Abstract
Following recent developments in micro-scale energy systems, such as microthruster and igniters among others, there is now considerable interest in using tertiary nanothermites to meet the increasing demand in high energy density propulsion systems. The first objective of this research is to compare and analyze the thermal behaviour of different nanothermite tertiary compositions based on nano-aluminium (n-Al), graphene oxide (GO), various salt and metallic oxidizers. The second objective is to identify the thermite reaction mechanism through correlations with the activation energy and exothermic peaks. Thermogravimetry analysis coupled with a differential scanning calorimeter (TGA/DSC) was employed to elucidate the reaction process of these nanothermite compositions, while bomb calorimetry was used to measure their heat of combustion. The apparent kinetics parameters were calculated using Kissinger and Ozawa approaches. The results demonstrate that the addition of GO enhances the reactivity of nanothermites with both salt and metallic oxidizers by reducing the reaction onset temperature, activation energy and increasing the heat release. For nanothermites with oxidizing salts, the heterogeneous solid–gas reaction mechanism plays a more important role than the condensed phase reactions. In general, nanothermites based on oxidizing salts are more reactive than those with metallic ones, as indicated in both theoretical and experimental data. Among them, the GO/Al/KClO4 nanothermite exhibits the highest heat release (9614 J g−1), while the GO/Al/K2S2O8 nanothermite shows the lowest onset temperature and activation energy (380 °C and 105 kJ mol−1). This study provides benchmark information for optimizing the tertiary nanothermites design, use, storage and handling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal properties of nanothermite, such as ignition temperature and specific heat released, are important criteria that form part of the practical nanothermite usage as well as a key to exploring the reaction mechanism. Based on this, many studies have been carried out to improve their efficient combustion and ignition characteristics [1,2,3,4,5,6].
Nanothermites are solid-phase energetic mixtures traditionally composed of metal and metal-oxide components at nanometric scale. Compared to traditional micron-scale thermite powders, nanothermites offer improvements in the reaction rate, heat release, pressure and thrust output by leveraging: large specific surface area, a decrease in the required activation energy, a fast energy release and a high energy density [7, 8]. Despite their advantages over microthermites, nanothermites still suffer from relatively long ignition delays, slow combustion kinetics, particle agglomeration before ignition, and incomplete combustion which have limited their applicability [8,9,10,11,12].
A growing body of literature can be found on the thermal behaviour and combustion mechanism of nanothermite compositions. A solid-state reaction is the most common initiating reaction for thermite compositions, followed by the movement of the resulting reactive species towards each other [13]. The way and nature of movement of the species is still being debated [1,2,3,4,5]. Granier et al. assessed the ignition process and thermal properties of nanothermite based on n-Al and molybdenum oxide (MoO3) and they found that n-Al reduced the ignition time significantly. In addition, they reported that the combustion of Al/MoO3 will not occur until the temperature reaches the melting point of either Al or MoO3 followed by a diffusion-controlled reaction [13]. Based on what was reported in [8, 13], nanothermites will ignite around the melting temperature of Al (660 °C). In [10], the authors investigated the thermal properties of nanothermites based on a wide range of oxidizers which release oxygen at a widely different range of temperatures to find the relationship between the oxidizer oxygen release temperature and nanothermite ignition temperature. They concluded that the presence of free oxygen is not necessary to initiate many types of Al-based nanothermites. They also suggested a condensed phase reaction mechanism, which does not depend on free oxygen or melted Al. A condensed phase reaction mechanism is confirmed by many studies to be the governing mechanism for nanothermite combustion [14,15,16,17].
Recently, graphene and its derivatives have been attracting much attention for improving ignition and the combustion characteristics of nanothermite due to a large specific area and unique thermal, electrical and mechanical properties [18,19,20,21]. Furthermore, the abundance of oxygenic functional groups on the basal plane and at the edges of GO makes it an energetic material (EM) in and of itself, capable of severe exothermic breakdown when heated, and hence it is a promising contender for nanothermite components [22, 23].
To this day, discussions of the effect of graphene and its derivatives, particularly GO, on the combustion properties and thermal behaviour of nanothermites have been limited to those based on certain types of metallic oxidizers, like Fe2O3 and Bi2O3 [24, 25]. On the other side, the effect of GO on the thermal behaviour of nanothermites based on oxidizing salts and some types of metallic ones has not been explicitly studied from a thermal and mechanistic point of view. Hence, given the importance of gaining a more in-depth understanding of thermite materials for the development of new thermite compositions, it is vital to explore how physical and chemical characteristics of the new components affect the thermite reaction. Additionally, it is important to determine the reaction mechanism and understand the thermal behaviour of these new nanothermites to know how they can be used to adapt to future applications from a performance standpoint and practical safety concerns related to large-scale industrial use.
In the present work, we investigate the thermal behaviour and reaction characteristics of tertiary nanothermites based on n-Al, GO and different types of salt and metallic oxidizers. Experimental and theoretical investigations were performed on eight nanothermite compositions containing five oxidizing salts and three metallic oxides, respectively. Five controlled (reference) samples were prepared and analysed with oxidizing salts for comparison purposes. All compositions were characterized using TGA/DSC and a bomb calorimeter. The controlling mechanisms of different nanothermite compositions are discussed based on the measured kinetic parameters.
