Abstract
The oxidative stability of biodiesel/diesel blends is usually evaluated from the released gaseous phase change characteristics, without analyzing what happens in liquid phase. Depending on the storage and/or heating conditions, gums and insoluble organic particulates may be formed in liquid phase by oxidation. To contribute to the evaluation of this oxidized heavier components formation in liquid phase, a new thermal analysis quantitative method was developed by the authors, which was applied to diesel oil/soybean biodiesel blends having from 5 to 20 vol% of the latter. The method consists on obtaining differential thermogravimetric curves (DIFTG), subtracting the TG curve obtained in inert atmosphere from the TG curve of the same sample obtained in air, which, actually, shows how and how much accumulated oxidized mass is being formed in liquid phase during analysis. Corresponding thermal effects are quantified by differential DTA (DIFDTA) curves, subtracting respective DTA curves in N2 from those in air. The results show that, as the biodiesel content is increased, a higher amount of heavy oxidized products remains in liquid phase of the blend, up to the respective much higher decomposition temperatures. This fact is confirmed by estimating the activation energies as a function of the mass loss conversion degree, which shows that the higher is the formation rate of the liquid phase oxidized mass in a blend, the higher is the activation energy needed for its decomposition and the higher is the blend ignition temperature, which is a polynomial function of second order of the biodiesel content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diesel oil is a fossil fuel composed from 10 to 22 carbon atoms per hydrocarbon molecule, being a complex mixture of paraffinic, naphthenic and aromatic hydrocarbons [1]. Biodiesel is composed of alkyl esters of long chain carboxylic acids [2] and derived from organic matter of animal or vegetable origin biomasses, which have been used as a fuel, in addition to being renewable. Soy is one of the most important seeds in the world, with a high protein content surpassing several types of other existing oilseeds. In Brazil, it is the most used raw material for biodiesel production [3].
Biodiesel, mixed with diesel oil, tends to improve the characteristics of the resulting fuel, increasing lubricity of low sulfur diesel oil [4]. The consumption of this mixture is viable because it results in lower CO2 emission, high energy value, biodegradability and insignificant sulfur content in its chemical composition [2]. The 2017 current Brazilian legislation requires vehicular fleets, captive or specific, to use diesel with at least 8% in volume of biodiesel, a content that will be increased progressively up to 10 vol% in 2019 [5].
In diesel engines, the sprayed oil is compressed and mixed with air, which increases the mixture temperature promoting its spontaneous combustion (autoignition) [6]. Thus, any fuel oxidation interferes in its ignition delay and in the combustion process. Unstable diesel fuels can form soluble gums or insoluble organic particulates, which may occur by fuel heating during fuel system recirculation. Both may contribute to injector deposits and to form particulates clogging fuel filters [7].
The oxidative stability of biodiesel is the main parameter to assure its quality as a fuel, which depends on its chemical composition and physicochemical properties. Depending on the number of existing unsaturated bonds in its molecule, there are different levels of stability to oxidation [8], which may occur in organic substrates (unsaturated fatty acids) by hydrolysis and/or by reaction with oxygen [9]. Biodiesel can be highly reactive due to the presence of free radicals that generate unpaired electrons, turning the molecule highly reactive [10]. Volatile and non-volatile compounds are generated by increasing the rate of the peroxides formed by decomposition [9].
The high reactivity of biodiesel in oxidative ambient is due to the presence of double bonds in its structure, mainly in the presence of water, air and/or metals [8]. Often formation of hydroperoxides occurs, which, being unstable, easily react, generating by-products of oxidation, which form residues during combustion causing problems and/or operating failures, negatively affecting the combustion performance [11].
