Abstract
In this paper, the effects of adding vitamin C to biomedical ultra-high molecular weight polyethylene (B-UHMWPE) on thermal behavior and thermal degradation kinetics are investigated. The kinetic studies were conducted using Ozawa–Flynn–Wall (OFW), corresponding to pre-exponential factor (A) and activation energy (Ea). Compounds with 1.0 and 2.0% mass vitamin C exhibited a lower decomposition rate. Activation energy results from the OFW and Kissinger methods were close to each other and showed a dependence on the degree of conversion (α), with Ea being an increasing function of conversion degree to B-UHMWPE and a decreasing function for the compounds. Finally, the pre-exponential factor increases with the addition of vitamin C, favoring its interaction with the free radicals originated from the thermal degradation of B-UHMWPE, also suggesting a reduction in its decomposition rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomedical ultra-high molecular weight polyethylene (B-UHMWPE) has been a widely used polymer in orthopedic applications since the 1990s, especially for joint implants (hip and knee). To this end, the polymer undergoes an ionizing radiation process to create crosslinks on its surface, which increases its resistance to adhesive or abrasive wear [1]. On the other hand, the formation of free radicals also occurs in the radiation process, which leads to oxidation of its macromolecules [2]. Stabilization of crosslinked UHMWPE was also achieved in the 1990s by a re-fusion process that removed residual free radicals. However, this process led to a decrease in the crystallinity of the polymer and a consequent loss in its long-term mechanical strength and toughness [3]. The great interest in rendering the surface of the implant more resistant to wear is due to a concern with debris (i.e., polymer particles) which detach from the implant during its life. These particles can cause tissue osteolysis close to the implanted material, aseptic loosening and instability in the implanted part with consequent fracture [4, 5]. Several studies have shown that the stabilization of radiated UHMWPE has been achieved by adding vitamin E (α-tocopherol), and satisfactory results in improving mechanical resistance and toughness have been observed in experimental and clinical applications [1, 6,7,8].
The antioxidant action of vitamin E is achieved through the donation of a hydrogen atom to the oxidized molecule which occurs without advancing oxidation in its vicinity [9]. Certainly, the stabilization mechanism involves macroradical action from the irradiated UHMWPE, which then reacts with a hydrogen radical from the hydroxyl phenol group of vitamin E [10]. As a consequence, vitamin E stabilizes the peroxyl and alkyl radicals that are present in the polymer [11]. More recently, other natural or synthetic antioxidants have also been investigated to increase the thermal stability of biomedical UHMWPE [12, 13]. In addition, vitamin E has also been used as an antioxidant in other plastics and rubbers [14,15,16]. It is important to note that vitamin E is used in smaller dosages than synthetic antioxidants that have shown excellent antioxidant action [17]. Alternatively, Shen et al. [12] investigated the stabilization of irradiated UHMWPE with natural phenols with multiple phenolic hydroxyl groups and demonstrated significant results.
Several mathematical methods have been used to estimate the activation energy, pre-exponential factor and conversion function to material degradation. Among the most studied methods, Ozawa–Flynn–Wall (OFW), Coats–Redfern (CR) and Kissinger methods can be mentioned. The OFW method evaluates the decomposition of different components in different time periods, allowing for an estimation of the decomposition activation energy (Ea) at each stage of degradation [18]. The Kissinger method evaluates one value of the activation energy in the temperature at maximum degradation rate, being more appropriate for a monotonic material that does not degrade in multiple stages [19].
To the best of the authors’ knowledge, no studies have been conducted to investigate the kinetic parameters associated with the degradation of biomedically modified UHMWPE with vitamin C (ascorbic acid), which is also an important antioxidant that reacts with peroxide radicals from the oxidative stress of organism cells [20, 21]. Moreover, the chemical structure of vitamin C can have a different effect on the thermal stabilization of UHMWPE.
