Abstract
Stability of nanofluids is one of the major challenges for their real-world applications and benefits. Although ultrasonication and addition of surfactant are commonly used to obtain better stability of nanofluids, there is a lack of adequate knowledge on the effects of various parameters and duration of ultrasonication as well as some other influences of surfactant. The effect of ultrasonication on the dispersion of nanoparticles and agitation as well as temperature on the thermal conductivity measurements of aqueous TiO2 nanofluids was experimentally studied. An UV–Vis absorbance analysis was performed to identify the degree of dispersion of nanoparticles (stability) and also to determine the right amplitude as well as the duration of the ultrasonication. In addition, agitation of nanofluids during the measurement of thermal conductivity showed a serious adverse effect as significant fraction of nanoparticles adhered to both the probe and the wall of the sample container. Furthermore, present results showed that the enhanced thermal conductivity of this nanofluid further increases noticeably with increasing temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, nanofluids have received huge interest from researchers and industrial people and thus extensive research works have been performed on various areas of these engineered fluids [1,2,3]. Despite some good developments in some areas, the real usages of nanofluids in applications particularly in thermal management systems remain very challenging and beyond the reach mainly due to not yet well-understanding the underlying mechanisms and also not having long-term stability of nanofluids [4, 5]. In spite of nanofluids exhibiting superior thermophysical properties such as thermal conductivity, thermal diffusivity, heat capacity and viscosity, there remains controversy and serve inconsistencies in reported results on these properties [3, 6,7,8,9,10,11]. If nanoparticles are not well-dispersed and stable for long period in host fluids, nanofluids can neither exhibit desired high thermophysical properties nor can be used in real applications particularly in closed systems. Thus, it is of great importance to make sure that the added nanoparticles are properly (homogenously) dispersed in base fluids and nanofluids have long stability. However, it is very challenging to achieve a long-term stability of nanofluids as many factors such as nanofluids preparation, nanoparticles types, size shapes, purity and degree of agglomerations as well as properties of base fluids are involved in this process. Various techniques, which include sonication, surfactant addition, agitation and surface treatment of nanoparticles, are commonly employed for better stability of sample nanofluids. However, in most cases nanofluids undergo sonication as well as having addition of various surfactants into them. Both of these means need to be carefully studied and understood before applying them to the preparation of nanofluids for their better stability and properties without changing the chemistry of nanofluids and the original structures of nanoparticles [4, 5, 12]. On the one hand, addition of surfactant should be avoided as it can change the chemical composition and some properties of the nanofluids at different conditions (temperature and pressure) besides making nanofluids a three-phasic (nanoparticles, base fluids and surfactant) complex system. In case surfactant is to be used, its adverse effects due to adding wrong and excessive amount (must be close to its critical micelle concentration) need to be accounted for.
On the other hand, though ultrasonication is most widely used in nanofluids studies, there is a lack of adequate knowledge on the effects of its various parameters and duration of use. For instance, the effects of amplitude, frequency, power settings, etc., of the ultrasonicator and its duration of use on the samples and their properties particularly on thermal conductivity are neither well understood nor carefully studied in the literature. Ultrasonication treatment for dispersion of particles in liquids can be done through both direct sonication using ultrasound probe (or horn) and indirect sonication (ultrasonic bath). However, according to a standard dispersion protocol probe-based direct ultrasonication is recommended for better dispersion of nanoparticles [13]. Since such ultrasonication can not only breakdown the agglomerations but can also create agglomeration of the particles and may also result in some kind of chemical reactions, it is very important to determine the optimal sonication parameters or conditions which also differ considerably for different systems (e.g., brand, models, types, etc.). For nanofluids’ sonication treatment, such parameters include sonication time, amplitude, probe type and dimensions, container geometry and tip immersions, volume and concentration of sample nanofluids as well as sample temperature. These parameters or conditions demonstrate that dispersion of nanofluids using ultrasonication is a complete procedure, which requires careful optimization and good knowledge about the whole procedure. In addition, the parameters optimization and ultimate dispersion results of the ultrasonication treatment need to be properly assessed by the post-processing of the dispersion by determining the agglomeration size and distribution using other techniques and equipments such as dynamic light scattering (DLS) or UV–Vis absorbance and transmission electron microscopy. Despite the fact that most of the studies on nanofluids used ultrasonication for the dispersion of nanoparticles and that there are several studies specifically dedicated to dispersion and stability of nanofluids [14,15,16,17,18], no standard dispersion procedure or protocol for ultrasonication is available for nanofluids and therefore well dispersion of nanoparticles and long-term stability of nanofluids remains very challenging. Nonetheless, there are some standard operating procedures proposed for dispersion of nanopowders using ultrasonication for mostly environmental and health-related assessment (e.g., [13, 19]) that can provide useful information and can be adapted for better dispersion of nanoparticles and to obtain stable nanofluids.
