Abstract
Curing kinetic model is a crucial basis for the processing analysis and simulation of resin matrix composites. The existing dynamic and isothermal models have systematic errors for an actual curing cycle with heating and holding stages. In this study, a piecewise model was proposed to be consistent with curing cycles. First, the dynamic and isothermal models of an epoxy/amine system were developed by analyzing differential scanning calorimetry (DSC) data. Then they were applied to heating and holding stages, respectively, to establish the piecewise model, which was verified by carrying out cyclic DSC tests and comparing the accuracies of models. The prediction error of the proposed model could be lowered by over threefold relative to the existing models. Moreover, the proposed model had a favorable universality compared to the cyclic DSC test. These advantages could be conducive to the numerical simulation and parameter optimization of curing process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background of curing kinetic
The curing stage is important in the manufacturing of thermosetting resin matrix composite parts, which has a significant impact on the mechanical properties and structural integrity of the parts [1].
Curing is an exothermic reaction, and the thermal conductivity of the resin matrix is very poor. There is a coupling relationship between temperature and curing degree in the curing cycle, which will form an obvious temperature and curing degree gradient inside the part [2, 3]. Among them, the increase of peak temperature reduces the interlaminar shear strength of the parts [4]. The curing process involves the three-state transformation of the resin matrix, and its physicochemical properties are changed by temperature and curing degree [5, 6]. The complex stress generated in the parts causes potential damage to the structure and performance [7, 8]. Therefore, accurate expression of temperature and curing degree is of great significance for curing research and numerical prediction [9, 10].
By analyzing the curing dynamics of composites, a curing dynamics model can be established, which plays an important part in composite process simulation, and can quantify the curing rate, curing degree and curing heat release. It can be used for numerical simulation of curing process and optimization of curing cycle [11, 12], reducing temperature and curing degree gradient, which is very essential for obtaining parts with the least defects [13, 14]. Differential scanning calorimetry (DSC) is one of the most effective methods to analyze the curing reaction of resin system [15]. At present, DSC tests are usually carried out according to three temperature procedures: dynamic heating, isothermal holding and manufacturer's recommended curing cycle (MRCC).
Traditional methodologies and models
Dynamic DSC test: The dynamic curing kinetic of the resin system is analyzed by dynamic DSC. This method is simple and effective. Moreover, the dynamic model is in good agreement with the DSC results [16,17,18,19]. Hesabi et al. [20] analyzed the curing behavior of epoxy/dicyandiamide system, indicating that autocatalytic model can well simulate the experimental results.
Isothermal DSC test: The isothermal curing kinetic of the resin systems is analyzed by isothermal DSC. This method is not unified, and there are some problems such as the selection and realization of isothermal temperature. Xu et al. [21] used of autocatalytic model with diffusion factor has greatly improved the model prediction. Tezel et al. [22] used autocatalytic model to describe the isothermal curing process in a certain temperature range. Liu et al. [23] found that only the curing reaction at a certain temperature can be described by the autocatalytic model.
Dynamic and isothermal DSC tests: The curing kinetic of resin systems is analyzed by dynamic and isothermal DSC. For the same system, due to the difference of temperature program, the dynamic and isothermal kinetic models can only be well predicted under the corresponding temperature program [24,25,26]. Kim et al. [27] found that the linear baseline and correction baseline used in the dynamic DSC heat flow curve had little effect on the study of curing kinetic. The isothermal model accurately predicted the isothermal curing process and the total heat of dynamic scanning, but the time consistency with the dynamic experimental data was poor. Hayaty et al. [28, 29] developed the dynamic and isothermal autocatalytic models of epoxy/dicyandiamide system. However, the parameters of the two models were different. D'Elia et al. [30] considered the initial curing degree before isothermal curing when developing the isothermal model. And the model and experimental data fitted well in the range of low curing degree and high conversion. However, there was a difference when the curing degree is greater than 0.9. Newcomb [31] found that the coefficient of variation of dynamic reaction heat at the heating rate of 1–5 °C min−1 was less than 2%, and faster heating rate would lead to incomplete curing. The isothermal model with diffusion factor fitted well with the isothermal DSC data, but the model parameters were quite different from the dynamic model parameters. Xu et al. [32] found that after the glass transition of isothermal curing, the autocatalytic model would deviate significantly from the experimental data, which must be corrected by using the diffusion factor. It is also found that the total reaction order and activation energy of the dynamic and isothermal models were very similar. Tziamtzi and Chrissafis [33] accurately predicted the isothermal curing process of a bio-epoxy resin within a certain temperature range based on dynamic DSC data alone and autocatalytic model with diffusion factor, preventing the time-consuming and inaccurate isothermal experiment.