The oxidation behavior of biodiesel has been studied extensively, mostly by analyzing what happens to the gaseous phase due to this oxidation, by several methods, to mainly evaluate oxidation stability temperatures or oxidation times and/or oxidizing gas pressure drop at a determined temperature, among which are PetroOXY (ASTM D7545), Pressure Differential Scanning Calorimetry, PDSC (ASTM D5483), low P-DSC and Rancimat method—EN 14214 [12,13,14,15,16]. Leonardo et al. [17] reported that during thermal analyses in air of biodiesel, oxidation in liquid phase generates heavy oxidized components, a fact that does not occur when the same sample is analyzed in same operating conditions in nitrogen. They also reported that these high molecular heavier compounds, which initially dissolve in the liquid phase, sediment during their concentration process, as the low molecular compounds are released in the first step of mass loss due to vaporization, forming carbonaceous residues during their thermal decomposition at higher temperatures.
The authors of the present article also noticed that when analyzing their biodiesel, diesel or the blend samples in air, the mass loss during the evaporation step was little lower when analyzed in nitrogen. This indicates in these cases that, actually, in the presence of air, the oxidation in liquid phase forms heavier products, which are volatilized and/or decomposed only at higher temperatures than those of non-oxidized corresponding components in inert ambient.
Thus, in the present article, to contribute to a better evaluation of these heavier oxidized components formation process, a new thermal analysis method is presented, which quantifies the oxidized mass as it is being formed in the liquid phase of those blends, when heated in oxidant atmosphere. The method is based on Differential Thermogravimetry (DIFTG), which consists on subtracting the TG curve obtained in inert atmosphere from the TG curve obtained in air. The method was applied to TG curves, in nitrogen and air, of diesel oil, soybean biodiesel obtained by methyl route and to their blends. The resulting DIFTG curve shows the oxidized mass increase in liquid phase as is being formed, in air ambient, followed by its decrease due to its decomposition and burnout, as the temperature is increased. Corresponding thermal effects are shown by Differential DTA (DIFDTA) subtracting respective original DTA in N2 from that in air.
To avoid any misunderstanding of the innovative characteristics of the developed method, it must be noted that a differential thermogravimetric curve (DIFTG) is not equivalent to a derivative thermogravimetric (DTG) curve, as sometimes is stated incorrectly. A DIFTG curve shows the difference between two thermogravimetric (TG) curves at a same temperature or time, while a DTG curve shows the rate of mass loss at any point of a TG curve, which is, actually, the derivative of the function that represents the TG curve at any of its points, as a function of time or temperature.
It is also important to note that this liquid phase oxidation has not been quantified as it occurs so far, by any of the existent methods to evaluate oxidative stability of bio or mineral fuels. The method was applied to the studied samples, in which results are evaluated and discussed in the present paper.
To see how this heavy oxidized component formation affects the activation energy of the pyrolysis as a function of the conversion degree (α) of each studied case, isoconversional methods were applied to thermogravimetric (TG) data obtained in each ambient. In this paper, only the Osawa Flynn–Wall with Doyle approximation method [18] activation energy results are shown, because the results of the two other applied methods were very similar, as well as their respective curves as a function of the conversion degree. As will be shown, the higher obtained activation energies in air than in nitrogen atmosphere, for a same degree of conversion, during the vaporization or thermal decomposition of the remaining liquid phase, confirmed the occurrence of the oxidation in the respective liquid phases [19].
Materials and methods
Samples preparation
All specifications of the diesel and biodiesel soybean biodiesel commercial samples of the present study are in accordance with the requirements of the limits allowed by the Brazilian National Agency of Petroleum, Natural Gas and Biofuels (ANP) and the physicochemical standards according to the specifications determined by ASTM, ABNT and ISO normative entities. In addition to diesel oil (B0) and biodiesel (B100), blends of these fuels containing 5, 10,15 and 20% of biodiesel, named respectively as B5, B10, B15 and B20, were analyzed as well.
Thermal analysis
Simultaneous thermogravimetric/differential thermal analyses (TG/DTA) of all samples were performed in a TA Instruments, model-Q600, simultaneous TG/DTA/DSC equipment. Heating rates of 10, 15 and 20 °C min−1 were used from 25 to 600 °C, using 5 mg samples in open aluminum crucibles. As purge gases, 100 mL min−1 of nitrogen or air were used, to have, respectively, inert and oxidizing ambient during the analyses. A new aluminum crucible was always used for each analysis. This procedure was adapted to avoid that possible residues from an analysis could interfere into the subsequent one. Data obtained from TG/DTG/DTA curves were treated using the TA Instruments Universal Analysis software.