Materials and methods
Materials
Graphite flakes, phosphoric acid (H3PO4), sulphuric acid (H2SO4), hydrogen peroxide (H2O2), and acetone used in the preparation of GO were purchased from Sigma-Aldrich. Oxidizing salts, such as potassium perchlorate (KClO4) and ammonium nitrate (NH4NO3), were brought from Defence Research and Development Canada. All other salts and metallic oxidizers, namely potassium periodate (KIO4), potassium persulfate (K2S2O8), potassium permanganate (KMnO4), iron oxide (Fe2O3), copper oxide (CuO) and tungsten oxide (WO3), were purchased from Sigma-Aldrich. All oxidizers are fine powder in order of 5–10 μm according to their suppliers and used without further purification. The 40 nm Al nanoparticles with 78 mass % active Al content and 30–50 m2 g−1 surface area were purchased from Us Research Nanomaterials Inc.
Preparation of GO and nanothermite compositions
The detailed procedures preparation of the GO and nanothermite compositions can be found in our previously published article [26]. Different nanothermite compositions and their reactions are shown in Table 1.
Homogeneity of nanothermites
Morphology and homogeneity of nanothermite samples were studied by FEI Quanta FEG 450 SEM operated at 15 kV coupled with EDS measurements.
Thermal analysis and reaction calorimetry
Simultaneous TGA/DSC was performed to assess the thermal characteristics of nanothermite compositions. 5 mg nanothermite heated at varied rates (2, 5, 8, 10 °C min−1) in an alumina crucible under nitrogen (100 mL min−1) from 30 °C to 1100 °C. Oxygen bomb calorimeter (model: Parr™ 1341 Plain Jacket Calorimeter) used to measure heat of combustion of nanothermite mixture. Five successive tests were used to compute the average heat of combustion, which was then compared to the theoretical value.
Results and discussion
Structure and chemical characterization
The CHNSO Elemental Analyser EA3000 (EuroVector) was used to examine the composition of prepared GO. Table 2 summarizes the mass fractions (wt.%) of C, H, N, S, and O.
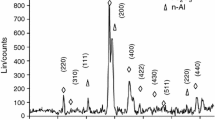
The homogenous distribution of each component throughout the thermite samples is an important performance factor. The quality of dispersion in the samples was evaluated using SEM–EDS analysis, as shown in Fig. 1, in which the GO/Al/KIO4 was selected as a representative for all of the samples.
The EDS findings in Fig. 1 demonstrate the spatial homogeneity of the elemental composition of GO/Al/KIO4 nanothermites. As can be observed, there were no agglomerations of fuel or oxidizer, indicating that the particles were well mixed and distributed, validating the preparation procedure. Furthermore, this good homogeneity paves the way for the next step of thermal characterization.
Thermal behaviour
Simultaneous DSC/TGA was performed for prepared GO and different nanothermite formulations. Thermal behaviour of GO is illustrated in Fig. 2.
TGA curve shows that the mass loss of GO happens in three stages. The evaporation of the interstitial water entrapped inside GO, which corresponds to the endothermic in the DSC curve, causes the first mass loss of up to 100 °C. Second mass loss from 100 to 50 °C is ascribed to degradation of oxygenated groups of GO and production of graphite (C). The second mass loss is consistent with the exothermic peak observed slightly above 200 °C in DSC curve. The breakdown of carbonyl groups produced on the surface of GO during the oxidation process results in third mass loss up to 1100 °C [27, 28]. The thermal decomposition of GO/Al/KClO4 nanothermite is illustrated in Fig. 3.
As shown in Fig. 3a, the GO/Al/KClO4 nanothermite decomposes in two phases. Above 200 °C, a first and steep mass loss is linked to GO decomposition as previously clarified. The primary thermite reaction in GO/Al/KClO4 exhibits a second mass loss from 400 to 550 °C [29].
Figure 3b depicts three endothermic and one exothermic event in the thermal breakdown of Al/KClO4. At around 302 °C, a first endothermic peak appears, which is attributed to the phase transition of KClO4 from a rhombic to a cubic structure [30]. The major thermite reaction between Al and KClO4 is represented by an exothermic second peak, which is accompanied by a substantial mass loss between 500 and 560 °C. The melting of the unreacted Al and KCl reaction product at 660 and 770 °C, respectively, is attributed to the third and fourth endothermic peaks [30].
In the case of GO/Al/KClO4, there was a new exothermic peak at 210 °C, corresponding to the decomposition of GO and confirms TGA findings [31]. The main thermite reaction peak is divided into two consecutive overlapping peaks. First, a decreased and broadened thermite reaction peak indicates staged reaction pathways between decomposition product of GO (graphite) and nanothermite components (i.e., C–O, C–KClO4 reactions). These reactions effectively help accelerate the occurrence of the subsequent thermite reactions (Al–KClO4 and Al–O). Moreover, C–O and C–KClO4 reactions clarify the increase in energy released and the decrease in its reaction onset temperature with the addition of GO. The thermal decomposition of GO/Al/K2S2O8 nanothermite is illustrated Fig. 5.