Cyclic DSC test: The actual curing cycle includes dynamic heating and isothermal holding stages, and the prediction effect using only dynamic or isothermal models is poor [34]. Therefore, the development and verification of the actual curing kinetic model need to be combined with the actual curing cycle. Faria et al. [35] developed a method to investigate curing kinetic by using cyclic DSC test under the MRCC temperature program. Mphahele et al. [36] determined the curing degree at a specific time point under MRCC conditions by measuring the residual heat of the resin system. There are few studies on the development of curing kinetic model by cyclic DSC test, which is related to its complexity and is not conducive to parametric modeling. Therefore, the methodology in previous literature cannot provide an effective approach to obtain the consistent model for various curing cycles conveniently. Moreover, the investigation method by using cyclic DSC test has to be constrained to the specific curing cycle without a universality to be consistent with various cycle parameters. Nevertheless, the method of cyclic DSC test could be employed to measure the systematic error of a concerned model of curing kinetic.
Motivation of a new model
In the actual curing process, the heating rate and holding temperature of each part of the composite parts are different. For larger and thicker parts, this difference will be more significant under the influence of mold thermal hysteresis and peak temperature. Therefore, the curing kinetic model needs to be related to the heating rate and holding temperature in the curing cycle. However, the single curing kinetic models lack this correlation with the actual curing cycle, and lack of error quantification and verification in predicting the actual curing process.
In this study, a piecewise model is proposed, which applies the dynamic and isothermal models to the heating and holding stages of the actual curing cycle, respectively. The model parameters were linear with the heating rate and holding temperature of the actual curing cycle. And the validity of the piecewise model is verified by cyclic DSC test. The advantages and accuracy of the piecewise model were verified by comparing the prediction errors of the dynamic, the isothermal and the piecewise models.
Theoretical and experimental methods
Reference models
The curing degree α is defined as the heat H(t) generated at time t divided by the total heat HT in the whole reaction process [15]:
where HT is the area under the DSC time–heat flow curve. Derive the reaction rate with respect to time:
It is generally considered that the reaction rate is a function of temperature and conversion, the basic kinetic equation:
where K(T) is the temperature-dependent rate constant, which can be described by the Arrhenius equation:
where A represents the pre-exponential factor, E the apparent activation energy, R the universal gas constant and T the absolute temperature.
By substituting the Arrhenius temperature coefficient, Eq. (3) can be written as Eq. (5), which represents the general rate equation for the kinetic study:
where f(α) is the reaction model depending on the reaction mechanism. The analysis of curing kinetic shows that the epoxy/amine system is controlled by autocatalytic reaction [37]. In this study, the following empirical formula is adopted:
where n and m are the reaction orders relating to the effects of unreacted reactants and catalytic effect of the product of the reaction, respectively.
For the isothermal curing kinetic model, the autocatalytic model with additional factor f1(α) can be used to simulate the isothermal curing kinetic of epoxy system [31]:
and
where C and αc are two temperature-dependent empirical constants.
Taking the logarithm of Eq. (4) to obtain Eq. (9) [13]:
The activation energy and pre-exponential factor of the isothermal model can be solved according to Eq. (9).
Kinetic parameters cannot be determined from a single DSC curve unless at least one kinetic parameter is known. For dynamic curing kinetics model parameters, when the activation energy is determined by Kissinger and Ozawa method [38], as follows Eqs. (10) and (11), respectively, the other parameters can be obtained as:
and
where Tp is the peak temperature of the DSC heat flow curve; C is an empirical constant, which depends on the pre-exponential factor, the reaction model f(α) and the activation energy.