Differential TG and DTA curves
For a same organic function set of substances, the higher is the organic chain and corresponding molecular weight of a substance, the lower will be its vapor pressure as a function of temperature, the higher will be its thermal stability and the higher will be its volatilization or decomposition temperature [20].
When an organic substance is oxidized without suffering any volatilization or decomposition, usually it is because more stable oxidized products are formed in liquid phase than the corresponding non-oxidized ones. Also, when oxidation occurs with simultaneous decomposition, usually lighter products are formed, as well as heavier products in the liquid phase than the decomposed ones [20, 21]. In any case, the thermal stability of the oxidized products will depend on their respective molecular weight and structure, which will determine the respective volatilization or decomposition temperatures [21].
During thermal analyses of the analyzed samples in air, for a same sample and temperature, the mass losses during the evaporation or decomposition steps before autoignition were systematically lower than in analyses performed in nitrogen. This occurrence as previously discussed indicates that during analyses in air, some of the biodiesel and/or diesel components were oxidized forming heavier products, which were remaining in liquid phase [22], volatilizing or decomposing only at higher temperatures than the non-oxidized corresponding components in nitrogen.
The mass of oxidized products which remained in liquid phase was estimated by Differential Thermogravimetry (DIFTG), subtracting the TG curve in nitrogen of a sample from its TG curve in air. As shown and discussed in the following experimental results, this procedure allows one to obtain the accumulated oxidized mass curve in liquid phase as a function of temperature, which, in the analyzed samples, still occurs even at little higher temperatures than the corresponding autoignition points. The method allows obtaining the liquid phase oxidation profile of each case, which shows respective percentual mass gain during the formation of the oxidized heavy components and subsequent mass loss, during their decomposition and/or burnout in air. Differential DTA (DIFDTA) curves were also obtained by the difference between respective DTA curves in air and nitrogen, which show respective thermal effects during the formation, decomposition and burnout of these liquid phase oxidized products.
Activation energy and ignition delay
To determine the activation energy (Eα) of the transformations in air and nitrogen, as a function of the conversion degree, the Ozawa Flynn–Wall Method with Doyle approximation kinetic isoconversional method was applied [19, 22,23,24]. It is important to note that from the obtained activation energy (Eα) values in air, it is possible to also verify the influence of the addition of biodiesel on the thermal behavior and combustion of the blends, as a function of the conversion degree (α), as well as on the ignition delay of the studied blends, through Eq. 1 [19]:
where τ is the ignition delay, p is the pressure, T is the temperature, R is the universal constant of gases, Eα is the activation energy at a conversion degree and b is the kinetic coefficient.
It will be seen that the higher is the oxidized state of the liquid phase of a diesel/biodiesel blend, the higher will be the activation energy to promote its vaporization or decomposition, after which, depending on the operating conditions, ignition and combustion of the released gases may occur. From Eq. 1, it can be seen that the higher is the activation energy, the higher will also be the ignition delay, because the higher will be the vaporization or thermal decomposition temperatures of the heavier oxidized products formed in liquid phase. Thus, the higher is the formation of these products, the higher will be the interference in the performance of the diesel engines in which they will be formed.
The Ozawa, Flynn and Wall method with Doyle approximation is an isoconversional kinetic method applied to TG curve data of a same sample obtained by at least three different β heating ratios, which estimate the value of the activation energy (Eα) as a function of conversion degree (α) by Eq. 2:
where A is the pre-exponential factor, R is the Universal Constant of gases, T, is the absolute temperature, β is the heating rate and α conversion degree and g(α) is a function of α.