This study aims to identify the influence of ultrasonication and agitation on the dispersion of TiO2 nanoparticles as well as thermal conductivity of their water-based nanofluids. The effect of temperature on thermal conductivity of this nanofluid is also studied besides including preliminary findings of using an ionic liquid (IL) as surfactant in nanofluid.
Sample preparation and experiments
Sample nanofluid was prepared with a two-step method by dispersing a very low concentration (0.1 w/w %) of purchased titanium oxide (TiO2) nanoparticles (IoLiTec, Germany) in water (Milli-Q®). The prepared nanofluid then underwent ultrasonication (Hielscher UP200Ht ultrasonic processor) at various amplitudes and different short time periods (0.5 to 6 min using a pulse cycle of 0.5 s on and 0.5 s pause in order to avoid over-heating of the sample), and the optimum ultrasonication amplitude (%) was obtained. Before each sample preparation, an internal/automatically calibration of the equipment was performed according to the specification of the manufacturer.
A schematic chart of the experimental methodology employed in this study is shown in Fig. 1. Besides visualization, UV–Vis absorbance analysis was performed to identify the optimum dispersion and amplitude of the ultrasonicator and the maximum time of stability of the suspension as well. A double-beam UV–Vis spectroscopy (Hitachi 100-40) at ambient temperature (20 to 25 °C) was employed to determine the effects of added TiO2 nanoparticles and sonication time on the dispersion behavior of nanofluids at λ = 520 nm. It is known that the UV–Vis absorption spectroscopy provides useful information on the degree of dispersion of nanoparticles in nanofluids.
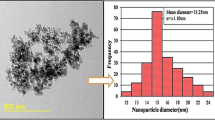
According to the supplier (IoLiTec, Germany), the TiO2 nanoparticles have a spherical shape with an average diameter of 20 nm. The TiO2 nanoparticles morphology was characterized with a scanning electron microscope (SEM) (JEOL, JSM-7100F). However, as can be seen from the SEM images presented in Fig. 2, the nanoparticles have an elongated shape with a diameter between 50 and 200 nm with an approximate average diameter of 100 nm.
After sonication, the thermal conductivity of sample nanofluids with agitation as well as at different temperatures was measured by using a Hukseflux TPSYS08 device in conjunction with probe (non-steady-state thermal conductivity probe). The sample container was a stainless steel cylindrical vessel with a volume capacity of 125 mL.
Results and discussion
Effect of ultrasonic amplitude
As mentioned before, employing sonication (ultra) during nanofluids sample preparation is the most effective and used technique. However, most of the studies failed to realize the importance of parameters of the sonicator used and thus the values of these parameters are missing in most of published articles in the literature. One of such key parameters is the amplitude of the ultrasonicator which mainly controls the degree of dispersion and duration of sonication needed for a particular sample. In this study, sample nanofluids underwent ultrasonication at different amplitudes (20, 40 and 60%). Determination of UV–Vis absorbance with respect to sample elapsed time showed the smallest variation in absorbance at 60% amplitude which indicates the best dispersion of nanoparticles as depicted in Fig. 3 with the sample and sonication information in the inset table. Thus, the optimum amplitude setting of 60% was used in this study.
UV–Vis absorbance and visual inspection
After completing each sonication, the UV–Vis absorbance of sample nanofluids was determined to evaluate the level of dispersion of nanoparticles. Table 1 shows the magnitude of UV–Vis absorbance of a sample nanofluid on the day of preparation and after 5 days at different sonication times. Table 1 indicates an increase in degree of agglomeration/sedimentation of this nanoparticle after 5 days. The increased sedimentation of nanoparticles can more clearly be evidenced from the visual inspection as shown in Fig. 4 which contains images of sample nanofluid at four different sonication times (s) at the day of preparation and after 5 days of preparation of the sample. As can be seen from Fig. 4, almost all nanoparticles are settled down at the bottom of the cuvettes within 5 days. This connotes that more measures have to be taken in order to obtain better dispersion of nanoparticles and thus longer stability of nanofluids.