Sample preparation and measurement
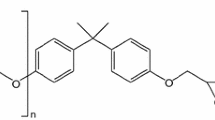
The epoxy/amine prepreg of 3233/CF3011 was produced by Beijing Institute of aeronautical materials, where 3233 represents a medium temperature curing resin system, which was similar to hexply1454 prepreg [28]. The epoxy resin is bisphenol A diglycidyl ether (DGEBA), with an equivalent mass of 175–180 g equiv-1 and chemical formula: The curing agent is latent dicyandiamide (C2H4N4). CF3011 refers to a carbon fiber cloth. The MRCC of the prepreg consists of a heating stage from room temperature to 125 °C at a heating rate of 2–4 °C min−1 and a holding stage for 90–120 min.
Thermogravimetric analysis (TGA) was performed using Mettler Toledo TGA2 at a heating rate of 10 °C min−1 under nitrogen and air atmospheres from 25 to 80 °C to analyze the thermal stability of the epoxy/amine prepreg.
The curing kinetic of the prepreg was analyzed by DSC using Mettler Toledo DSC3. The sample mass was between 14 and 18 mg, and all tests set up a holding stage for 3 min to stabilize the sample temperature with a constant nitrogen flow of 50 mL min−1. Since the difference between the linear baseline and the corrected baseline is very small and the corresponding reaction heat is very close, the linear baseline is used in the DSC heat flow curve analysis.
Three types of DSC experiments were carried out as follows.
Dynamic DSC test. The temperature range of − 30 to 260 °C at different heating rates: 2, 3, 4, 5, 10, 15, and 20 °C min−1.
Isothermal DSC testing. According to the implementation methods of isothermal temperature, isothermal DSC testing can be divided into three types: (1) Rapidly heating up to the isothermal temperature at a heating rate of 50 °C min−1; (2) Raise the temperature to isothermal temperature at a heating rate of 20 °C min−1; (3) Isothermal DSC testing is achieved by preheating to an isothermal temperature [39]. Using the second method, the total heat consists of the exothermic heat in the heating stage, the transition stage, the isothermal stage and the residual heat. After the holding stage, it is rapidly cooled to room temperature at the rate of − 20 °C min−1, and then dynamically heated to 260 °C at the rate of 10 °C min−1 for the determination of residual heat and glass transition temperature Tg. According to the principle of sub-Tg curing, four temperatures of 120, 125, 130 and 135 °C were selected as the isothermal test range [40,41,42].
Cyclic DSC test: The samples were firstly heated at 3 °C min−1 to holding temperature (120, 125, 130 and 135 °C), then hold for 90 min. Finally, they were dynamically heated to 260 °C at the rate of 10 °C min−1 for the determination of residual heat.
Measurement and calculation results
Thermogravimetric analysis
TG and derivative thermogravimetric (DTG) curves are shown in Fig. 1, and the TGA data are shown in Table 1. In N2 atmosphere, the initial decomposition temperature of the prepreg was 315 °C, indicating that it has excellent thermal stability [43]. Since the mass loss of the prepreg was less than 1 mass.% in the temperature range of 25–260 °C, the mass change of the prepreg during DSC test and actual curing can be ignored [44].
Dynamic curing kinetic model
The dynamic DSC test results are illustrated in Fig. 2. The DSC heat flow curve, reaction heat and initial reaction temperature all increased along with the increase of heating rate. The total heat obtained by calculating the exothermic peak area was 128.4 ± 1.9 J g−1, and the coefficient of variation was 1.5%, indicating that the correlation between the total heat of reaction and the heating rate was negligible in this heating rate range.
The curve of curing rate was solved through the curve of heat flow. With the increase of heating rate, “S”-shaped and “ ∩ ”-shaped curing curves were displayed at higher temperature as shown in Fig. 3.
The Kissinger and Ozawa methods assume that the conversion at each DSC exothermic peak was invariant and independent of the heating rates [45]. Figure 4 presents the linear fitting, which determined the activation energies to be 86.75 and 93.71 kJ mol−1, respectively. Combining the results of two methods, the activation energy was assumed as 90.23 kJ mol−1.
The kinetic parameters A, m and n were solved by multivariate nonlinear fitting method, and the linear relationship between the parameters and the heating rate β was established, as shown in Table 2. The parameters have obvious linear regularity. The reaction rate-dependent (RD) model can directly reflect the changes of model parameters with heating rate, which was convenient for parameter adjustment and optimization in model application.
Using the RD model parameters shown in Table 2 of the dynamic curing kinetic model at each heating rate, the comparison diagram between the dynamic model and the experimental values and the error distribution diagram of predicting the curing degree were obtained, as shown in Fig. 5.
