Abstract
The static melting method is used to prepare KNO3–NaNO3–NaCl ternary mixed molten salt containing 5–20 mass% NaCl, and the thermal physical parameters such as specific heat capacity, melting point, and latent heat of phase change are measured by differential scanning calorimetry. The microstructures of the mixed molten salts are characterized by XRD and SEM. The experimental results show that compared with the base salt, after adding different mass fractions of NaCl, the melting point of the molten salts was reduced by about 24.8%, and the latent heat of phase change was increased by 22.71–52.53 J g−1, the solid and liquid state specific heat capacity of molten salts increased by 3.4% and 2.3% after the addition of 10 mass% NaCl. The addition of 15 mass% NaCl formed a special chlorine salt layer on the surface of the molten salt. This structure reduced the intermolecular voids and enhanced the intermolecular force, which may be the reason for the increase in specific heat capacity and latent heat of phase change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of the economy, environmental problems are becoming more and more prominent, and the application of clean energy and renewable energy is increasingly important [1]. Solar power generation and heating are currently one of the key development of energy-saving and clean energy technologies, which may become the main energy source of the future [2]. It has characteristics such as intermittency, which will broaden the scope of its application if combined with thermal storage technology. Phase change heat storage in heat storage technology has become a hot research topic due to the advantages of high heat storage density, the phase change temperature of molten inorganic salt phase change material is generally in the range of 100–1000 °C, and its advantages are low cost, high use temperature, good safety, and large latent heat, high energy storage density, and good thermal stability [3,4,5].
Among the many inorganic salts, nitrates have a melting point of around 300 °C and the upper limit of use temperature is about 600 °C. Its properties make them suitable for use as medium and low-temperature thermal storage materials. Hitec molten salt (7% NaNO3–53% KNO3–40% NaNO2) and Solar Salt composite molten salt (40% KNO3–60% NaNO3) have excellent overall performance and are widely used in Solar Power Station [6, 7]. The study of molten nitrate salt systems is still one of the hot spots in science, focusing on the physical properties, thermodynamic properties, operating temperature, microstructure, etc. [8]. At present, although nitrates and their composites have been widely used as medium and low-temperature thermal storage media, the high melting temperature, relatively low latent heat of phase change and specific heat will affect the scope of application of nitrates in thermal storage systems and the application effect [9, 10].
Domestic and international scholars have used a variety of approaches to increase the performance of nitrates, such as adding nanoparticles to molten salts to improve their thermophysical properties [11, 12] or using chlorine salts to improve the performance of nitrates [13]. Gasanaliev et al. [14] researched mixed molten salts of nitrate and chloride and found that the addition of chloride reduces the phase change temperature and that the latent heat of phase change of the mixed molten salt system is maintained. Chlorinated salt systems are not only diverse, but also have a wide temperature range (150–900 °C and above) [15], normally have a high specific heat capacity and latent heat of phase change, better thermal stability, and high thermal conductivity, making them promising materials for high-temperature thermal storage. Among them, NaCl is low cost and abundant [16], suitable for large-scale applications. Zhong et al. [17] prepared 58.1% LiNO3–41.9% KCl and 87% NaNO3–13% NaCl mixed molten salts, and the test results show that the produced phase change materials demonstrated good thermal stability. Li et al. [18] prepared a binary molten salt of 88% LiNO3–12% NaCl with only 1.2 °C supercooling degree.
The purpose of this paper is to investigate the effect of adding NaCl particles on the thermal properties of KNO3–NaNO3 binary nitrate and to improve the performance of the thermal storage materials. The thermal properties of the mixed molten salts were measured by differential scanning calorimetry, the physical phase and crystallization of the materials were analyzed by XRD, and the microstructure was characterized by SEM. The experimental results are used to improve the properties of nitrate salts and broaden the application of nitrate molten salts in phase change heat storage and solar power generation.
Experimental method
Experimental materials
KNO3 (melting point 334.0 °C, latent heat of phase change 90.0 J g−1) and NaNO3 (melting point 306.8 °C, latent heat of phase change 162.5 J g−1) were selected as the phase change material. The purity was greater than or equal to 99%. The mixing ratio of KNO3 and NaNO3 was 74.7:25.3. NaCl was a modified salt with a purity greater than or equal to 99%.