This paper investigates the effects of vitamin C on the thermal stability of B-UHMWPE measured by TG/DTG analysis, as well as the corresponding decomposition kinetic parameters that are evaluated by the Ozawa–Flynn–Wall (OFW) and Kissinger methods.
Experimental
Materials
Biomedical ultra-high molecular weight polyethylene (B-UHMWPE) was kindly donated by the Division of Orthopedic Technology of Baumer SA (Brazil), and vitamin C (ascorbic acid) with 98.3% purity was obtained from Galena Chemicals and Pharmaceuticals (Brazil). Both solids’ components were mixed in different ratios (0.5, 1.0 and 2.0% mass of vitamin C), resulting in powders of B-UHMWPE/Vit C compounds that were labeled as 0.5% Vit C, 1.0% Vit C and 2.0% Vit C, respectively.
Preparation of the samples
For compression molding, B-UHMWPE and B-UHMWPE/Vit C were pressed under 10 MPa and 160 °C for 6 min in a manual hydraulic press in accordance with Souza et al. [22]. The resulting film samples were between 0.31 and 0.35 mm thick.
Characterization of the samples
For thermal characterization, TG analysis was performed on a DTG-60H Shimadzu Thermogravimetry apparatus to study the thermodegradation kinetics of the pure and modified polymer. Approximately 6.0 mg of sample was loaded and heated from 100 to 700 °C with a heating rate of 5, 15 and 30 °C min−1 under an inert atmosphere of 50 mL min−1 of argon flow, considering that Tinitial is the initial temperature and Tmax is the temperature at maximum degradation rate.
For morphological characterization, atomic force microscopy (AFM) analyses were performed on a Confocal Raman-AFM Microscope, model Alpha 300 AR, from WITEC Raman Imaging. An aluminum cantilever (k = 0.2 Nm−1) was used; and 256 points per line were obtained at a scanning speed of 1 s/line for every image. Scanning electron microscopy (SEM) analysis was performed on a Leo 1430 unit from Zeiss detecting secondary electrons. Vitamin C sample was metalized with gold prior to analysis.
Theoretical approach
The thermogravimetry technique provides mass loss data with high reproducibility, allowing for calculations of kinetic parameters to be accurately performed. In this context, mass loss data are recalculated to provide the degree of conversion (α) of a solid sample during its thermal decomposition [23].
Because the heating rate is linear, α is defined as
where m0 is the initial mass, mT is the mass at the decomposition temperature and mf is the mass at the end of the thermal decomposition. The conversion represents the decomposed amount of the sample.
The rate of decomposition is a function of temperature and conversion:
It is possible to rewrite the right side of Eq. (2) by two functions, where the first is temperature-dependent and the second depends on the conversion:
The function k(T) in Eq. (3) is temperature-dependent and is generally expressed by the Arrhenius equation:
where (A) is the pre-exponential factor (min−1), Ea is the activation energy (kJ mol−1), R is the universal gas constant (8.314 J mol−1 K−1) and f(α) is the differential conversion function that is proportional to the concentration of non-degraded material, given by
where n is the order of the decomposition.
For thermal degradation, considering a constant heating rate (linear temperature distribution in time), the heating rate β (K min−1) can be written as follows:
The solid-state non-isothermal decomposition reaction rate is expressed from Eq. (4) as
Integrating both sides of Eq. (7) from the initial temperature T0, and corresponding the degree of conversion when α = 0 to the temperature of the peak T leads to the following expression:
The evaluation of kinetic parameters requires a solution of temperature that is the dependent part of Eq. (8). Consequently, many techniques have been used to solve it, including methods that are based on data from either a single rate or from several rates. For instance, the Kissinger method is a differential-based method, while both OFW and CR methods are integral methods that use various heating rates to determine the values of the kinetic parameters. Due to the mathematical complexity of Eq. (8), Ea and (A) parameters are generally evaluated using mathematical software, such as MATLAB and MAPLE. Although the Kissinger and OFW methods use multiple heating rates, each one adopts different assumptions to interpret the degradation mechanisms of monotonic and multicomponent materials. Studies have shown that in the case of modified polymers as well as composites and blends, more accurate and better understood results are obtained when multiple heating rates are used [24].