Agitation effect on thermal conductivity
In order to try to extend the stability time of sample nanofluid during the measurement of the thermal conductivity, a magnetic stirring bar was employed inside the sample container and effect of agitation on the thermal conductivity was also studied. As commonly used, a magnetic stirring bar was employed inside the sample container used in Hukseflux (TPSYS08) device that was used to measure the thermal conductivity of the nanofluid under agitation. A cycle of 50 min of agitation and 15 min of stabilization without agitation, following with the measurement of the thermal conductivity, was used. No noticeable effect of such agitation on the thermal conductivity of this nanofluid was observed as reported in Fig. 5. It can also be seen (Fig. 5) that at the day of preparation (day 1) the thermal conductivity of nanofluid without agitation is slightly higher than that of nanofluids with agitation. This is because of losing nanoparticles to the container wall and probe resulting to decrease the real concentration of nanoparticles in the local measurement region where the measurement probe was inserted. However, almost no change in thermal conductivity of nanofluids at both conditions (with and without agitation) was observed in the following days (days 2 and 3). Nonetheless, all these thermal conductivity data fall within the measurement uncertainties. During thermal conductivity measurement while agitating the nanofluids it was interesting to observe that nanoparticles were adhered to the measurement probe as well as to the wall of the cell container as shown in Fig. 6. This is probably due to the centrifugal force generated by a continuous and homogeneous agitation. This demonstrates that any kind of such agitation during the measurement will neither increase the stability nor have any positive effect on the thermal conductivity of nanofluids.
Temperature effect on thermal conductivity
After performing ultrasonication, thermal conductivity of nanofluid was measured at different temperatures ranging from room temperature to 60 °C. Figure 7 demonstrates that thermal conductivity of water increases due to addition of small concentration (0.1 w/w %) of TiO2 nanoparticles and it increases with temperature. It is also apparent that the enhancement of the thermal conductivity of nanofluids relative to base fluid (water) increases as the temperature increases. This is interesting as nanofluids can perform better heat transfer (like cooling) at high-temperature conditions.
It was found that nanoparticles were adhered to the probe’s surface and the sidewall of the vessel. This result might have affected quantitatively the measurements presented here but not qualitatively.
Using an ionic liquid as dispersant
Our other ongoing study on the measurement of thermal conductivity of the binary mixture of water and 1-ethyl-3-methylimidazolium methanesulfonate ([EMIM][MeSO3]) showed thermal conductivity enhancement of about 5% for the mixture with a mass fraction 2% of this ionic liquid (IL) in comparison with the base fluid (water). Thus, in order to try to increase the enhancement of the thermal conductivity and also to try to extend the stability time of the nanofluid, a base fluid with a composition of 2.24% IL and 97.76% of water was used for the preparation of a new sample nanofluid having 0.1 mass% of TiO2 nanoparticles. However, the results show a rapid decrease in the stability time for less than 1 h making it impossible to measure the thermal conductivity since there is not enough time for temperature stabilization. This could be possibly due to the fact that the ionic liquid forms micelles around the TiO2 nanoparticles. It is possible to see in Fig. 8 (particularly from close-up view in Figure (b)) the formation of deposits in the wall of the cuvette, something that did not happen for the nanofluid prepared with only water (Milli-Q®). Nonetheless, the present IL may not be suitable for TiO2 nanoparticles, and other types of ILs need to be tried in order to identify ILs as dispersing agent for nanofluids.
Conclusions
Several key factors such as ultrasonication for stability, temperature, agitation and surfactant (a new kind, i.e., IL) are experimentally investigated, and they not only influence the stability and thermophysical properties but also possess serious challenges toward the real-world application of nanofluids.
This study demonstrates that regardless of the brand or model of ultrasonication device used, the proper amplitude setting and sonication duration are very crucial for the better dispersion and stability of nanoparticles without any adverse effect.
UV–Vis absorbance is a good and easy technique to study the time duration of the stability and its determination with respect to time showed that the best dispersion of nanoparticles can be obtained at 60% amplitude setting of ultrasonicator for this nanofluid.
The agitation (by magnetic stirrer) was found to have no noticeable influence on the thermal conductivity of this nanofluid. In addition, using agitation to the sample nanofluid during the measurement of thermal conductivity nanoparticles was found to adhere to the measurement probe as well as the wall of the sample container leading to deterioration of the stabilization of the nanofluid. Although the results are not very conclusive, such study on the influence of agitation of nanofluids during measurement is of first kind.
Furthermore, the thermal conductivity of this nanofluid was observed to be higher than that of the pure water, and the enhanced thermal conductivity increased noticeably with increasing temperature.
The preliminary effort to use IL as a surfactant in aqueous nanofluids was not successful for the present IL, and thus no conclusions can be made on this.
The occurrence of particle adhesion to the thermal conductivity probe has to be accounted in the future measurements by considering the extra thermal conductance in the temperature measurement.
