWA wood was lower than that of the FW. From a macroperspective, this helped not only shorten the burning time but also increased the thermal reaction rate.
It can be observed from Fig. 12 that, under the effect of water evaporation and porosity, the continuous temperature changed law of the water evaporation stage was different when the deepening of DAWA degrees changed under different heating rates. Under the same heating rate, the range of the continuous temperature of the DAWA wood during the volatile precipitation is smaller than that of the FW. At the beginning of the DAWA process, the continuous temperature of 10 and 30 ℃ min−1 decreased rather expeditiously. Specifically speaking, this basically took place for 30 times before the temperature range changes started rebound. Under different heating rates, with the deepening of the DAWA process, the overall change in the trend of the temperature duration during the volatile combustion stage of wood was consistent, and the temperature duration was the minimum at 30 times. Except for the condition of 40 ℃ min−1, at other heating rates, the continuous temperature range of volatile combustion stage reached the maximum value at the 50th time.
In the early stage of ageing, the continuous temperature range of the volatile combustion stage changes slightly. Noticeably, at the 40 ℃ min−1, the continuous temperature range of the volatile combustion stage hardly changed under the 40th–60th times of DAWA. This suggests that when the heating rate was large enough, the impact of DAWA on the continuous temperature range of this stage gradually decreased. As shown in Fig. 11, the DAWA narrowed the continuous temperature range of wood in the carbon burning stage. Undoubtedly, at 30 and 40 ℃ min−1, with the deepening of the DAWA process, the stage duration basically changed horizontally, and at 40 ℃ min−1, it started from the 10th time. While at 30 ℃ min−1, it commenced from the 30th time. With the increase of the heating rate, the fluctuation of the temperature duration of the carbon combustion stage in the dry and wet alternate ageing was gradually lessened. In general, the effect of heating rate on the evolution of DAWA wood in various heat flow stages was consistent. With the consideration given to the continuous temperature range of each stage of the reaction, the principle influence stage that caused the reaction of DAWA wood to advance was the second stage. This was mainly due to the less volatile gas released from the pyrolysis of DAWA wood in the second stage. Also, the increasing difficulty of thermal reaction resulted in the increased continuous temperature range of volatile combustion reaction in the third stage.
Analysis of DAWA degree on wood thermal energy release
The influence of the various DAWA degree on the characteristics of thermal energy release from the wood was mainly analysed from four aspects: The thermal energy release curve, the stage characteristics, the total thermal energy release, and the thermal energy release ratio of different thermal energy release stages.
Analysis of thermal energy release change with temperature
Converting the horizontal coordinate of the heat flow curve to time and integrating the curve yielded the change in the release of the thermal energy during combustion of DAWA wood, as rendered in Fig. 13. The exothermic process of both the wood with different degrees of wet and dry ageing and the FW shared similar patterns. At the initial stage of the experiment, the amount of thermal energy release was extremely small, and the amount of thermal energy increases slowly with the increased in temperature. Obviously, there was an excessive period of thermal energy release growth pattern between 330 and 360 ℃. When the temperature was less than 330 ℃, the amount of thermal energy released all increased exponentially with the increased of temperature, and the difference between the release of the thermal energy of the FW and the DAWA wood gradually became larger. When the temperature was greater than 360 ℃, the difference between the release of the thermal energy of various wet and dry ageing wood maintained a certain range. When the temperature was greater than 360 ℃, the release of the thermal energy increased linearly. In addition, the difference between the release of the thermal energy of the DAWA wood remained within a certain range.