Ozawa–Flynn–Wall method (OFW)
The OFW method estimates activation energy as a function of decomposition progress based on weight loss versus temperature data at different heating rates [25]. It does not require prior knowledge of the degradation mechanism steps adopted for the analysis. The activation energy is evaluated for different conversion degree (α), enabling to ascertain the complexity of the decomposition mechanism [26, 27]. The OFW method assumes that the parameters (A), g(α) and Ea are independent of the absolute temperature (T) [25] and is based on the Doyle approximation [28]; therefore, the right-hand value of Eq. (8) can be expressed with respect to the P function:
Substituting Eq. (9) into Eq. (8) provides:
In this case, when (A) and Ea are independent of α, Eq. (10) can be written in logarithmic form as follows:
If E/RT > 20, Eq. (11) can be reduced to:
By plotting ln β versus 1/T for Eq. (12), the slope and the intercept of its linear regression give the values of Ea and (A), respectively.
Kissinger method
This is a differential method based on several heating rates, denoted as β. It assumes that the reaction occurs in the temperature of the peak of the first mass loss derivative (DTG), which corresponds to a maximum temperature (Tmax) that is obtained at different rates [29]. Moreover, the Kissinger method considers Ea/RT “>” 1.
The maximum value of the conversion degree is obtained by defining the time derivative of Eq. (4) as equal to zero, such that \(\frac{\text{d}}{{{\text{d}}t}}\left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}} \right) = 0\). This can be rewritten as
Applying derivatives of the product of functions, the following occurs:
Substituting Eq. (9) into Eq. (10), as follows:
When a natural logarithm is applied to both sides, Eq. (11) becomes
Because the heating rate is constant, \(\frac{{{\text{d}}T}}{{{\text{d}}t}} = \beta\) and \(\frac{\text{d}}{{{\text{d}}t}}\left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}} \right) = 0\), and T(t) = Tmax and α(t) = αmax. Kissinger’s approach assumes that the decomposition product n \((1 - \alpha_{\hbox{max} } )^{{\text n} - 1}\) is independent of rate. Thus, the value of Ea can be calculated from the slope of the linear fitting of the ln (β/T 2max ) versus 1/Tmax plots.
The Kissinger model gives average degradation Ea values in the temperature interval where it is applied. In this way, it is appropriate to provide a single value of Ea because we are limited in evaluating complex processes of materials [19, 27].
Results and discussion
Thermal stability
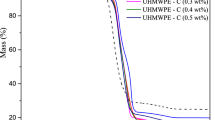
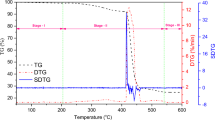
The effect of adding vitamin C to B-UHMWPE was investigated in terms of thermal behavior and thermodegradation kinetics. Figures 1 and 2 show the resulting mass loss (%) and DTG curves as a function of temperature, with varying heating rates of 5, 15 and 30 °C min−1. The unmodified polymer and B-UHMWPE/Vit C (concentrations of 0.5, 1.0 and 2.0 mass% of vitamin C) were analyzed under an argon atmosphere. Table 1 shows the Tinitial and Tmax values of the samples according to the increase in heating rates. From the curves depicted in Fig. 1, it is suggested that the samples present the typical behavior of all polyethylenes, showing only one stage of decomposition [30]. As the heating rate increases, all curves shift to the right, denoting that higher temperatures are required for the sample degradation process. An explanation for this effect was provided by Tang et al. [31], who attributed these shifts to the behavior of the polymer molecules, which did not have enough time to release the heat that was gained from the increased heating rate. Since heat diffusion is slow, it also results in slower decomposition rates at the higher decomposition temperatures of the samples.