Samples preparation
Prior to samples preparation, the three pure salts were first dried in a drying oven for 24 h at a drying temperature of 120 °C to completely remove the crystalline water from the samples. The mixed molten salts were prepared by the static melting method [19], and five groups of binary nitrates were prepared in the ratio of (KNO3:NaNO3) 74.7:25.3, and NaCl was added to four of the group at 5 mass%, 10 mass%, 15 mass%, and 20 mass%. The five groups of mixed salts were stirred and pulverized in a stainless steel mortar one by one, then placed in a corundum crucible and heated from room temperature to 400 °C in a muffle furnace at a heating rate of 5 °C min−1 and kept for 2 h to ensure complete fusion. Then, the temperature was decreased from 400 to 50 °C at a rate of 5 °C min−1 and held for half an hour. After standing and cooling to room temperature, took it out and ground, sealed, and dried it for storage.
Differential scanning calorimetry
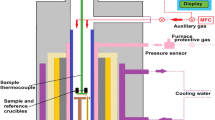
The specific heat capacity, melting point, and latent heat of phase change of binary nitrates and four groups of ternary mixed molten salts with different mass fractions of NaCl were measured by a TG/DSC simultaneous thermal analyzer (METTLER TGA/DSC2 1600LF). The specific heat capacity of the sample was measured by the sapphire method [20] and calculated by comparing the DSC heat flow curves of the standard sapphire sample and the sample to be measured. In this study, the accuracy of temperature measurement is approximately ± 0.1 K, the accuracy of mass measurement is within ± 0.01 mg, and the accuracy of calorimeter measurement is within ± 0.5%. The test principle is as follows:
Based on the error transfer formula, the uncertainty for specific heat capacity can be derived as follows:
In the formula, Cp is specific heat capacity, J g−1 °C−1, Φ is heat flow, mW, m is mass, g, Reference is pure sapphire in the form of a crystal.
A certain mass (difference in mass from the sapphire < 1 mg) of the dried sample was placed in an aluminum crucible and sealed. The sample was placed in the apparatus furnace, and the temperature was increased from 50 to 400 °C at a rate of 10 °C min−1, kept it constant at 400 °C for 10 min, then cooled it from 400 to 50 °C at a rate of 10 °C min−1. Nitrogen was used as the experimental protective gas and purge gas during the measurement, with a flow rate of 25 mL min−1.
Scanning electron microscope (SEM)
The ternary mixed molten salt with the addition of NaCl had an increased specific heat capacity and latent heat compared to the normal binary nitrate. In order to determine the reason for the increase in specific heat capacity and latent heat and to investigate the mechanism, the microscopic surface morphology of binary nitrate and ternary molten salt in the solid state was characterized using an electron microscope (JEM-2100F/HR). In this paper, the sample powder was attached to the sample holder by tape, and the surface of the mixed nitrate was sprayed with gold to observe the morphology of the sample more clearly.
Analysis of experimental results
Melting point and latent heat of phase change
The DSC curves for the binary molten salt of 74.7% KNO3–25.3% NaNO3 and the ternary molten salt of KNO3–NaNO3–NaCl with different mass fractions of NaCl added are shown in Fig. 1. It can be seen that the addition of NaCl can lower the melting point of the binary nitrate and increase its latent heat of melting, improving its thermophysical properties. According to experimental measurements, the melting point of 74.7% KNO3–25.3% NaNO3 was 277.6 °C. The melting points of ternary mixed melting salts with different mass fractions of NaCl added from 5 to 20% were 209.2 °C, 209.2 °C, 209.0 °C, and 207.7 °C, respectively, and the melting points were all reduced by about 24.8%, which means that the change in melting point of the ternary mixed salt with the change in mass fraction of NaCl was not significant. The latent heat of melting increased by the addition of 5 mass% and 15 mass% NaCl reached peaks of 143.43 J g−1 and 139.60 J g−1, respectively, and the latent heats of phase change increased by 57.8% and 53.6%, respectively. That was considerably higher than the latent heat of phase change given in the literature [2] for 53% KNO3–40% NaNO2–7% NaNO3 (80.0 J g−1), 54% KNO3–46% NaNO3 (100.0 J g−1), and 54% NaNO3–46% KNO3 (117.0 J g−1).