Under the heating rate of 10 ℃ min−1, the thermal energy of the different DAWA woods cross each other as the temperature rose. This showed different growth characteristics within different temperature stages. At the temperature lower than 200 ℃, the release of the thermal energy of DAWA wood is lower than that of the FW. When the temperature transitions started from 340 to 360 ℃, the release of the thermal energy from the wood treated with the DAWA method for 60 times was gradually greater than that of the FW. Also, the wood treated with the DAWA process for 40 times showed the lowest thermal energy release. As can be seen from the previous section, the thermal diffusion capacity of the wood was gradually weakened when treated with the alternate DAWA for 50 times; the release of the thermal energy from the wood treated with the DAWA process for 50 times is always smaller than that of the other types of woods treated with the DAWA process after the change in the patterns of the release of the thermal energy. This was influenced by the significant reduction of moisture at the beginning of the alternate DAWA, and the release of the thermal energy from the word treated with the DAWA process for 20 times was always larger than that of the other types of woods treated with the DAWA process. It was therefore clear that the change in the release of the thermal energy for the wood treated with the DAWA process between 20 and 50 times could be obvious. In addition, the amount of the variation in the release of the thermal energy from the wood treated with the DAWA can be possibly determined as the threshold value for the amount of the release of the thermal energy through which the wood treated with the DAWA process could be affected. In addition, the amount of the release of the thermal energy from the FW is mainly found not only in the middle of all the multifarious degrees of DAWA but also in the middle of the range.
In a side-by-side comparison, although the release of the thermal energy at 10 ℃ min−1 was not the greatest at the low temperature stage, the release of its thermal energy was always progressively greater than all of the other heating rates when the temperature was increased. No consistent changes were found in the patterns at the other heating rates for the wood with treated different degrees of wet and dry ageing; the release of the thermal energy at 40 ℃ min−1 for the wood treated with the DAWA process between 20 and 60 times gradually became lower than that obtained at the other heating rates as the temperature increased. The release of the thermal energy at 30 ℃ min−1 for the wood treated with the DAWA process between 30 and 60 times was always progressively greater than that released at 20 ℃ min−1.
Thermal energy release stage evolution
According to the change in the trend of heat release, the release of the thermal energy curve was mainly divided into different stages by the tangent method, as in Fig. 14. The thermal energy release progression was divided into the slow exothermic stage, the accelerated exothermic stage and the rapid exothermic stage. According to the exothermic process and the comparison made with previous studies [52, 53], in this study, Ta and Tb are defined as the end temperature of dehydration and the ignition temperature.
The change in the boundary temperature dehydration and the temperature, along with the ignition temperature of wood with various degrees of DAWA, was acquired (Fig. 15). The trend of the change in dehydration and temperature at low heating rates was opposite to that of other heating rates. This was mainly because initial DAWA caused greater change in the wood moisture, and when the temperature was low, the time needed for the thermal reaction in the wood is long enough, so that the final dehydration temperature was lower. As the DAWA progressed, the changes in the wetness and swelling of the micro-cracks tended to equalise, and the end of dehydration temperature also changed less. It was worth noting that the first point of the decrease in the end-of-dehydration temperature gradually progressed to depth with the initial ageing. The curves can be successfully fitted by the sinusoidal damping functions at 20, 30, and 40 ℃ min−1, with a better fit at 30 ℃ min−1 and an average fit at 20 and 40 ℃ min−1. This indicated that the final temperature of the dehydration was also cyclical and was damped with the increased ageing due to the creeping plastic deformation in the walls of the wood cells during the cycle of suction-desorption. The final temperature of the dehydration was also cyclical and damped with the increase in the ageing activity. As shown in Fig. 16, the overall temperature for ignition increased when the strength in the DAWA activity increased. This was because the fitted curves showed a linear increase at 10 and 30 ℃ min−1 and an exponential increase at 20 and 40 ℃ min−1. The deeper the ageing, the smaller the change in the temperature of ignition at the same rate of warming. This meant that the change in ignition stabilised when the thermal transport capacity of the wood reached its maximum.