The TGA graphs of Al/K2S2O8 nanothermites with and without GO show that decomposition occurs mainly in three stages and is demonstrated in Fig. 4a. First, the mass loss in nanothermite samples occurred in two mass reduction sub-steps at 270 °C and 310 °C. This mass loss is attributed to the thermal decomposition of K2S2O8 to K2S2O7 and O2. Second, mass loss occurring in the temperature range of 420–610 °C is assigned to the major thermite reaction. The final decomposition step began at ~ 1050 °C and corresponded to the decomposition of K2SO4 to SO2, O2 and K.
The thermal decomposition of GO/Al/K2S2O8 nanothermite is characterized by three endothermic and three exothermic peaks appearing in Fig. 4b. The first endothermic peak is displayed at approximately 340 °C and is ascribed mainly to the K2S2O7 phase change and decomposition of K2S2O8 to K2S2O7 and O2 (small amount) [32]. The second endothermic peak appears at ~ 640 °C and corresponded to the melting of unreacted Al. The third endothermic peak characterized the melting of K2SO4 followed by decomposition to SO2, O2 and K [32]. On the other side, GO showed a non-negligible exothermic peak at 200 °C, suggesting the removal of the oxygenated group in GO and confirming TGA findings. The second exothermic peak mainly resulted from the decomposition reaction of K2S2O8 to K2S2O7 and O2. This reaction is highly exothermic in comparison with the other metallic oxidizers, which have an endothermic decomposition reaction. Nanothermite of GO/Al/K2S2O8 had an exothermic peak at ~ 410 °C resulting in a 12% mass loss and suggesting the thermite reaction occurrence. Inclusion of GO on Al/K2S2O8 enhances the reactivity of the nanothermite due to the increasing heat released and the decreasing reaction onset temperature. The addition of GO improved the total energy output from GO/Al/K2S2O8; however, it still had a lesser effect compared to GO/Al/KClO4. The lesser effect of GO on GO/Al/K2S2O8 can be illustrated by the capability of K2S2O8 to oxidize nano-C in the mixed suspension before ignition [33, 34]. Therefore, the GO/Al/K2S2O8 nanothermite loses the advantage of the high energy output from other suggested reaction paths, like (C–O, C–KClO4) in GO/Al/KClO4, in accelerating the main thermite reaction. So, the enhanced available energy of the GO/Al/K2S2O8 nanothermite will be mainly directed to improving both the fuel-oxidizer contact and the reactant mass diffusion. The decomposition of the GO/Al/KIO4 nanothermite and its reference sample is shown in Fig. 5.
Figure 5a clarifies that the GO/Al/KIO4 nanothermite decomposes in two steps. First, KIO4 decomposes to KIO3 and O2, which occurs at temperature 340 °C. Second, the decomposition step at temperature 550 °C represents the main thermite reaction with a mass loss of 15%.
The thermal decomposition of the GO/Al/KIO4 nanothermite is illustrated in Fig. 4b and exhibits three exothermic and two endothermic peaks. The exothermic peak appearing at a temperature of 340 °C is earmarked for the decomposition of KIO4 to KIO3 and releases oxygen. This temperature is less than the one reported in literature [35], which can be ascribed to the effect of GO as EM by itself and its high surface area, which enhances the contact between the fuel and oxidizer. Although the decomposition of KIO4 is exothermic (80 J g−1), it is still less reactive than K2S2O8 but more reactive than other metallic oxidizers. The endothermic peak displayed at temperature 530 °C is characterized by the decomposition of KIO3 to potassium iodide (KI) and oxygen. The other two exothermic and endothermic peaks resulted from the decomposition of GO at 200 °C, the main thermite reaction at 550 °C and the melting of unreacted Al at 660 °C. It clearly appears in Fig. 5b that GO not only enhanced the reactivity of the Al/KIO4 nanothermite by shifting the reaction onset temperature to a lower one, but also increased the energy released, which was calculated from the integration of the area under the peak. The heat released for the GO/Al/KIO4 nanothermite increased by approximately 30% compared to the reference one. Furthermore, the increasing percentage of the heat released in GO/Al/KIO4 is less than that in the case of GO/Al/KClO4. The high enthalpy of the side reaction (C-KClO4), which was recorded at approximately 154 kJ mol−1, is two times larger than (C-KIO4), which has an enthalpy of reaction equal to 72 kJ mol−1) [36], and is considered the main reason for the lower increase in the energy output of GO/Al/KIO4 compared to GO/Al/KClO4. Figure 6 demonstrates the TGA/DSC of GO/Al/KMnO4 nanothermites.
The mass loss of GO/Al/KMnO4 is considered in two phases. The first phase is attributed to the decomposition of KMnO4 at a temperature of 260 °C to K2MnO4 and O2 accompanied by a mass loss of about 10% [37]. In addition, further decomposition of K2MnO4 to K3MnO4 and O2 occurs at 520 °C followed by the main thermite reaction as illustrated in Fig. 6a [38, 39].