In Fig. 2, both DTG curves of B-UHMWPE and B-UHMWPE/Vit C are shown. Only one DTG peak was observed in all samples, denoting typical thermal degradation behavior in a single step. In this kind of process, degradation occurs by random chain scission and by intermolecular transfer, which are indicative of a first-order reaction [32]. The values of the temperatures Tinitial and Tmax at different heating rates are summarized in Table 2. It may be seen that the average values of the initial and maximum decomposition temperatures of the B-UHMWPE lies between 414.8 and 482.0, whereas for B-UHMWPE/Vit C compounds with 0.5, 1.0 and 2.0%, those values lie between 412.0 and 483.6, 360.6 and 478.5, and 413.1 and 484.5 °C, respectively. Thus, the B-UHMWPE thermal stability was not practically altered with addition of 0.5 and 2.0% of Vitamin C. In contrast to the expected, the addition of 1.0% of Vitamin C declined the initial decomposition temperature of B-UHMWPE about 54.2 °C.
Thermal decomposition kinetics
In this study, the Ozawa–Flynn–Wall (OFW) method gives the activation energy values (Ea) and pre-exponential factor (A) of B-UHMWPE and B-UHMWPE/Vit C as a function of the conversion degree (α). Alternatively, the Kissinger method determines Ea at Tmax. Both methods are used at heating rates of 5, 15 and 30 °C min−1 to investigate the degradation process of the samples from lower to higher rates. The OFW integral method gives kinetic parameters from Eq. (12) at various stages of the sample degradation process. Figure 3 shows typical linear plots between ln β and 1/T obtained at three decomposition rates for OFW method. For each plot, both values of Ea and A were determined from the slope of the linear regression and its intercept with the y-axis, respectively. Activation energy was also evaluated using the Kissinger method, Eq. (14), from the graph of ln (β/T 2max ) versus 1/Tmax. The values of maximum degradation temperature of the samples are shown previously in Table 1. The kinetic parameters evaluated from both methods and their respective linear correlation coefficient (r2) are shown in Tables 3 and 4. It is seen that the fitted curves show linearity with r2 > 0.99 to temperature lying in a range from 25 to 700 °C. This suggests that the calculated Ea values are reliable for describing the decomposition kinetics of B-UHMWPE and its compounds with vitamin C. Figure 3 shows a spacing pattern between parallel straight lines for all samples, indicating that the processing of samples was suitable for homogeneity [19]. According to [33], the constancy of the activation energy value and its independency with the extent of degradation means that the degradation kinetics corresponds to a single-reaction mechanism.
Table 2 shows the values of Ea, α and r2 using the OFW method for pure polymer and B-UHMWPE/Vit C. The results showed that activation energy of all samples depend on the conversion degree (α). Moreover, two stages of activation energy were observed for B-UHMWPE: Ea increased with conversion until 60%, whereas Ea remained practically constant for α > 70%. In fact, this behavior is a typical feature for several polymers [33, 34]. For example, Peterson et al. [33] studied the degradation kinetics of polyolefins and suggested two degradation stages, where the initial lower values of activation energy were initiated by weak links in the first, and higher values of Ea in the second stage were somewhat hindered by degradation previously initiated due to random chain scission at lower temperatures (early stage).
The activation energy values for 1.0 and 2.0 compounds were reduced with the conversion degree increasing. B-UHMWPE with 0.5% vitamin C concentration had its activation energy slightly decreased when α was increased. The compound with 1.0% particularly showed a pronounced reduction in Ea with α. Indeed, the decrease in the Ea values with α was also observed by Chrissafis et al. [35] in other polymeric systems. Furthermore, activation energy values of UHMWPE/Vit C were higher than pure polymer for all evaluated conversion degrees. These results suggest that the addition of Vitamin C to B-UHMWPE could modify the mechanisms of the thermal degradation of the pure polymer.