Total thermal energy release
As can be observed from Fig. 17, the overall trend of wood changed with the deepening of the degree of the DAWA at various heating rates was basically the same. This exhibited a fluctuating form. At 20, 30, and 40 ℃ min−1, the release of the total thermal energy from the wood decreases with the intensifying of the extent of the DAWA. From the comparison of the amount of the total release of the thermal energy, the total release of the thermal energy from the wood treated with the DAWA was basically smaller than that of FW. In terms of the magnitude of the curve fluctuation, the total release of the thermal energy from the wood was smaller than that of the FW. As observed from the fluctuation of the curve, the greatest fluctuation was found in the wood treated with DAWA between 10 and 20 times due to the substantial reduction of moisture at the beginning of the alternating dry and wet cycle. As the DAWA progresses, the fluctuation tended to become smaller and smaller. It was worth noting that the total release of the thermal energy from the wood neither increased nor decreased monotonically with the increase of the rate of warming. This was because during the high temperature stage all structures of the wood were subject to intense oxidation reactions and so their thermal energy production was more dictated by the internal control of the wood and less affected by changes in external conditions.
Percentage of thermal energy release in different exothermic stages
As revealed in the previous section, in this study, there were segmental boundary temperatures in the exothermic combustion of the wood treated with DAWA. To further appraise the effect of the changes in the degree of the wood treated with ageings on the different exothermic stages involved in the combustion, the amount of thermal energy released from the initial exothermic temperature to the segmental boundary temperature was reckoned for the different types of woods treated with DAWAs and the fresh types of woods, and the percentage of the total release of the thermal energy accounted for by each stage. As showcased in Table 4, there were some differences in the percentage of the release of the thermal energy at each stage for the wood treated with the DAWA and the FW at the same rate of warming. Overall, the percentage of wood involved in the slow exothermic stage was around 1–8%, within the accelerated exothermic stage around 12–18% and in the rapid exothermic stage between 73 and 84%.
Within the slow exothermic stage, the percentage of the release of the thermal energy gradually increased as the rate of warming increases. To have the 20 times excluded from this aspect, the percentage of the release of the thermal energy in the slow stage of the exothermic of wood treated with the DAWA basically decreased as the degree of ageing increases. In addition, the thermal effect of desorption during the ageing induced the shedding of volatile substances from the surface of the cell walls. In addition, the proportion of the release of the thermal energy at 10 ℃ min−1 for different types of woods treated with DAWA was less than 2%. Moreover, the percentage of DAWA was closer for 10–30 times and closer for 40–60 times, depending on the wood’s thermal transport capacity. During the accelerated exothermic stage, the percentage of the release of the thermal energy at 10 and 40 ℃ min−1 increased as the wood treated with the DAWA increased. Overall, the proportion of wood in the rapid exothermic stage decreases as the rate of warming increased. At 10 ℃ min−1, the proportion of wood in the rapid exothermic stage decreases as the degree of DAWA deepens. However, at 20 ℃ min−1 it gradually increases. At 40 ℃ min−1, the proportion of thermal energy release in DAWA wood at 10–50 times varied almost horizontally. In terms of the volatility, as the degree of DAWA deepened, the proportion at 30 ℃ min−1 fluctuated more strongly, and so the percentage of pine fluctuated above and below that of the FW.
Apparent activation energy distribution of wood thermal energy release in various DAWA degrees
Through a kinetic sense, the apparent activation energy refers to the minimum amount of energy required to convert an inactive molecule into an active molecule, and the amplitude of the apparent activation energy is nearly related to the rate of the chemical reaction and the ease of the reaction. To better understand the effect of the DAWA on the distribution of apparent activation energy in the various stages of the thermal energy release from the wood, both the accelerated stage of the exothermic of wood and the rapid stage of the exothermic of wood are determined as a complete reaction, respectively. That is, the reaction order of the exothermic stage of wood from dehydration temperature to ignition temperature was 1, and the reaction order involved in the exothermic stage from ignition temperature to the end of combustion was 1. According to Friedman differential equivalent conversion method [54, 55], the distribution of apparent activation energy of the wood treated with DAWA in the reaction to the various exothermic stages is calculated. Figures 18 and 19 represent the linear regression diagram of pine wood treated with different degrees of DAWA under different conversion degrees at accelerated exothermic stage and the rapid exothermic stage, correspondings. The two exothermic stages were analysed as follows.