The prediction error of the dynamic model was less than 2.2%, and the model significantly underestimated the curing degree in the later stage of curing. In the actual curing cycle, the curing temperature in the isothermal stage was in the range of 120–135 °C, thus the prediction error of the model could be less than 1%.
Isothermal curing kinetic model
The isothermal DSC test results shown in Fig. 6 indicated that the curing reactions were autocatalytic. Along with the increase of temperature, both the curing rate and maximum curing degree increased. The total heat of isothermal DSC test was 131.68 ± 0.92 J g−1, and the coefficient of variation was 0.7%, indicating that the correlation between total heat and isothermal curing temperature could also be ignored in this temperature range.
According to the corresponding relationship between curing degree and temperature of dynamic 20 °C min−1 DSC, the heat release of isothermal DSC in the heating stage was less than 2%. Therefore, the curing degree at the heating stage was recorded as 0 to study the change of curing degree in holding stage.
In order to better reflect the isothermal curing process, the autocatalytic model with extended terms as shown in Eq. (7) was used for modeling. By solving the model parameters, a temperature-dependent (TD) model could be developed, as shown in Table 3.
Among them, αc was far less than the curing degree corresponding to the glass transition at the later stage of curing, which indicated that the extended model played a greater role in the early stage of curing.
By developing the linear relationship between 1/T (K−1) and lnK as shown in Eq. (9), the activation energy and pre-exponential factor were determined as E = 86.62 kJ mol−1, A = 5.23E9 (s−1), respectively. By comparing the parameters of dynamic model and isothermal model, the effect of temperature on curing reaction could not be ignored.
The TD model parameters were used in the isothermal curing kinetic model at different temperatures. The comparison diagram between the predicted value of the model and the experimental value and the error diagram of the predicted value of curing degree are shown in Fig. 7. It can be found that the maximum curing rate was reached when the curing degree was about 18–26%.
As shown in Fig. 7c, the variation trend of prediction error at isothermal 125–135 °C was basically the same, and the maximum error was less than 1%. The maximum error of isothermal 120 °C increased to 2.7% at the later stage of curing. The reason was that there was a large residual heat due to the low curing temperature, which was quite different from the other three groups.
Establishment and verification of piecewise model
The curing degree of the prepreg was about 49–64% when it was heated to 125 °C at 2–3 °C min−1, so the two stages of heating and holding the curing degree in the actual curing cycle could not be ignored.
According to the actual curing cycle, the dynamic and isothermal models were applied to the dynamic heating and isothermal holding stages, respectively, and the piecewise model was established with the final curing degree αp in the heating stage as the piecewise point:
and
where A, E, m1 and n1 are the parameters of the dynamic model, respectively; and K, m2, n2 and f1(α) are the parameters of isothermal model and additional factor, respectively; αp represents the final curing degree of the heating stage; TC is the temperature in holding stage. Tonset is the temperature at which the dynamic stage of the curing cycles begins; β is the heating rate in heating stage of the curing cycle.
In the curing cycle at different holding temperatures, the experimental values of curing degree were measured by cyclic DSC test to verify the piecewise model.
According to the initial curing temperature and baseline slope of dynamic DSC experiment, the heating phase baseline of cyclic DSC heat flow curve was determined, and the horizontal baseline was used in isothermal phase. The experimental value of curing degree was obtained by integration.
In the four groups of curing cycles with different holding temperatures, the comparison results of the predicted curing degree values and experimental values of the isothermal, dynamic and piecewise models are shown in Fig. 8. The prediction errors of the three models are shown in Fig. 9.
In the setting of initial conditions, since the baseline established by DSC determines that the scope of curing research was above the initial curing temperature of the resin system, the initial temperature was set to 87.87 °C to avoid the accumulation of model initial error during the heating process from room temperature. Due to the calculation of the model, the initial curing degree cannot be zero, which was consistent with the order of magnitude of the initial curing degree measured by DSC.
The cyclic DSC test proved the validity of the piecewise model in the actual curing process. If only the isothermal or dynamic model was used, the predicted curing degree deviated greatly from the experimental value. The piecewise model combined the advantages of the dynamic and isothermal models in the heating and holding stages, respectively, and can accurately predict the development trend of curing degree in the actual curing process.