An antioxidant effect of vitamin C can be suggested to the compounds. Antioxidants were incorporated into the polymers at low concentrations. Therefore, in order to better evaluate the antioxidant effect of vitamin C on the degradation of B-UHMWPE, low, intermediate and high concentrations of 0.5, 1.0 and 2.0%, respectively, were used in this study. All of the evaluated compounds presented higher Ea values than those obtained from the pure polymer throughout the studied conversion range and heating rates. As a result, the vitamin C reduced the decomposition rate of B-UHMWPE.
The mechanism of thermal stabilization caused by vitamin C in its compounds with B-UHMWPE may be similar to that of UHMWPE/vitamin E compounds, which is already thoroughly discussed in the current literature [9, 12, 36, 37]. From this perspective, the stabilization of the polymer occurs from the reaction of peroxy (–CHOO*–) and alkoxy (–CHO*–) radicals, with hydrogen being abstracted from the vitamin E phenol group [9, 10, 18, 36]. It is possible that the structure of vitamin C allows even more hydrogen atom abstractions by radicals than that of vitamin E. This possibility of increasing the antioxidant capacity of vitamin C is supported by Shen et al. [11]. They observed that the multiple phenolic hydroxyl groups present in the phenols made them more effective than vitamin E on the stabilization of UHMWPE. Peltzer et al. [13] studied non-irradiated UHMWPE compounds with various concentrations of vitamin E (0.1, 0.3, 1.0 and 2.0%) and heating rates, which are compatible with those studied herein. They observed an 11% increase in the value of the activation energy for the compound with 1.0% vitamin E. In present study, 1.0% B-UHMWPE/vitamin C compounds showed an increase of 58%. The average activation energy values are shown in Table 3. These results support the hypothesis that the chemical structure of vitamin C enables the abstraction of more hydrogen atoms than vitamin E to increase the activation energy of thermal decomposition of B-UHMWPE.
Table 3 also shows the values of the pre-exponential factor (A) of the B-UHMWPE and its compounds. It is apparent that there is an increase in the values of (A) associated with an increase in vitamin C concentration in the compounds. According to Lee et al. [38], a decrease in (A) is related to a reduction in the macromolecule mobility of the polymer. Thus, B-UHMWPE/Vit C compounds had its molecular mobility increased. In this connection, the molecular mobility of the polymer can interact with the vitamin C. Consequently, contact points may have been established between the polymer and vitamin. Therefore, the reactions among the hydrogen atoms that were abstracted from vitamin C can be favored, and the macroradicals that resulted from the thermal degradation of the polymer.
Table 4 presents the activation energy values of the pure B-UHMWPE and its vitamin C compounds as estimated from the Kissinger method. In this case, the analysis is done at maximum decomposition rate. The obtained Ea values are close to the average values obtained by the OFW method, as shown in Table 4. Similar trends were observed in the results obtained by both methods. All studied compounds presented higher values of activation energy than the pure polymer. AFM images depicted in Fig. 4a, b show that vitamin C particles cannot be identified clearly in the film surface topography, suggesting that they could be buried beneath the surface. AFM images show that B-UHMWPE/Vit C compounds exhibited flat surfaces associated with polymer and round-shaped protuberances (clear contrast). Moreover, SEM micrograph in Fig. 5 shows a round-like morphological aspect of pure vitamin C, indicating that the round-shaped protuberances in AFM images refer to vitamin C particles covered by B-UHMWPE as shown by arrows in Fig. 4a, b. Based on these assumptions, vitamin C particles distributed inside polymer collaborated with the increase in Ea values of the compounds. Therefore, vitamin C has potential as an antioxidant for biomedical UHMWPE used as implant material.