Accelerated exothermic stage
As illustrated in Fig. 20, the energy needed for the apparent activation of the wood treated, respectively, with the DAWA for 10, 20, 30, and 60 times, and the FW in the accelerated exothermic stage showed an overall opposite trend. The apparent activation of energy of the wood treated with the DAWA increased gradually with the advance of reaction, while that of FW decreased gradually. Because of the DAWA process, the thermal transport capacity of the wood was ameliorated, and so the energy required for reaction is reduced. With the increase of the conversion degrees, the volatile matter gradually burned completely, and the energy demand increased. However, FW required an immense amount of energy during the initial volatile combustion in the accelerated exothermic stage. With the progress of the reaction, wood pores were deformed due to the factors of the temperature and gas volatilisation.
The reaction conditions were strengthened, and the energy required for the reaction is gradually reduced, which was shown as the apparent activation energy was gradually ameliorated. The change in the trend of the apparent activation energy of the wood treated with the DAWA for 50 times was consistent with that of the FW. However, we found that the apparent activation energy of the FW in the accelerated exothermic stage was 73.23–139.21 kJ mol−1, while that of the wood treated with the DAWA wood for 50 times was 38.78–51.88 kJ mol−1, which belonged to the wood with the lowest apparent activation energy distribution among the wood with various DAWA degrees. Comparatively, the apparent activation energy of the wood treated with the DAWA for 50 times was less than twice that of the FW. It was worth noting that with the rise of conversion degree, the apparent activation energy distribution of the wood treated with the DAWA for 40 times first increased and then decreased. This finally reached the maximum at the conversion degree of 0.4.
The authors believed that the continuous process of the DAWA could have a comprehensive impact on the macro- and micro-aspects of wood. From the above analysis, we could know that, from the macrostructure to the micro pore development, no matter how many cycles of DAWAs, the essential properties of wood composition would damp this change, and then this performance would occur for the wood treated with ageings for 40 times, which corresponded to the evolution of the characteristics of the heat transfer for the wood treated with the DAWA in the previous experiment process. Moreover, clearly, except for the wood treated with the DAWA for 40 times, the apparent activation energy distribution of other ageing wood was smaller than that of the FW. On the other hand, although the energy required for the reaction increased when the conversion degree increased, the apparent activation energy distribution of wood with various DAWA degrees at the initial reaction (α = 0.1–0.2) was smaller than that of the FW. According to the calculation, with the deepening of the DAWA degree, they were curtailed by 77%, 69%, 75%, 36%, 62%, and 59%. The DAWA process had doubled the difficulty of the thermal reaction to the wood, and so this allowed the minimum demand of the energy for the exothermic reaction. The effect of initial DAWA (0–20 times) was more obvious. At the end of reaction (α = 0.9), the average apparent activation energy of the wood treated with the DAWA was 87.86 kJ mol−1, which is greater than that of the FW. Since the accelerated exothermic stage corresponded to the volatile combustion stage, the volatile combustion is chiefly a gas stage reaction, and the thermal reaction was more vigorous in the initial reaction. Therefore, the apparent activation energy at the conversion degree of 0.1–0.3 in the accelerated exothermic stage was altered greatly.
Rapid exothermic stage
As exhibited in Fig. 21, regarding the rapid exothermic stage, the apparent activation energy of different types of woods treated with DAWAs had the same overall change trend with the deepening of the ageing degree, and both of them continue to increase with the advancement of the reaction. As can be perceived from Fig. 21, in the early reaction stage of the rapid exothermic stage, the apparent distribution of the apparent activation energy of the wood treated with the DAWA demonstrated a small difference with that of the FW, indicating that the energy initially required for the wood to enter the stage of the carbon oxygen exothermic reaction would not change considerably with the deepening of the DAWA. It was believed that the modification of thermal transfer characteristics of cell wall pores caused by the DAWA would not be the main cause, contradictory to the chemical reaction, but this was ascribed to the immense amount of the release of gas and thermal energy from the wood in the late burning period. At the end of the rapid exothermic stage, the distribution of apparent activation energy of wood with different degrees of DAWAs is greater than that of the FW. This was mainly because the continuous DAWAs caused the surface of the wood to harden, the relative density to increase, and the macromolecules to fall off. However, this causes difficulty for the oxidation to increase. The apparent activation energy of 10, 30, 40, 50, and 60 times was 6.11, 3.56, 1.74, 2.51, and 2.30 times of that of FW, respectively. With the deepening of ageing, the effect of the DAWA on the energy demand at the end of the rapid exothermic stage was gradually lessened.