In addition, a small melting peak of KNO3–NaNO3 appears near 230.0 °C in the figure, and the latent heat value corresponding to this peak is not large, presumably due to the conversion of NaNO3 from the low-temperature stable phase II-NaNO3 second-order phase to the high-temperature phase I-NaNO3 [21], and the main peak appearing at 297.5 °C is the phase transition peak of the binary molten salt. With the addition of NaCl, the phase transition temperature of the ternary eutectic mixed molten salt is reduced to about 209.0 °C. It is slightly different from the crystal phase transition temperature of NaNO3, and the two peaks are connected together. Since there is no obvious demarcation between the two melting peaks, the latent heat values of the two peaks can be summed as the eutectic latent heat value of the mixed molten salt of the system. Since there is no obvious demarcation between the two melting peaks, the latent heat value of the two melting peaks can be summed up as the eutectic latent heat value of the mixed molten salt of the system [22]. Therefore, the calculation formula of the eutectic latent heat value \(\Delta H_{{\text{m}}}\) of each group of mixed molten salts is as follows:
In the formula, \(\Delta H_{1}\) is the latent heat value of the phase transition of the mixed molten salt, and \(\Delta H_{2}\) is the heat absorbed due to lattice transformation of NaNO3.
When NaCl was used as an additive, the number of anions and cations was larger than that of nitrates. At unit molar mass, the number of negative charges increases, which affected the electrostatic attraction between the molten salt ions, and chlorine salt as a heterogeneous salt disrupts the spatial dot matrix of the nitrate system, caused a change in its structure, and then, the lattice energy of the material also changes, raised the latent heat of the molten salt. The data of the melting point, peak temperature, melting endpoint, and latent heat of melting of the five mixed molten salts are listed in Table 1.
Specific heat capacity
Figure 2 and Table 2 are the specific heat capacity curves and average values of samples in solid and liquid phases with different mass fractions of NaCl. The average specific heat capacity of KNO3–NaNO3 binary molten salt in the solid phase is 1.17 J g−1 °C−1, and that in the liquid phase is 1.28 J g−1 °C−1. Compared with the base salt, when 10 mass% NaCl is added, the specific heat capacities of molten salt in the solid and liquid state increased by 3.4% and 2.3%, respectively. Experiments had proved that adding an appropriate amount of NaCl can effectively increase the specific heat capacity of binary nitrates. The increase in the specific heat capacity of the ternary molten salt may be due to the addition of an appropriate amount of NaCl, which changed the internal structure of the binary mixed molten salt and increased the specific surface area of the sample.
Heat storage calculation
The heat absorbed or given off when the temperature changes from a base state (or reference state) to a certain temperature T without a phase change or chemical change in the substance is the sensible heat of the substance. In the calculation of sensible heat, the average heat capacity is generally chosen for calculation, the equation for the sensible heat is as follows:
In the formula, \(Q_{{\text{p}}}\) is the sensible heat, J.
Ideally, the total heat stored in molten salt for unit mass at 150–350 °C is the sum of sensible heat and latent heat, and the calculation results are shown in Table 3. As it is obvious, the ternary molten salt with 10 mass% and 15 mass% NaCl has strong heat storage capacity due to the high latent heat and specific heat of phase transformation.
X-ray diffraction (XRD)
Figure 3a shows the XRD analysis of four groups of mixed molten salts after 10 thermal cycles. It can be seen from the diagram that the major crystalline phases of the four molten salts both are KNO3 and NaNO3, and there is no irregular characteristic peak, indicating that the molten salt does not appear decomposition phenomenon, and there is no chemical reaction to produce new substances, only physical mixing. The material exhibits good thermal stability and chemical compatibility. However, the intensity of the characteristic peaks is different, and the peak intensity of molten salt with 15 mass% NaCl is the highest. Figure 3b shows the XRD spectrum of the ternary nitrate molten salt containing 15 mass% NaCl. There are several independent narrow peaks and higher peaks, indicating that the sample has better crystallinity.
The eutectic of NaNO3 and KNO3 at 29.36° indicates that the two salts penetrate each other and the structure is stable. Multiple diffraction peaks of NaCl appear in the spectrum in the range of 33.60–50.00°. NaCl binds better with both nitrates and can increase the phase interface area, which has a certain effect on increasing the specific heat and latent heat of phase change of nitrates.