The actual curing cycle includes heating and holding stages. There were systematic errors when dynamic or isothermal models were used alone. The reason was that there was a great difference between the temperature program when the model was developed and applied. The piecewise model applied the dynamic and isothermal models to the heating and holding stages to adapt to the actual curing cycle. Thus, the systematic error of a single model in the actual curing cycle was eliminated.
The isothermal model obviously overestimated the curing degree in the heating stage, and the error increased with the increased of holding temperature. The reason was that the temperature range of the model was limited, and there was a large error when isothermal model parameters were extended to actual curing cycles. The applicability of the model parameters became worse with the increased of holding temperature.
The dynamic model obviously underestimated the curing degree in the holding stage, and the error decreased with the increased of holding temperature. The reason was that after entering the holding stage, the curing rate was only determined by the curing degree, rather than the original temperature and curing degree. With the increase of holding temperature, the application range of the model in the holding stage decreases and the curing rate in the holding stage decreases. The applicability of the model parameters became better with the increased of holding temperature.
In four groups of curing cycles with different holding temperatures, the mean errors of curing degree predicted by isothermal, dynamic and piecewise models are shown in Table 4. The overall errors of the three models were 1.63, 1.07 and 0.34%, and the standard deviations were 0.24, 0.33 and 0.11%, respectively, as shown in Fig. 10. Compared with the isothermal and dynamic models, the error of the piecewise model was reduced by 79.1 and 68.2%, and the standard deviation of the error was reduced by 54 and 66%, which also demonstrated the advantages of the piecewise model.
Conclusions
In this study, the RD dynamic model and TD isothermal model of epoxy/amine system were developed by DSC method. The dynamic and isothermal model were applied to the heating and holding stages of the actual curing cycle. Taking the final curing degree in heating stage as the piecewise point, the piecewise model was established. The validity of the piecewise model was verified by carrying out cyclic DSC tests. In the actual curing cycle, the mean errors of isothermal, dynamic and piecewise models were 1.63, 1.07 and 0.34%, respectively. The prediction error of the proposed model could be lowered by over threefold relative to the existing models, which verified the accuracy of the proposed model. In addition, the method of establishing piecewise model was universal, which can be used to develop other systems. These advantages could be conducive to the numerical simulation and optimization of the curing process.
References
Hui XY, Xu YJ, Zhang WC, Zhang WH. Multiscale collaborative optimization for the thermochemical and thermomechanical cure process during composite manufacture. Compos Sci Technol. 2022;224:109455.
Mesogitis TS, Skordos AA, Long AC. Stochastic simulation of the influence of cure kinetics uncertainty on composites cure. Compos Sci Technol. 2015;110:145–51.
Lorenz N, Muller-Pabel M, Gerritzen J, Muller J, Groger B, Schneider D, et al. Characterization and modeling cure- and pressure-dependent thermo-mechanical and shrinkage behavior of fast curing epoxy resins. Polym Test. 2022;108:107498.
Sorrentino L, Esposito L, Bellini C. A new methodology to evaluate the influence of curing overheating on the mechanical properties of thick FRP laminates. Compos Part B-Eng. 2017;109:187–96.
Yuksel O, Sandberg M, Baran I, Ersoy N, Hattel JH, Akkerman R. Material characterization of a pultrusion specific and highly reactive polyurethane resin system: elastic modulus, rheology, and reaction kinetics. Compos Part B-Eng. 2021;207:108543.
Mohseni M, Zobeiry N, Fernlund G. Process-induced matrix defects: post-gelation. Compos Part a-Appl S. 2020;137:106007.
Zbed RS, Le Corre S, Sobotka V. Process-induced strains measurements through a multi-axial characterization during the entire curing cycle of an interlayer toughened Carbon/Epoxy prepreg. Compos Part a-Appl S. 2022;153:106689.
Mesogitis TS, Skordos AA, Long AC. Uncertainty in the manufacturing of fibrous thermosetting composites: a review. Compos Part a-Appl S. 2014;57:67–75.
Thomas R, Wehler S, Fischer F. Predicting the process-dependent material properties to evaluate the warpage of a co-cured epoxy-based composite on metal structures. Compos Part a-Appl S. 2020;133:105857.