Conclusions
The thermal degradation behavior of the B-UHMWPE/Vit C compounds occurred in only one stage, requiring higher temperatures for degradation with an increasing of heating rate. The addition of vitamin C increased the activation energy of the pure polymer. The pre-exponential factor (A) indicated that the mobility of polymer chains increased, favoring the interaction of vitamin C with the free radicals that were originated from thermal degradation of the polymer, thereby stabilizing the compounds. Analogous tendency was observed from activation results of the OFW and Kissinger methods. The addition of 1.0% vitamin C resulted in activation energy values higher than the pure polymer and other compounds. The incorporation of vitamin C decreased the B-UHMWPE decomposition rate even at a lower concentration of 0.5%. These results favor the perspective that vitamin C can act as an antioxidant for B-UHMWPE.
References
Bracco P, Oral E. Vitamin E-stabilized UHMWPE for total joint implants: a review. Clin Orthop Relat Res. 2011;469:2286–93.
Oral E, Greenbaum ES, Malhi AS, Harris WH, Muratoglu OK. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials. 2005;26:6657–63.
Fu J, Doshi BN, Oral E, Muratoglu OK. High temperature melted, radiation cross-linked, vitamin E stabilized oxidation resistant UHMWPE with low wear and high impact strength. Polymer. 2013;54:199–209.
Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20:1659–88.
Paxton EW, Inacio M, Slipchenko T, Fithian DC. The Kaiser Permanente national total joint replacement registry. Perm J. 2008;12:12–6.
Oral E, Wannomae KK, Hawkins N, Harris WH, Muratoglu OK. Alpha-tocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials. 2004;25:515–22.
Kurtz SM, Dumbleton J, Siskey RS, Wang A, Manley M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J Biomed Mater Res A. 2009;90:549–63.
Lerf R, Zurbrugg D, Delfosse D. Use of vitamin E to protect cross-linked UHMWPE from oxidation. Biomaterials. 2010;31:3643–8.
Turner A, Okubo Y, Teramura S, Niwa Y, Ibaraki K, Kawasaki T, Hamada D, Uetsuki KK, Tomita N. The antioxidant and non-antioxidant contributions of vitamin E in vitamin E blended UWMWPE for total kneep replacement. J Mech Behav Biomed. 2014;31:21–30.
Costa L, Carpentieri I, Bracco P. Post electron-beam irradiation oxidation of orthopedic Ultra-High Molecular Weight Polyethylene (UHMWPE) stabilized with vitamin E. Polym Degrad Stab. 2009;94:1542–7.
Bracco P, Brunella V, Zanetti M, Luda MP, Costa L. Stabilisation of ultra-high molecular weight polyethylene with vitamin E. Polym Degrad Stab. 2007;92:2155–62.
Shen J, Gao G, Liu X, Fu J. Natural polyphenols enhance stability of crosslinked UHMWPE for joint implants. Clin Orthop Relat Res. 2015;473:760–6.
Peltzer M, Wagner JR, Jiménez A. Thermal characterization of UHMWPE stabilized with natural antioxidants. J Therm Anal Calorim. 2007;87:493–7.
Al-Malaika S, Ashley H, Issenhuth S. The antioxidant role of α-tocopherol in polymers. I. The nature of transformation products of α-tocopherol formed during melt processing of LDPE. J Polym Sci Polym Chem. 1994;32:3099–113.
Al-Malaika S, Goodwin C, Issenhuth S, Burdick D. The antioxidant role of α-tocopherol in polymers II. Melt stabilising effect in polypropylene. Polym Degrad Stab. 1996;64:145–51.
Litwinienko G, Dabrowska M. Thermogravimetric investigation of antioxidant activity of selected compounds in lipid oxidation. J Therm Anal Calorim. 2001;65:411–7.
Al-Malaika S, Issenhuth S, Burdick D. The antioxidant role of vitamin E in polymers V. Separation of stereoisomers and characterisation of other oxidation products of dl-α-tocopherol formed in polyolefins during melt processing. Polym Degrad Stab. 2001;73:491–503.
Kaya I, Dogan F, Gul M. A new schiff base epoxy oligomer resin: synthesis, characterization, and thermal decomposition kinetics. J Appl Polym Sci. 2011;121:3211–22.