Figure 6b indicates that the thermal decomposition of the GO/Al/KMnO4 nanothermite has three exothermic peaks resulting from the decomposition of GO, KMnO4 and the main thermite reaction. The reactivity of the GO/Al/KMnO4 nanothermite is enhanced by the addition of GO and using n-Al. This appeared in the thermite exothermic peak in the DSC plot compared with the previous results reported by Chen Wei et al., which stated that the reaction of the micro-Al size with the decomposition products of KMnO4 will not occur until adding Mg as a secondary fuel to enhance micro-Al sensitivity to react with KMnO4 [40]. The other two endothermic peaks are characterized by the second decomposition step of K2MnO4 to K3MnO4 and O2 and the melting of unreacted Al fuel. Thermal analysis for the decomposition of the GO/Al/NH4NO3 nanothermite and its reference sample is demonstrated in Fig. 7.
The thermal gravimetric analysis of GO/Al/NH4NO3 is reflected in two steps as shown in Fig. 7a. The first step is attributed to the decomposition of NH4NO3, within which several thermal events occur, begins with crystallographic transformation at 67 °C and ends with maximum rate of decomposition at ~ 290 °C (~ 60% mass loss). The second step is assigned to the main thermite reaction starting at 620 °C and ending at 750 °C with a 7% mass gain.
Figure 7b shows the DSC curve of GO/Al/NH4NO3 spanning a temperature range of 30 °C to 1100 °C, where multiple thermal events occur. During the heating of GO/Al/NH4NO3, four endothermic and three exothermic peaks are recognized, with just one exothermic peak for the Al/NH4NO3. The unexpected small peaks at low temperature in DSC were likely resulted from the heat activated and insignificant atomic re-arrangement within the ternary mixtures. The crystallographic transformations IV → II and II → I produce the first two endothermic peaks at 67 and 127 °C, respectively. Near 170 °C, the third endothermic peak, which corresponds to the melting point of ammonium nitrate, occurs, and breakdown begins. At around 290 °C, the fourth peak, which indicates to the maximal rate of decomposition, is attained [41]. On the other side, the first exothermic peak is characterized by the decomposition of GO and appears at 200 °C. The second exothermic peak may be attributed to the intermediate reaction between the decomposition products of GO and Al and both first and second exothermic peaks do not appear in the Al/NH4NO3 reference sample. The third exothermic peak is assigned to the main thermite reaction between the decomposition products of NH4NO3 and Al fuel. So, we can observe that the inclusion of GO has a good catalytic effect on the performance of Al/NH4NO3 nanothermite. The effect of GO on the thermal decomposition of nanothermites based on metallic oxidizers (Fe2O3, CuO and WO3) and n-Al is shown in Fig. 8.
The thermal decomposition of GO/Al/ Fe2O3 occurs with two exothermic peaks as shown in Fig. 8a. Generally, the initial energy released was observed at a temperature of 550 °C in the first exothermic peak and a milder energy was released in the second exothermic hump, which represents the main thermite reaction at a temperature of 690 °C [25]. The effect of the addition of GO on Al/Fe2O3 is noted in enhancing the reactivity of Al/Fe2O3 by increasing the energy output in the first decomposition step and shifting the main thermite reaction to occur on the first stage and, consequently, increase the energy output.
Figure 8b illustrates the thermal decomposition of GO/Al/CuO with three remarkable exothermic peaks and one endothermic peak, which corresponds to the melting of unreacted Al. The first exothermic peak is characterized by the decomposition of GO at approximately 200 °C. The second small exothermic peak at 300–350 °C is attributed to the moisture removal with heat treatment [42]. The third exothermic peak with a maximum peak temperature at 565 °C is assigned to the major thermite reaction of GO/Al/CuO. The heat released from GO/Al/CuO is 2.7 times greater than that of Al/CuO measured by Sharma et al. [42]. The exothermic energy release of the GO/Al/CuO nanothermite increased and the reaction onset temperature decreased with the addition of GO due to increased intimate contact and effective heat transfer between the Al and CuO by the GO assisted oxidation of Al nanoparticles through oxygen liberation from GO decomposition.
Figure 8c shows that DSC curve of GO/Al/WO3 is punctuated by four exothermic peaks and only one endothermic peak which is ascribed to the melting of Al. As mentioned before, the exothermic peak at 200 °C is assigned to the decomposition of GO. Three consecutive broad peaks after the melting of Al are described by the thermite reaction. Based on previous studies, the main thermite reaction of Al/WO3 occurred at approximately 1000 °C and released most of thermite reaction heat [43]. Here, it is noted that the addition of GO enhanced the energy released in the first and second steps and decreased the energy output from the third peak, which means that ignition occurs at a lower temperature and overall reactivity will be enhanced.
Heat of combustion of nanothermites
The combustion energy was determined using the usual operating techniques provided in reference [44]. Each test was carried out five times, with the average heat of combustion determined. The sample mass for the first two tests was 0.1 g, then raised to 0.3 g for the next two tests, and finally to 0.5 g for the last test. Table 3 lists the experimentally determined heat released values.