Ding AX, Li SX, Wang JH, Ni AQ, Zu L. A new path-dependent constitutive model predicting cure-induced distortions in composite structures. Compos Part a-Appl S. 2017;95:183–96.
Yoon JS, Kim K, Seo HS. Computational modeling for cure process of carbon epoxy composite block. Compos Part B-Eng. 2019;164:693–702.
McCoy JD, Ancipink WB, Clarkson CM, Kropka JM, Celina MC, Giron NH, et al. Cure mechanisms of diglycidyl ether of bisphenol A (DGEBA) epoxy with diethanolamine. Polymer. 2016;105:243–54.
Song YJ, Liu M, Zhang LY, Mu CZ, Hu X. Mechanistic interpretation of the curing kinetics of tetra-functional cyclosiloxanes. Chem Eng J. 2017;328:274–9.
Kim KW, Kim DK, Kim BS, An KH, Park SJ, Rhee KY, et al. Cure behaviors and mechanical properties of carbon fiber-reinforced nylon6/epoxy blended matrix composites. Compos Part B-Eng. 2017;112:15–21.
Hardis R, Jessop JLP, Peters FE, Kessler MR. Cure kinetics characterization and monitoring of an epoxy resin using DSC, Raman spectroscopy, and DEA. Compos Part a-Appl S. 2013;49:100–8.
Xu MZ, Luo YS, Lei YX, Liu XB. Phthalonitrile-based resin for advanced composite materials: curing behavior studies. Polym Test. 2016;55:38–43.
Zu Y, Zong LS, Wang JY, Jian XG. Kinetic analysis of the curing of branched phthalonitrile resin based on dynamic differential scanning calorimetry. Polym Test. 2021;96:107062.
Bai Y, Yang P, Zhang S, Li YQ, Gu Y. Curing kinetics of phenolphthalein-aniline-based benzoxazine investigated by non-isothermal differential scanning calorimetry. J Therm Anal Calorim. 2015;120(3):1755–64.
Chen MF, Peng SQ, Zhao MY, Liu YH, Liu CP. The curing and degradation kinetics of modified epoxy-SiO2 composite. J Therm Anal Calorim. 2017;130(3):2123–31.
Hesabi M, Salimi A, Beheshty MH. Effect of tertiary amine accelerators with different substituents on curing kinetics and reactivity of epoxy/dicyandiamide system. Polym Test. 2017;59:344–54.
Xu XQ, Zhou Q, Song N, Ni QR, Ni LZ. Kinetic analysis of isothermal curing of unsaturated polyester resin catalyzed with tert-butyl peroxybenzoate and cobalt octoate by differential scanning calorimetry. J Therm Anal Calorim. 2017;129(2):843–50.
Tezel GB, Sarmah A, Desai S, Vashisth A, Green MJ. Kinetics of carbon nanotube-loaded epoxy curing: rheometry, differential scanning calorimetry, and radio frequency heating. Carbon. 2021;175:1–10.
Liu FY, Yu WW, Wang YJ, Shang RJ, Zheng Q. Curing kinetics and thixotropic properties of epoxy resin composites with different kinds of fillers. J Mater Res Technol. 2022;18:2125–39.
Song XX, Xu SA. Curing kinetics of pre-crosslinked carboxyl-terminated butadiene acrylonitrile (CTBN) modified epoxy blends. J Therm Anal Calorim. 2016;123(1):319–27.
Monteserin C, Blanco M, Laza JM, Aranzabe E, Vilas J. Thickness effect on the generation of temperature and curing degree gradients in epoxy-amine thermoset systems. J Therm Anal Calorim. 2018;132(3):1867–81.
Bornosuz NV, Gorbunova IY, Petrakova VV, Shutov VV, Kireev VV, Onuchin DV, et al. Isothermal kinetics of epoxyphosphazene cure. Polymers-Basel. 2021;13(2):297.
Kim J, Moon TJ, Howell JR. Cure kinetic model, heat of reaction, and glass transition temperature of AS4/3501-6 graphite-epoxy prepregs. J Compos Mater. 2002;36(21):2479–98.
Hayaty M, Beheshty MH, Esfandeh M. Cure kinetics of a glass/epoxy prepreg by dynamic differential scanning calorimetry. J Appl Polym Sci. 2011;120(1):62–9.