The distribution of the apparent activation energy of the wood treated with the DAWAs for 20 times decreased first and then increased with the advancement of the reaction, but the overall apparent activation energy was the closest to that of the FW. The apparent activation energy of the wood treated with the DAWA for 40 times began to increase promptly when the conversion degree was low, but it increased slowly at the end of the stage. Therefore, it is worth mentioning that at the end of the rapid exothermic stage (α = 0.8–0.9), the apparent activation energy distribution of FW could had a decreasing trend, while that of the wood treated with the DAWA was not the case. It was mainly related to the difficulty of carbon black participating in the reaction of the oxidation in the late burning stage of wood. The ageing of wood was affected by the early rapid exothermic stage because its energy demand increased with the progress of the reaction in the rapid exothermic stage.
Average apparent activation energy analysis
The average apparent activation energy of different exothermic stages was estimated to change with the degree of the DAWA (Fig. 22). It was found that the effect of DAWA on the reaction to the energy requirement of wood at different exothermic stages is characterised by the degree of the DAWA. In the stage of the exothermic acceleration, the average apparent activation energy fluctuated with the deepening of DAWA. The apparent activation energy from the wood treated with the DAWA for 40 times was the largest. According to the basic physical properties mentioned above, it was believed that the stage of the exothermic acceleration can be dominated by volatile precipitation and combustion, while the gas release and diffusion can be directly affected by the changes of wood cell wall pores. Besides, as revealed earlier in this study, the thermal diffusivity of wood reached the extreme value when the wood was treated with the DAWAs for 40 times; that is, the volatiles emitted and diffused the most, resulting in an increased energy demand for combustion reaction.
Within the rapid exothermic stage, the average apparent activation energy gradually decreased with the deepening of the DAWA. The average apparent activation energy of wood was the largest when treated with ageings for 10 times alternately. This was mainly related to the difficulty of oxidation reaction of wood macromolecular surface groups. When compared with the fluctuation amplitude of the curve, the interference of the DAWA on the average apparent activation energy in the rapid exothermic stage was smaller than that in the accelerated exothermic stage. According to the change in the curve trend, the authors presumably associated that with the deepening of the DAWA, and the difference between the average apparent activation energy of the rapid exothermic stage and that of FW would tend to a smaller fluctuation range. Thus, after the wood was treated with the DAWA process more than 40 times, the overall DAWA no longer interfered with the demand for the reaction to the carbon and oxygen energy in the rapid exothermic stage of the wood.
Conclusions
In this study, four conclusions were drawn:
-
As revealed by both the SEM and the thermal property experiments, the walls of the wood cells were influenced by the elastic stress and mechanical adsorption creep stress in the process of the suction and desorption cycle, so the external pore deformation process of wood was damping and periodic. This led to the thermal transport characteristics of wood and showed that the sinusoidal damping characteristics changed with the degree of ageing.
-
Because of the evolution of the heat transfer characteristics, the thermal transport process of the wood treated with the ageing method changed slowly to varying degrees, resulting in a low heating rate. The peak temperature of the wood treated with the DAWA process was less than that of the FW. This helped shorten the time required to reach the peak temperature, and strengthened the burning intensity. When the heating rate was too fast, the dry and wet alternate ageing played a vital factor hindering the occurrence of peak temperature.