Results demonstrated in Table 3 point out that the energy of combustion increased with the addition of GO. Again, this would be ascribed to the effect of GO on decreasing the diffusion distance between fuel and oxidizer, improving the dispersion of n-Al particles and preventing their agglomeration through the course of oxidizers. In addition to the exothermic decomposition of GO at 200 °C, which consequently catalyses the thermite reaction through C–O and C-oxidizer reaction pathways. As a result, GO serves as a secondary fuel, providing extra energy to the system while also assisting in the completion of n-Al combustion and compensating for the loss in active Al content caused by the Al2O3 shell. Among the other samples, the heat generated by the GO/Al/KClO4 nanothermite has the highest value, showing that the combustion of Al in GO/Al/KClO4 is more complete than in other oxidizers.
The literature data provided in [47, 48] verified our observation that the theoretical and experimental heat of reaction values varied significantly. Furthermore, our measured heat of combustion for Al/KClO4 is very similar to the literatured value, indicating that the testing methodologies were successful and reliable.
The difference in heat of reaction between measured and theoretical values is attributed to the fact that the latter is always calculated for Al metal, not n-Al, which has completely different combustion behaviour. Also, in theoretical calculations, we do not take in our consideration the effect of alumina layer that serves as diffusion barrier, heat sink, hindering the combustion reaction of n-Al and this will lead to an increase the calculated energy output values [26]. Furthermore, discounting the influence of heat loss produced by phase transitions results in a misleading increase in theoretically computed heat of combustion values [45, 46]. Finally, one of the other causes of a discrepancy between theoretical and experimental heat of reaction is measurement mistakes, such as thermometer reading errors.
Nanothermite reaction kinetics
To ensure safe processing, handling and storage of nanothermites, in addition to avoiding hazards associated with their thermal decomposition, stability and ignition, a kinetics evaluation should be carried out. Arrhenius parameters for the thermal decomposition reaction of nanothermite compositions were calculated using Kissinger’s method according to Eq. (1) [49].
where β is the heating rate of the DSC experiment; Tp is the maximum temperature of the exothermic decomposition peak at the differential heat rate of the DSC experiment; E, A and R the activation energy, the pre-exponential factor and the universal gas constant, respectively. Calculated value of activation energy was confirmed through Ozawa’s method according to Eq. (2).
For each heating rate and nanothermite composition, ln (β/T 2p ) was plotted against (1/Tp) as illustrated in Fig. 9.
Figure 9 demonstrates the relationship between ln (β/T 2p ) and (1/Tp) is a straight line obtained by linear regression through the data points. The slope and intercept of the line are used to compute activation energy and pre-exponential factors for each composition. The results tabulated in Table 4 represent the kinetic parameters among all nanothermite samples.
Results demonstrated in Table 4 show that the absolute values of the correlation coefficients of all nanothermites are greater than 0.99. Furthermore, the highest activation energy and pre-exponential factor achieved by a nanothermite is based on GO/Al/NH4NO3, while those based on GO/Al/K2S2O8 represent the lowest one. Generally, nanothermites based on oxidizing salts have a lower activation energy compared with those based on metallic oxidizers, such as Al/Fe2O3, Al/Bi2O3, Al/MnO3, Al/NiO, and Al/MnO2, which were reported at 248, 222, 205, 217, and 342 kJ mol−1, respectively [15, 50, 51]. As well, the addition of GO could reduce the activation energy of nanothermites. For example, composition based on GO/Al/KClO4 has an activation energy (135 kJ mol−1), which is lower than all metallic oxides based nanothermites and even lower than this (Al/KClO4) without GO, which recorded (145 kJ mol−1) [30]. Reducing the activation energy of Al nanoparticle-based thermites can be illustrated as follows. Firstly, before the thermite reaction occurs, nanothermite components aggregate forming agglomerations. Then, the thermal decomposition of GO happens with the liberation of oxygen through its oxygenated function groups, which could be disruptive to the aggregation of nanothermite constituents. To some extent, this step is similar to a second mixing cycle in that it disperses the nanothermite mixture more evenly. Furthermore, the exothermic combustion process will benefit from the gaseous oxygen generated by GO degradation. The exothermic nature of the decomposition of oxygenated salts, which typically occurs before the main thermite reaction as shown in the TGA/DSC data, might represent a sort of pre-ignition that decreases the system's activation energy.
Nanothermite reaction mechanism
Although it is difficult to determine the reaction mechanisms of thermite formulations that have been developed because of their energetic nature, it is necessary to have a solid understanding of their reaction mechanisms in order to know the impact of nanothermite compositions on the ignition and reaction characteristics and, hence, control their combustion behaviour. Generally, whatever the type of oxidizer, thermite reaction mechanisms can be mainly classified into either condensed phase reaction (solid–solid reaction) or heterogeneous reaction (gas–solid reaction) mechanisms. The condensed phase reaction governs most thermite composites based on metallic oxidizers [52,53,54,55]. In this mechanism, the decomposition of the oxidizer to liberate oxygen will not occur and the reaction ensues at the interface between the two solid reactants (fuel and oxidizer) through direct contact and ion transfer. In the heterogeneous reaction mechanism, on the other hand, first the oxidizer decomposes to release oxygen, then gaseous oxygen migrates to n-Al particles and diffuses through the alumina shell until reaching the Al core where ignition takes place.