Thumsorn S, Yamada K, Yew WL, Hamada H. Thermal decomposition kinetic and flame retardancy of CaCO3 filled recycled polyethylene terephthalate/recycled polypropylene blend. J Appl Polym Sci. 2013;127:1245–56.
Hazewindus M, Haenen GRMM, Weseler AR, Bast A. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and a-tocopherol. Food Chem. 2012;132:654–8.
Ameta RK, Singh M. A thermodynamic in vitro antioxidant study of vitamins B (niacin and niacin amide) and C (ascorbic acid) with DPPH through UV spectrophotometric and physicochemical methods. J Mol Liq. 2014;195:40–6.
Souza VC, Oliveira JE, Lima SJG, Silva LB. Influence of Vitamin C on morphological and thermal behaviour of biomedical UHMWPE. Macromol Symp. 2014;344:8–13.
Pielichowski K, Njuguna J. Thermal degradation of polymeric materials. Shawbury: Rapra Technology Ltd; 2005.
Kim JY, Kim DK, Kim SH. Thermal decomposition behavior of poly(ethylene 2,6-naphthalate)/silica nanocomposites. Polym Compos. 2009;30:1779–87.
Flynn J, Wall LA. A Quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Polym Lett. 1966;4:323–8.
Ozawa T. A new method of analyzing thermogravimetric data. B Chem Soc Jpn. 1965;38:1881–6.
Vyazovkin S. Advanced isoconversional method. J Therm Anal Calorim. 1997;49:1493–9.
Doyle CJ. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Chao M, Li W, Wang X. Thermal decomposition kinetics and anti-oxidation performance of commercial antioxidants. J Therm Anal Calorim. 2015;120:1921–8.
Tang W, Li XG, Yan D. Thermal decomposition kinetics of thermotropic copolyesters made from trans-p-hydroxycinnamic acid and p-hydroxybenzoic acid. J Appl Polym Sci. 2004;91:445–54.
Denq BL, Chiu WY, Lin KF. Kinetic model of thermal degradation of polymers for nonisothermal process. J Appl Polym Sci. 1997;66:1855–68.
Peterson JD, Vyazovkin S, Wight CA. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly(propylene). Macromol Chem Phys. 2001;202:775–84.
Shih YF. Thermal degradation and kinetic analysis of biodegradable PBS/multiwalled carbon nanotube nanocomposites. J Polym Sci Polym Phys. 2009;47:1231–9.
Chrissafis K, Paraskevopoulos KM, Pavlidou E, Bikiaris D. Thermal degradation mechanism of HDPE nanocomposites containing fumed silica nanoparticles. Thermochim Acta. 2009;485:65–71.
Jahan MS, Walter BM. Macroradical reaction in ultra-high molecular weight polyethylene in the presence of vitamin E. Radiat Phys Chem. 2011;80:281–5.
Oral E, Rowell SL, Muratoglu OK. The effect of a-tocopherol on the oxidation and free radical decay in irradiated UHMWPE. Biomaterial. 2006;27:5580–7.
Lee JY, Liao Y, Nagahata R, Horiuchi S. Effect of metal nanoparticles on thermal stabilization of polymer/metal nanocomposites prepared by a one-step dry process. Polymer. 2006;47:7970–9.
Acknowledgements
The authors are grateful to Brazilian Coordination for the Improvement in Higher-Level Personnel for scholarships. Moreover, characterizations provided by the Fast Solidification Laboratory of the Federal University of Paraiba and Northeast Center for Strategic Technologies (CETENE) are acknowledged as well.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, V.C., Santos, E.B.C., Mendonça, A.V. et al. Thermal behavior and decomposition kinetic studies of biomedical UHMWPE/vitamin C compounds. J Therm Anal Calorim 134, 2097–2105 (2018). https://doi.org/10.1007/s10973-018-7321-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7321-9