-
In the stage of the acceleration of the exothermic process, with the influence of the evolution of the wood cell wall pore on the thermal transport characteristics, the initial process of the DAWA (10–30 times) led to the opposite trend of the apparent activation energy of the wood and the FW as a whole, and the apparent activation energy gradually increased with the promotion of the reaction. With the deepening of ageing, the influence of the DAWA on the energy demand of oxidation reaction at the end of the rapid exothermic stage gradually decreased.
-
The average apparent activation energy analysis showed that the influence of the DAWA on the reaction to energy demand of the exothermic behaviour in various stages of the wood was periodic. The initial stage of the DAWA showed a great impact on the acceleration of the exothermic stage, while the middle of the DAWA had a great impact on the rapid exothermic stage.
References
Hua YW, Chun Q. Influence of Pu-zuo on progressive collapse behaviour of ancient southern chinese timber buildings built in the song and yuan dynasties: experimental research. Eng Fail Analy. 2022;137:106405.
Si S, Tang Q, Li X. The accelerated thermo-oxidative ageing characteristics of wood fiber/polycaprolactone composite: effect of temperature, humidity and time. J Renew Mater. 2021;9(12):2209.
Liu XY, Timar MC, Varodi AM. A comparative study on the artificial UV and natural ageing of beeswax and Chinese wax and influence of wax finishing on the ageing of Chinese ash (Fraxinus mandshurica) wood surfaces. J Photoch Photobio B. 2019;201:111607.
Liu XY, Timar MC, Varodi AM. Effects of ageing on the color and surface chemistry of paulownia wood (P. elongata) from fast growing crop. Bio Resour. 2016;11(4):9400–20.
Irmouli Y, George B, Merlin A. Artificial ageing of wood finishes monitored by IR analysis and color measurements. J Appl Polym Sci. 2012;124(3):1938–46.
Boratynski E, Jankowska A, Szczesna M. Influence of the accelerated ageing red oak wood on compressive strength along the fibers. Ann Warsaw Univ Life Sci-SGGW Warsaw. 2010;71:47–50.
Liu XY, Timar MC, Varodi AM. An investigation of accelerated temperature-induced ageing of four wood species: colour and FTIR. Wood Sci Technol. 2017;51(2):357–78.
Liu R, Zhu H, Li K. Comparison on the ageing of woods exposed to natural sunlight and artificial xenon light. Polymers. 2019;11(4):709.
Bao M, Rao F, He S. A note on the surface deterioration of scrimber composites exposed to artificial ageing. Coatings. 2019;9(12):846.
Garcia RA, Oliveira LJ, Nascimento AM. Color stability of weathered heat-treated teak wood. Maderas-Cienc Tecnol. 2014;16(4):453–62.
Matsuo M, Yokoyama M, Umemura K. Ageing of wood: analysis of color changes during natural ageing and heat treatment. Holzforschung. 2011;65(3):361–8.
Lovaglio T, Gindl-Altmutter W, Meints T. Wetting behaviour of alder (Alnus cordata (Loisel) duby) wood surface: effect of thermo-treatment and alkyl ketene dimer (AKD). Forests. 2019;10(9):770.
Pei S, Zhao Y, Luo YF. Effect of temperature and cyclic hygrothermal ageing on the interlaminar shear strength of carbon fiber/bismaleimide (bmi) composite. Mater Design. 2011;32:4341–7.
Kránitz K, Sonderegger W, Bues CT. Effects of ageing on wood: a literature review. Wood Sci Technol. 2016;50:7–22.
Bach QV, Tran KQ, Skreiberg O. Comparative study on the thermal degradation of dry and wet-torrefied woods. Appl Energy. 2017;185:1051–8.
Sand U, Sandberg J, Larfeldt J. Numerical prediction of the transport and pyrolysis in the interior and surrounding of dry and wet wood log. Appl Energy. 2008;85(12):1208–24.
Salin JG. Fibre level modelling of free water behaviour during wood drying and wettin. Maderas-Cienc Tecnol. 2011;13(2):153–62.
Cai L, Oliveira L. A simulation of wet pocket lumber drying. Dry Technol. 2008;26(5):525–9.