TGA/DSC will be used to identify the thermite reaction mechanism by correlating the activation energy and exothermic peaks. From TGA/DSC outputs, the relationship between the oxygen release temperature of different salt and metallic oxidizers and the onset temperature of the corresponding nano-Al-fuelled thermites is demonstrated in Fig. 10.
Figure 10 illustrates that all of the oxidizing salts, in addition to CuO based nanothermites, have an onset temperature before the melting point of Al. These results indicate that the heterogeneous solid–gas reaction is the dominant reaction mechanism and the oxygen release from oxidizers, in addition to the rate of Al diffusion outwards from the alumina shell will be the rate determining steps of the reaction. In [34], the authors found two contradictive effects. On the one hand, the ignition of nanothermites based on Al and any type of salts (KClO4, KNO3, K2S2O8, etc.) approximately occurred near the melting point of Al. On the other hand, the reaction onset temperature of those based on carbon as a fuel instead of Al rose close to the oxygen release temperature from the oxidizers. Thus, the addition of GO in the developed nanothermites illustrates the decrease in the activation energy and onset temperature due to the reactivity of carbon, which has no oxide shell and the fuel is directly accessible to the oxidizer. Furthermore, the presence of GO in the energetic composite decreases the dependence of the ignition mechanism on the Al diffusion rate and makes it more reliant on the oxygen release temperature. Also, GO shifted the onset temperature of both Al/Fe2O3 and Al/WO3 nanothermites to a lower temperature in the direction of the solid–gas reaction mechanism. The condensed phase reaction mechanism, however, is their primary ignition mechanism where the ignition occurs at a temperature higher than the melting point of Al. Based on the above, the expected reaction mechanism is explained in Fig. 11.
Here, we summarize the steps of the ignition process, which confirms the TGA/DSC findings and calculated activation energy results. First, the ignition process starts by the decomposition of GO at 200 °C with the liberation of oxygen. Then, the reaction between the nanothermite’s oxidizer and carbon to form CO and CO2 gaseous products occurs and releases energy. The formed CO and CO2 play an important role in enhancing the gas–solid reaction mechanism and gas diffusion within the developed nanothermite composites. Moreover, they both improved the energy release as clarified in Eqs. (1&2) and resulted in less agglomerated reaction products [56, 57].
Afterwards when the temperature reached the oxidizer decomposition temperature (TOX), decomposition occurred with the liberation of oxygen. Finally, the released oxygen diffused through the alumina shell and interacted with outwardly diffusing Al atoms to ignite the nanothermite composite. In the case of the GO/Al/KClO4 nanothermite, KClO4 melted prior to ignition and the melting of Al and hence, the initiation of the thermite happened by the interaction of bound oxygen in the molten KClO4 and diffused Al in the alumina shell. Once the ignition begins, the heat released from the reaction catalyses the generation of gaseous oxygen, which will then be complete and be the dominant reaction mechanism.
Conclusions
In this paper, the thermal behaviour reaction mechanism study and kinetic analysis of the tertiary nanothermite mixtures based on Al, GO and different salt and metallic oxidizers were performed using DSC, TGA and reaction bomb calorimetry. The findings of this study showed that the initial decomposition temperature of all prepared nanothermites shifted to a lower temperature compared to their reference formulations. Additionally, the heat of combustion significantly improved and the GO/Al/KClO4 nanothermite achieved the highest value (9.6 kJ g−1). This is in a good agreement with the TGA/DSC analysis and theoretical calculations. Thermite reactions between n-Al and various salts occurred before the n-Al melted, according to the TGA/DSC measurements. As a result, the heterogeneous (solid–gas) reaction mechanism controls compositions based on oxidizing salts, while the condensed phase reaction mechanism controls compositions based on metallic oxides. Finally, the kinetic parameters of nanostructure thermites were estimated by applying the Kissinger and Ozawa isoconversional method. The activation energy measured by the Ozawa method is slightly higher than that of the Kissinger method, but still has reference value to some extent. The tertiary mixes' possible causes and processes were investigated. The nanothermite components were mixed more uniformly without noticeable agglomerates due to exothermic thermal degradation of GO and oxygenated salts, activation energy was lowered, and the overall combustion process was enhanced. These findings should serve as guidelines for formula design, safety, storage, and handling of nanothermites, resulting in improved performance in actual applications.
References
Trunov MA, Schoenitz M, Dreizin EL. Effect of polymorphic phase transformations in alumina layer on ignition of aluminium particles. Combust Theory Model. 2006;10(4):603–23.
Rai A, Park K, Zhou L, Zachariah MR. Understanding the mechanism of aluminum nanoparticle oxidation. Combust Theory Model. 2006;10(5):843–59.
Schoenitz M, Patel B, Agboh O, Dreizin EL. Oxidation of aluminum powders at high heating rates. Thermochim Acta. 2010;507–508:115–22.
Chowdhury S, Sullivan K, Piekiel N, Zhou L, Zachariah MR. Diffusive vs ex- plosive reaction at the nanoscale. J Phys Chem C. 2010;114(20):9191–5.
Levitas VI, Asay BW, Son SF, Pantoya M. Melt dispersion mechanism for fast reaction of nanothermites. Appl Phys Lett. 2006;89:071909.