Hayaty M, Beheshty MH, Esfandeh M. Isothermal differential scanning calorimetry study of a glass/epoxy prepreg. Polym Advan Technol. 2011;22(6):1001–6.
D’Elia R, Dusserre G, Del Confetto S, Eberling-Fux N, Descarnps C, Cutard T. Cure kinetics of a polysilazane system: experimental characterization and numerical modelling. Eur Polym J. 2016;76:40–52.
Newcomb BA. Time-temperature-transformation (TTT) diagram of a carbon fiber epoxy prepreg. Polym Test. 2019;77:105859.
Xu HJ, Tian GF, Meng Y, Li XY, Wu DZ. Cure kinetics of a nadic methyl anhydride cured tertiary epoxy mixture. Thermochim Acta. 2021;701:178942.
Tziamtzi CK, Chrissafis K. Optimization of a commercial epoxy curing cycle via DSC data kinetics modelling and TTT plot construction. Polymer. 2021;230:124091.
Shin DD, Hahn HT. A consistent cure kinetic model for AS4/3502 graphite/epoxy. Compos Part a-Appl S. 2000;31(9):991–9.
Faria H, Pereira CMC, Pires FMA, Marques AT. Kinetic models for the SR1500 and LY556 epoxies under manufacturer’s recommended cure cycles. Eur Polym J. 2013;49(10):3328–36.
Mphahlele K, Ray SS, Kolesnikov A. Cure kinetics, morphology development, and rheology of a high-performance carbon-fiber-reinforced epoxy composite. Compos Part B-Eng. 2019;176:107300.
Vidil T, Tournilhac F, Musso S, Robisson A, Leibler L. Control of reactions and network structures of epoxy thermosets. Prog Polym Sci. 2016;62:126–79.
Barros JJP, Silva IDD, Jaques NG, Wellen RMR. Approaches on the non-isothermal curing kinetics of epoxy/PCL blends. J Mater Res Technol. 2020;9(6):13539–54.
Bernath A, Karger L, Henning F. Accurate cure modeling for isothermal processing of fast curing epoxy resins. Polymers-Basel. 2016;8(11):390.
Jensen M, Jakobsen J. Enthalpy relaxation and post shrinkage of sub and sup-Tg cured epoxy. J Non-Cryst Solids. 2018;498:60–3.
Jahani Y, Baena M, Barris C, Perera R, Torres L. Influence of curing, post-curing and testing temperatures on mechanical properties of a structural adhesive. Constr Build Mater. 2022;324:126698.
Zhao L, Hu XA. Autocatalytic curing kinetics of thermosetting polymers: a new model based on temperature dependent reaction orders. Polymer. 2010;51(16):3814–20.
Xu YJ, Chen L, Rao WH, Qi M, Guo DM, Liao W, et al. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem Eng J. 2018;347:223–32.
Kudisonga C, Villacorta B, Chisholm H, Vandi LJ, Heitzmann M. Curing kinetics of a siloxane pre-ceramic prepreg resin. Ceram Int. 2021;47(14):20678–85.
Zheng T, Xi H, Wang ZX, Zhang XH, Wang Y, Qiao YJ, et al. The curing kinetics and mechanical properties of epoxy resin composites reinforced by PEEK microparticles. Polym Test. 2020;91:106781.
Acknowledgements
This study was supported by National Natural Science Foundation of China under Grant No. 52275441 and Natural Science Foundation of Shenzhen City under Grant No. WDZC20200817152115001. The authors would like to thank Testing Technology Center of Materials and Devices, Tsinghua Shenzhen International Graduate School (https://mdtc.sz.tsinghua.edu.cn) for the assistances of DSC measurements and analysis.
Author information
Authors and Affiliations
Contributions
LZ Investigation, Software, Writing—Original Draft. PF Conceptualization, Writing—Review and Editing, Supervision. JX Methodology, Writing—Review and Editing. YL Investigation, Writing—Review and Editing. WG Methodology, Writing—Review and Editing. GL Writing—Review and Editing, Supervision. FF Conceptualization, Writing—Review & Editing, Project administration.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Feng, P., Xu, J. et al. A piecewise kinetic model consistent with curing cycle of epoxy/amine composite. J Therm Anal Calorim 148, 12781–12793 (2023). https://doi.org/10.1007/s10973-023-12550-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12550-1