Haberle I, Haugen NEL, Skreiberg O. Drying of thermally thick wood particles: a study of the numerical efficiency, accuracy, and stability of common drying models. Energy Fuel. 2017;31(12):13743–60.
Thybring EE, Thygesen LG, Burgert I. Hydroxyl accessibility in wood cell walls as affected by drying and re-wetting procedures. Cellulose. 2017;24(6):2375–84.
Osawa T, Maeda K, Tsunetsugu Y. Influence of surface checks on wood moisture content during wetting and re-drying. Eur J Wood Prod. 2019;77(4):681–9.
Kumar M, Shakher C. Experimental characterisation of the hygroscopic properties of wood during convective drying using digital holographic interferometry. Appl Opt. 2016;55(5):960–8.
Bryś A, Bryś J, Ostrowska-Ligęza E. Wood biomass characterisation by DSC or FT-IR spectroscopy. J Therm Anal Calorim. 2016;126(1):27–35.
Gündüz G, Niemz P, Aydemir D. Changes in specific gravity and equilibrium moisture content in heat-treated fir wood. Dry Technol. 2008;26:1135–9.
Bhuiyan M, Hirai N, Sobue N. Changes of crystallinity in wood cellulose by heat treatment under dried and moist conditions. J Wood Sci. 2000;46(6):431–6.
Akguel M, Guemueskaya E, Korkut S. Crystalline structure of heat-treated Scots pine [Pinus sylvestris L.] and Uludag fir [Abies nordmanniana (Stev.) subsp. bornmuelleriana (Mattf.)] wood. Wood Sci Technol. 2007;41(3):281–9.
Yin Y, Berglund L, SalméN L. Effect of steam treatment on the properties of wood cell walls. Macromolecules. 2011;12(1):194–202.
Jalaludin Z, Hill CAS, Xie Y. Analysis of the water vapour sorption isotherms of thermally modified acacia and sesendok. Wood Mater Sci Eng. 2010;5(3/4):194–203.
Ding T, Li GT. Influence of steam pressure on physical and mechanical properties of heat-treated mongolian pine lumber. Eur J Wood Prod. 2011;69(1):121–6.
Zhang XX, Wang HK. Investigating the water vapor sorption behaviour of bamboo with two sorption models. J Mater Sci. 2018;53(11):8241–9.
Mantanis GI, Papadopoulos AN. The sorption of water vapour of wood treated with a nanotechnology compound. Wood Sci Technol. 2010;44(3):515–22.
Engelund ET, Thygesen LG, Svensson S. A critical discussion of the physics of wood-water interactions. Wood Sci Technol. 2013;47(1):141–61.
Callum AS, Norton A, Newman G. The water vapor sorption behaviour of natural fibers. J Appl Polym Sci. 2009;112(3):1524–37.
Miyoshi Y, Sakae A, Arimura N, Kojiro K, Furuta KY. Temperature dependences of the dynamic viscoelastic properties of wood and acetylated wood swollen by water or organic liquids. J Wood Sci. 2018;64:157–63.
Tsyganova S, Mazurova E, Bondarenko G, Chesnokov N. Influence of prolonged exposure of wood to water on wood structure and biochar properties. Wood Sci Technol. 2016;50:963–72.
Upadhyay PC. Effect of hygrothermal environment on the bending of PMC laminates under large deflection. J Reinf Plast Comp. 2000;19(6):465–91.
Kaiyuan L, Dennis SW, Wang JH, Ji J. Modelling pyrolysis of charring materials: determining flame heat flux using bench-scale experiments of medium density fibreboard (MDF). Chem Eng Sci. 2015;123:12339–48.
Xu QF, Chen LZ, Harries KA, Zhang FW, Liu Q, Feng JH. Combustion and charring properties of five common constructional wood species from cone calorimeter tests. Constr Build Mater. 2015;96:416–27.
Boiger G. A thermo fluid dynamic model of wood particle gasification and combustion processes. Int J Multiphys. 2014;8(2):203–30.