LeSergent L., Tailoring the ignition and reaction properties of Cu2O thermite nanolaminates, In mechanical engineering-nanotechnology. 2018, Waterloo: Waterloo, Ontario, Canada. pp. 1–97
Kim SH, Zachariah MR. Enhancing the rate of energy release from nanoenergetic materials by electrostatically enhanced assembly. Adv Mater. 2004;16(20):1821–5.
Pantoya ML, Granier JJ. Combustion behavior of highly energetic thermites: nano versus micron composites. Propellants Explos Pyrotech. 2005;30(1):53–62.
Dreizin EL. Metal-based reactive nanomaterials. Prog Energy Combust Sci. 2009;35(2):141–67.
Jian G, Snehaunshu C, Sullivan K, et al. Nanothermite reactions: Is gas phase oxygen generation from the oxygen carrier an essential prerequisite to ignition? Combust Flame. 2013;160(2):432–7.
Wu C, K.S., Chowdhury S, Jian G, Zhou L and Zachariah MR, Encapsulation of Perchlorate Salts within Metal Oxides for Application as Nanoenergetic Oxidizers,. Adv Funct Mater. 2012; 22(1): 78–85.
Kappagantula KS, Farley C, Pantoya ML, Horn J. Tuning energetic material reactivity using surface functionalization of aluminum fuels. J Phys Chem C. 2012;116:24469–75.
Granier JJ, Pantoya ML. Laser ignition of nanocomposite thermites. Combust Flame. 2004;138:373–83.
Dean SW, Pantoya ML, Gash AE, Stacy SC, Hope-Weeks LJ. Enhanced convective heat transfer in nongas generating nanoparticle thermites. J Heat Transf. 2010;132:111201.
Wen JZ, Ringuette S, Bohlouli-Zanjani G, Hu A, Nguyen NH, Persic J, Pe-tre CF, Zhou YN. Characterization of thermochemical properties of Al nanoparti-cle and NiO nanowire composites. Nanoscale Res Lett. 2013;8:184.
Sullivan KT, Chiou WA, Fiore R, Zachariah MR. In situ microscopy of rapidly heated nano-Al and nano-Al/WO 3 thermites. Appl Phys Lett. 2010;97(13):133104.
Sullivan KT, Piekiel NW, Wu C, Chowdhury S, Kelly ST, Hufnagel TC, Fezzaa K, Zachariah MR. Reactive sintering: an important component in the combustion of nanocomposite thermites Combust. Flame. 2012;159(2):15.
Balandin AA, Ghosh S, Bao WZ, Calizo I, Teweldebrhan D, Miao F, Lau CN. Superior thermal conductivity of single layer graphene. Nano Lett. 2008;8:902–7.
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA. Two-dimensional gas of massless diracfermions in graphene. Nature. 2005;438:197–200.
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS. Graphene based composite materials. Nature. 2006;442:282–6.
Lee C, Wei XD, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–8.
Yan Q-L, Gozin M, Zhao F-Q, Cohena A, Pang S-P. Highly energetic compositions based on functionalized carbon nanomaterials. Royal Soc Chem Nanoscale. 2016;8:4799–851.
Ferrari AC, Bonaccorso F, Fal’ko V, et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 2015;7:4598–810.
Thiruvengadathan R, Chung SW, Basuray S, Balasubramanian B, Staley CS, Gangopadhyay K, and Gangopadhyay S, A Versatile Self-Assembly Approach toward High Performance Nanoenergetic Composite Using Functionalized Graphene. Langmuir, American Chemical Society, 2014.
Yan N, Qin L, Hao H, Hui L, Zhao F, Feng H. Iron oxide/aluminum/graphene energetic nanocomposites synthesized by atomic layer deposition: Enhanced energy release and reduced electrostatic ignition hazard. Appl Surf Sci. 2017;408:51–9.
Fahd A, Dubois C, Chaouki J, Wen JZ, Youssef E. Synthesis and characterization of tertiary nanothermite CNMs/Al/KClO4 with enhanced combustion characteristics. Propellants Explos Pyrotech. 2021;46:995–1005.
Mokhtar MM, SAAEE, Hassaan MY, Morsy MS and Khalil MH, Thermally Reduced Graphene Oxide: Synthesis, Structural and Electrical Properties. Int J Nanoparticles Nanotech, 3:008, 2017. 3(1): p. 1–9.
Patil V, R.V.D., Rout TK, Banerjee S and Yadav GD Graphene oxide and functionalized multi walled carbon nanotubes as epoxy curing agents: a novelsynthetic approach to nanocomposites containing active nanostructured fillers, RSC Adv, 4: 49264–49272 (2014)
Peng-Gang Ren, D.-X.Y., Xu Ji, Tao Chen and Zhong-Ming Li (2011) Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology. 22: 1–8
Manoochehr Fathollahi HB. A comparative study of thermal behaviors and kinetics analysis of the pyrotechnic compositions containing Mg and Al. J Therm Anal Calorim. 2015;120:1483–92.
Yin K, Li H, Xia Y, Bi H, Sun J, Liu Z, Sun L. Thermodynamic and kinetic analysis of low-temperature thermal reduction of graphene oxide. Nano-Micro Lett. 2011;3(1):51–5.