Scanning electron microscope (SEM)
The microstructure of multivariate molten salt is closely related to its thermal properties. The reasons for the improvement of thermal properties can be studied by analyzing its microstructure. Figure 4 shows the SEM images of the KNO3–NaNO3–NaCl mixed molten salt magnified by 10,000 times after the addition of different mass fractions of NaCl. It can be seen from Fig. 4a that the microscopic surface of the binary nitrate is smooth, the structure is regular, there are gaps between the blocks, and there is no difference in the structure of each part, and the mixing effect of the two kinds of salts is good. Figure 4b shows the SEM images of the mixed molten salt with 5 mass% NaCl addition. Compared with the binary molten salt structure, the delamination phenomenon appears, partly keeping the regular shape and partly becoming serrated at the edges, and the gap between the structures is reduced. Figure 4c shows the SEM images of the molten salt with the addition of 10 mass% NaCl. The surface of the particles becomes uneven, and a large number of NaCl particles are attached to the surface of the binary nitrate, which increases the specific surface area of the mixed salt. Figure 4d shows the SEM images of the molten salt with the addition of 15 mass% NaCl. The addition of 15 mass% NaCl resulted in a more uniform distribution of NaCl particles on the surface of the molten salt, which forms a chloride salt layer structure on the nitrate surface, and a denser and tighter nitrate structure. By observing and analyzing SEM images, it can be seen that after adding NaCl, the surface of molten salt becomes irregular, forming a special chloride layer structure, and local stratification occurs. The comparison with the XRD experimental results verifies the phenomenon of the increased strength of the ternary molten salt eutectic structure. The special chloride salt layer structure increases the specific surface area and specific surface energy compared to the binary molten salt, which may also account for the increased latent heat of melting and specific heat capacity of the ternary molten salt.
Conclusions
Four different ternary molten salts were obtained by adding different mass fractions of NaCl to the binary nitrate 74.7% KNO3–25.3% NaNO3, and the melting points, latent heats of melting, and specific heat capacities of the five samples were measured by differential scanning calorimetry. X-ray diffraction and scanning electron microscopy were used to analyze the eutectic strength and microscopic morphology, and the following conclusions were obtained.
-
(1)
KNO3, NaNO3, and NaCl had excellent chemical compatibility with each other and did not react. The addition of NaCl at a mass fraction of 5–20 mass% significantly reduced the melting point of the binary nitrate and increased its latent heat of phase change, and there was no linear relationship between the change in melting point and the amount of additive. Compared to the base salt, the melting points were all reduced by approximately 24.8% and the latent heats of phase change increased by 52.53 J g−1, 37.86 J g−1, 48.70 J g−1, and 22.71 J g−1, respectively.
-
(2)
DSC test results showed that the ternary mixed molten salt with 10 mass%, 15 mass% NaCl had better performance with a melting point of about 209.0 °C, latent heat of melting of about 130.0 J g−1, and relatively high specific heat capacity. In the range of 150–350 °C, the total heat stored per unit mass of molten salt was the most, which were 386.83 J g−1 and 364.62 J g−1, respectively. It is a good phase change heat storage material.
-
(3)
According to the SEM images of the ternary mixed molten salt, it can be seen that when 15 mass% NaCl was added, the substance was uniformly dispersed and had a good eutectic structure. The chlorine salt forms a layered structure on the surface of the eutectic salt and the eutectic strength increased. The special chlorine salt layer structure increased the specific surface area and specific surface energy, which led to an increase in specific heat capacity and latent heat of phase change.
-
(4)
The addition of an appropriate amount of NaCl to the KNO3–NaNO3 binary nitrate can effectively improve the thermal properties of the nitrate and broaden its application range, which has a certain application value.
References
Hu Y, He Y, Zhang Z, Wen D. Effect of Al2O3 nanoparticle dispersion on the specific heat capacity of a eutectic binary nitrate salt for solar power applications. Energy Convers Manag. 2017;142:366–73.
Kenisarin MM. High-temperature phase change materials for thermal energy storage. Renew Sustain Energy Rev. 2010;14(3):955–70.
Nie B, Palacios A, Zou B, Liu J, Zhang T, Li Y. Review on phase change materials for cold thermal energy storage applications. Renew Sustain Energy Rev. 2020;134:110340.
Ren N, Wu YT, Ma CF, Sang LX. Preparation and thermal properties of quaternary mixed nitrate with low melting point. Sol Energy Mater Sol Cells. 2014;127:6–13.
Wanyu H, Quanying Y. Molten salt as a phase change material. Materials reports 2015;29(S1):128–130+140. (in Chinese)
Glatzmaier G. Summary report for concentrating solar power thermal storage workshop: new concepts and materials for thermal energy storage and heat-transfer fluids, May 20, 2011: National Renewable Energy Lab (NREL), Golden, CO (United States) 2011.