Wadhwani R, Sutherland D, Moinuddin KAM, Joseph P. Kinetics of pyrolysis of litter materials from pine and eucalyptus forests. J Therm Anal Calorim. 2017;130(3):2035–46.
Jin E, Chung YG. Combustion characteristics of Pinus rigida specimens treated with mixed phosphorus–nitrogen additives. J Ind Eng Chem. 2016;36:74–9.
Maake T, Asante J, Mwakikunga B. Fire performance properties of commonly used South African hardwood. J Fire Sci. 2020;38(5):415–32.
Wang HY, Tian Y, Zhang L. Experimental study of the characteristic parameters of the combustion of the wood of ancient buildings. J Fire Sci. 2019;37(2):117–36.
Song KL, Ganguly I, Eastin I, Dichiara A. High temperature and fire behaviour of hydrothermally modified wood impregnated with carbon nanomaterials. J Hazard Mater. 2020;384:12831–9.
Tanaka T. Simple geometrical model of thermal conductivity and bound-water diffusion coefficient in resin-rich regions of softwood plywood. Wood Sci Technol. 2018;52:331–42.
Mendez MAA, Martinez ESM, Morales JE, Orea AC, Fonseca MRJ. Photo thermal techniques applied to the determination of the water vapor diffusion coefficient and thermal diffusivity of edible films. Anal Sci. 2007;23:457–61.
Majka J, Rogoziński T, Olek W. Sorption and diffusion properties of untreated and thermally modified beech wood dust. Wood Sci Technol. 2022;56:7–23.
Chen L, Liu ZH, Nagarajaiah S, Sun LM, Zhao L, Cui W. Vibration mitigation of long-span bridges with damped outriggers. Eng Struct. 2022;271:114873.
Gallerand L, Legrand M, Dupont T, Leclaire P. Vibration and damping analysis of a thin finite-size microperforated plate. J Sound Vib. 2022;541:117295.
Kuznetsov GV, Syrodoy SV, Kostoreva ZhA, Malyshev DV, Gutareva NY. The effect of the distance between wood and coal particles on the characteristics of their joint ignition under conditions of high-temperature radiation-convective heating. J Energy Inst. 2021;97:13–26.
Manzello SM, Suzuki S. Influence of board spacing on mitigating wood decking assembly ignition. Fire Saf J. 2019;110:102913.
Xu T, Lei P. Experimental study on flame height and heat release rate estimation of diesel-wetted wood powder fire. Case Stud Therm Eng. 2022;33:101906.
Yang Y, Fu T, Song F, Song X, Wang XL, Wang YZ. Wood-burning processes in variable oxygen atmospheres: thermolysis, fire, and smoke release behaviour. Polym Degrad Stabil. 2022;205:110158.
Gözke G, Açıkalın K. Pyrolysis characteristics and kinetics of sour cherry stalk and flesh via thermogravimetric analysis using isoconversional methods. J Therm Anal Calorim. 2020;146:893–910.
Muigai HH, Choudhury BJ, Kalita P, Moholkar VS. Physico–chemical characterisation and pyrolysis kinetics of Eichhornia Crassipes, Thevetia Peruviana, and Saccharum Officinarum. Fuel. 2021;289:119949.
Acknowledgements
This study was sponsored by the Young Elite Scientists Sponsorship Program of China Association for Science and Technology (Grant no. 2021QNRC001).
Author information
Authors and Affiliations
Contributions
JS contributed to the conceptualisation, data curation, writing—original draft. JZ was involved in the validation, investigation, and funding acquisition. JD assisted in the supervision and funding acquisition. SL performed the formal analysis. GH contributed to writing—review and editing. HM was involved in the methodology, resources, and writing–review and editing. C-MS contributed to writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Jj., Zhao, Jy., Deng, J. et al. Study on the evolution of thermal behaviour of dry and wet ageing wood with ageing degrees. J Therm Anal Calorim 149, 2217–2238 (2024). https://doi.org/10.1007/s10973-023-12828-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12828-4