Zhou W, DeLisio JB, Li X, Liu L, Zachariah MR. Persulfate salt as an oxidizer for biocidal energetic nano-thermites. J Mater Chem. 2015;3(22):11838–46.
Liu P, Wang T. Ultrasonic-assisted chemical oxidative cutting of multiwalled carbon nanotubes with ammonium persulfate in neutral media. Appl Phys A (Mater Sci Process). 2009;97:771–5.
Zhou W, De Lisio JBD, Wang X, Zachariah MR. Reaction mechanisms of potassium oxysalts based energetic composites. Combust Flame. 2017;177:1–9.
Oxley JC, Smith JL, Porter MM, Yekel MJ, Canaria JA. Potential Biocides: Iodine-Producing Pyrotechnics. Propell Explos Pyrot. 2017;42:1–18.
Pauling, L, General Chemistry, Dover Books on Chemistry. 2014.
Herbstein FH, Ron G, Weissman A, The thermal decomposition of potassium permanganate and related substances. Part I. Chemical aspects. J. Chem. Soc. A, 1971: 1821–1826.
Brown ME, Sole KC, Beck MW. Isothermal DSC study of the thermal decomposition of potassium permanganate. Thermochim Acta. 1985;89:27–37.
Becerra ME, Arias NP, Giraldo OH, López Suárez FE, Illán Gómez MJ, Bueno López A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl Catal B Environ. 2011;102:260–6.
Wei C, JW, Pingyun L, Li L, Binhua C, Junjun D, Longxiang W, Yuan Y, Fengsheng L, Ignition and Combustion of Super-Reactive Thermites of AlMg/KMnO4. Rare Metal Mater Eng, 2013. 42(12): 2458–2461
Gunawana R, Freij S, Zhang D, Beach F, Littlefair M. Amechanistic study into the reactions of ammonium nitrate with pyrite. Chem Eng Sci. 2006;61:5781–90.
Sharma M, Sharma V, Effect of carbon nanotube addition on the thermite reaction in the Al/CuO energetic nanocomposite, in Philosophical Magazine 2017, Taylor & Francis Group. pp. 1921–1938
Yi W, Jiang W, Cheng Z, Chen W, An C, Thermite reactions of Al/Cu core-shell nanocomposites with WO3. Thermochimica Acta, 2007; 463: 69–76.
Shoemaker D.P., C.W.G., Steinfeld J.I., and Nibler J.W., Experiment 7, In Experiments in Physical Chemistry. 1981, McGraw-Hill: New York, NY. pp. 125–138
Fischer SH, G.M.C., A survey of combustible metals, thermites, and intermetallics for pyrotechnic applications, In 32nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference, July 1996: Lake Buena Vista, Florida, USA
Grubelich SH, Grubelich MC, Theoretical energy release of thermites, intermetallics, and combustible metals, In 24th International Pyrotechnics Seminar. July 1998: Monterey, CA.
Farley CW, MLP, Losada M, and Chaudhuri S, Linking molecular level chemistry to macroscopic combustion behavior for nano-energetic materials with halogen containing oxides. J Chem Phys, 2013. 139: 1–8.
Farley C, Reactions of Aluminum with Halogen Containing Oxides, In Mechanical Engineering. May 2013, Texas Tech University: USA. p. 74.
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem, 29(6): 1702.
Puszynski JA. Processing and characterization of aluminum-based nanothermites. J Therm Anal Calorim. 2009;96:677–85.
Song J, T.G., Yao M, Ding W, Zhang X, Bei F, Tang J, Huanga J and Yu Z, Thermal behavior and combustion of Al nanoparticles/MnO2-nanorods nanothermites with addition of potassium perchlorate. RSC Advances, 2019. 9: 41319–41325.
Jacob RJ, D.L.O.-M., Overdeep KR, Weihs TP, Zachariah MR, Incomplete reactions in nanothermite composites. J Appl Phys, 2017; 121: 054307.
Sullivan KT, W.-A.C., Fiore R, Zachariah MR, In situ microscopy of rapidly heated nano-Al and nano-Al/WO3 thermites. Appl Phys Lett, 2012; 97: 133104.
GC Egan, LaGrange T, Time-resolved nanosecond imaging of nanoscale condensed phase reaction. J Phys Chem, 2015; 119: 2792–2797.
Jacob RJ, Jian G, Guerieri PM, Zachariah MR. Energy release pathways in nanothermites follow through the condensed state. Combust Flame. 2015;162:258–64.
Sherif Elbasuney AF, Hosam E. Mostafa, Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture. Fuel. 2017;208:296–304.
Elbasuney S, Gaber Zaky M, Radwan M, Mostafa SF. Stabilized super-thermite colloids: a new generation of advanced highly energetic materials. Appl Surf Sci. 2017;36:328–419.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fahd, A., Dubois, C., Chaouki, J. et al. Combustion behaviour and reaction kinetics of GO/Al/oxidizing salts ternary nanothermites. J Therm Anal Calorim 147, 10245–10257 (2022). https://doi.org/10.1007/s10973-022-11259-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11259-x