Liu M, Tay NS, Bell S, et al. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew Sustain Energy Rev. 2016;53:1411–32.
Zhang Y, Li J, Gao L, Wang M. Nitrate based nanocomposite thermal storage materials: Understanding the enhancement of thermophysical properties in thermal energy storage. Sol Energy Mater Sol Cells. 2020;216:110727.
Chen X, Wu YT, Wang X, Ma CF. Experimental study on the specific heat and stability of molten salt nanofluids prepared by high-temperature melting. Sol Energy Mater Sol Cells. 2018;176:42–8.
Durth M, Prieto C, Rodríguez-Sánchez A, Patiño-Rodríguez D, Cabeza LF. Effects of sodium nitrate concentration on thermophysical properties of solar salts and on the thermal energy storage cost. Sol Energy. 2019;182:57–63.
Akilu S, Baheta A, Sharma K, Said M, editors. Experimental determination of nanofluid specific heat with SiO2 nanoparticles in different base fluids. AIP Conference Proceedings; 2017: AIP Publishing LLC.
Nithiyanantham U, González-Fernández L, Grosu Y, Zaki A, Igartua JM, Faik A. Shape effect of Al2O3 nanoparticles on the thermophysical properties and viscosity of molten salt nanofluids for TES application at CSP plants. Appl Therm Eng. 2020;169:114942.
Li Y, Yue G, Yu Y, Zhu Q. Preparation and thermal characterization of LiNO3–NaNO3–KCl ternary mixture and LiNO3–NaNO3–KCl/EG composites. Energy. 2020;196:117067.
Gasanaliev AM, Gamataeva BY. Heat-accumulating properties of melts. Russ Chem Rev. 2000;69(2):179.
He MZ, Yang L, Zhang Z. Supercooling characteristics of inorganic phase change material CaCl2·6H2O. J Chem Ind Eng. 2017;68(11):4016–24.
Arconada N, Arribas L, Lucio B, González-Aguilar J, Romero M. Macroencapsulation of sodium chloride as phase change materials for thermal energy storage. Sol Energy. 2018;167:1–9.
Zhong L, Zhang X, Luan Y, Wang G, Feng Y, Feng D. Preparation and thermal properties of porous heterogeneous composite phase change materials based on molten salts/expanded graphite. Sol Energy. 2014;107:63–73.
Li Y, Wang C, Liu G, Zhu Q, Qiu Z. Thermal property characterization of a low supercooling degree binary mixed molten salt for thermal energy storage system. Int J Thermophys. 2019;40(4):1–12.
Tian H, Wang W, Ding J, Wei X. Thermal performance and economic evaluation of NaCl–CaCl2 eutectic salt for high-temperature thermal energy storage. Energy. 2021;227:120412.
Galazutdinova Y, Vega M, Grágeda M, Cabeza LF, Ushak S. Preparation and characterization of an inorganic magnesium chloride/nitrate/graphite composite for low temperature energy storage. Sol Energy Mater Sol Cells. 2018;175:60–70.
D’Aguanno B, Karthik M, Grace A, Floris A. Thermostatic properties of nitrate molten salts and their solar and eutectic mixtures. Sci Rep. 2018;8(1):1–15.
Roget F, Favotto C, Rogez J. Study of the KNO3–LiNO3 and KNO3–NaNO3–LiNO3 eutectics as phase change materials for thermal storage in a low-temperature solar power plant. Sol Energy. 2013;95:155–69.
Acknowledgements
This research is supported by the Science and Technology Commission of Shanghai Municipality under the contract No. 20dz1205208, which is gratefully acknowledged by the authors.
Author information
Authors and Affiliations
Contributions
Y. Li prepared the samples and the manuscript. W.W. Tan and C.G. Wang contributed to the analysis and manuscript preparation. Q.Z. Zhu helped with the analysis through constructive discussions.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Authors: Y. Li, W.W. Tan, C.G. Wang, Q.Z. Zhu.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Tan, W.W., Wang, C.G. et al. Research on the effect of adding NaCl on the performance of KNO3–NaNO3 binary molten salt. J Therm Anal Calorim 148, 733–739 (2023). https://doi.org/10.1007/s10973-022-11791-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11791-w