Nonetheless, more extensive and systematic studies on the dispersion of nanoparticles and stability of nanofluids as well as their impact on the thermal conductivity of nanofluids are to be performed.
Change history
21 September 2020
A Correction to this paper has been published: https://doi.org/10.1007/s10973-020-10240-w
29 August 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10973-022-11565-4
References
Murshed SMS, Nieto de Castro CA. Nanofluids: synthesis, properties and applications. New York: Nova Science Publishers Inc.; 2014.
Murshed SMS, Nieto de Castro CA, Lourenço MJV, Lopes MLM, Santos FJV. A review of boiling and convective heat transfer with nanofluids. Renew Sustain Energy Rev. 2011;15:2342–54.
Murshed SMS, Nieto de Castro CA. Conduction and convection heat transfer characteristics of ethylene glycol based nanofluids—a review. Appl Energy. 2016;184:681–95.
Lourenço MJV, Vieira SI. Nanofluids preparation methodology. In: Murshed SMS, Nieto de Castro CA, editors. Nanofluids: synthesis, properties and applications, vol. 1. New York: Nova Science Publishers Inc.; 2014. p. 1–28.
Nieto de Castro CA, Vieira SI, Lourenço MJV, Murshed SMS. Understanding stability, measurements, and mechanisms of thermal conductivity of nanofluids. J Nanofluids. 2017;6:804–11.
Murshed SMS, Leong KC, Yang C. Thermophysical and electrokinetic properties of nanofluids—a critical review. Appl Therm Eng. 2008;28:2109–25.
Murshed SMS. Correction and comment on “thermal conductance of nanofluids: is the controversy over?”. J Nanoparticle Res. 2009;11:511–2.
Murshed SMS. Determination of effective specific heat of nanofluids. J Exp Nanosci. 2011;6(5):539–46.
Murshed SMS. Simultaneous measurement of thermal conductivity, thermal diffusivity, and specific heat of nanofluids. Heat Transf Eng. 2012;33:722–31.
Franca JMP, Vieira SIC, Lourenco MJV, Murshed SMS, Nieto de Castro CA. Thermal conductivity of [C4mim][(CF3SO2)2N] and [C2mim][EtSO4] and their ionanofluids with carbon nanotubes: experiment and theory. J Chem Eng Data. 2013;58:467–76.
Murshed SMS, Estellé P. A state of the art review on viscosity of nanofluids. Renew Sustain Energy Rev. 2017;76:1134–52.
Cacua K, Bioucas FEB, Murshed SMS, Lourenço MJV, Santos FJV, Nieto de Castro CA. Effects of agitation and ultrasonication on dispersion and thermal conductivity of aqueous TiO2 nanofluids. In: 1st European symposium on nanofluids; 2017, Lisbon.
Taurozzi JS, Hackley V, Wiesner M. Preparation of nanoparticle dispersions from powdered material using ultrasonic disruption. NIST Spec Publ. 2012;1200:2.
Rao Y. Nanofluids: stability, phase diagram, rheology and applications. Particuology. 2010;8:549–55.
Yu W, Xie H. A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater. 2012;2012:17.
Mahbubul IM, Saidur R, Amalina MA, Elcioglu EB, Okutucu-Ozyurt T. Effective ultrasonication process for better colloidal dispersion of nanofluid. Ultrason Sonochem. 2015;26:361–9.
Pradhan S, Hedberg J, Blomberg E, Wold S, Wallinder IO. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J Nanoparticles Res. 2016;18:285.
Sharma SK, Gupta SM. Preparation and evaluation of stable nanofluids for heat transfer application: a review. Exp Thermal Fluid Sci. 2016;79:202–12.
Alstrup JK, Kembouche Y, Loeschner K, Correia M. SOP for probe-sonicator calibration of delivered acoustic power and de-agglomeration efficiency for in vitro and in vivo toxicological testing, version 1.0. NANoREG. 2014.
Acknowledgements
Authors gratefully acknowledge the help of F.E.B. Bioucas from CQE@Ciencias (currently in FAU Erlangen-Nürnberg, Germany) in performing this work and also to Instituto Tecnológico Metropolitano de Medellín (Colombia) for the SEM analysis of the nanoparticles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The article to which this update relates has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10973-020-10240-w
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cacua, K., Murshed, S.M.S., Pabón, E. et al. RETRACTED ARTICLE: Dispersion and thermal conductivity of TiO2/water nanofluid. J Therm Anal Calorim 140, 109–114 (2020). https://doi.org/10.1007/s10973-019-08817-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08817-1