At a same conversion degree Eα and g(α) are constants. Thus, from Eq. 2, the activation energy (Eα) can be estimated by plotting ln(β) versus 1/T values for each selected conversion degree, at different heating rates, obtaining a straight line in which angular (a) and linear (b) coefficients are given by
The method was applied to the thermogravimetric analysis data of each of the analyzed samples, obtained using at 10, 15 and 20 °C min−1 heating ratios (β), at nine different α values (from 0.1 to 0.9). For each conversion degree, a straight line of ln (β) as a function of 1/T was obtained, from which the respective activation energy was calculated from its angular coefficient, applying Eq. 3. These determinations were done to evaluate how the content of biodiesel addition influences the ignition delay, since the higher is the activation energy of the first stages of mass loss, the higher will be that delay. A higher change of activation energy also indicates a higher change in the mechanism of transformations with mass loss.
Results and discussion
Thermal analysis
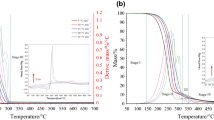
In the following, figures show the TG curves obtained in inert (N2) and oxidative (air) ambient for diesel oil (B0), biodiesel (BD) and their blends B5, B10, B15 and B20.
From Fig. 1, it can be seen that at 125 °C, due to the purge gas dragging action lowering respective partial pressures in gas phase, diesel lightest components evaporate from the beginning of the analysis. For biodiesel, due to the much lower vapor pressures of its components at same temperatures at atmospheric pressure, the mass loss due to its components evaporation just begins at that temperature.
Up to 225 and 250 °C, respectively, for diesel and biodiesel, from their DTA curves it can be seen that in all analyses, there are practically only endothermic effects due to the volatilization of respective components.
Up to 250 °C, B0 TG and DTG curves are similar in air and in N2 ambient, the mass loss at a same temperature being little higher in the last case. A same behavior in mass loss occurs for BD in both ambient up to 250 °C, after which, in air, a higher mass loss rate occurs, due to the combustion of the released gases from the decomposition of heavier carbonaceous products, as indicated by respective DTA exothermic peak in the upper part of Fig. 1.
Figures 2–5 show the TG, DTG and DTA curves for the blends B5, B10, B15 and B20 in oxidative (air) and inert (N2) ambient.
From their TG and DTG curves, in general, it can be noticed that the loss mass in air is little lower than in nitrogen ambient at a same temperature, due to the formation of heavier oxidized components which will vaporize or decompose thermally at higher temperatures. As seen in Fig. 1, this difference is more apparent for biodiesel.
From respective DTA curves, it can be seen a similar behavior in nitrogen and air for B5, B10. B15 and B20 blends. In air, all blend DTA curves first show a significant endothermic peak due to vaporization of the released gases, followed by a minor exothermic peak due to the heavy components burnout, which begins by autoignition. In N2 ambient, a broad endothermic peak of small intensity occurs about 250 °C, due mainly to their component evaporation and/or thermal decomposition
Differential TG and DTA curves
As previously defined, differential thermogravimetric curve (DIFTG) is that one obtained from the difference between two TG curves of a same sample obtained in different thermal analysis conditions. Actually it allows one to quantify transformations that cannot be seen using only conventional thermogravimetric (TG) or derivative thermogravimetric (DTG) curves. Figure 6 shows the B0, B5, B10, B15 and B20 DIFTG curves, which show the influence of the biodiesel content on the accumulated mass formation of oxidized species in the liquid phase of diesel and the studied blends. The curves are shown on the respective initial sample mass basis, which were vertically shifted for a better comparison among each other.
Figure 7 shows the B0, B5, B10, B15 and B20 DIFDTA curves, which show the influence of the biodiesel content on their respective ignition points, which increase, when the biodiesel content increases.
According to Fig. 6, it can be seen that diesel oil, even when non-blended with biodiesel, presents a significant accumulated formation of oxidized mass in the liquid phase (5.56%), which confirms the formation of higher molecular species, such as the cited soluble gums and insoluble organic particulates [7].
As biodiesel is added to diesel, in the case of B5 blend, the formation of a much lower accumulated oxidized mass in the liquid phase (1.14%) indicates that an effective interaction between diesel and biodiesel has occurred, which reduces the oxidation in liquid phase. Considering that soybean biodiesel comes with natural antioxidants [11], their action may also explain the decrease of this type of oxidized mass in liquid, which is confirmed by the fact that the autoignition temperature of this mixture occurs at practically the same temperature as that of diesel and according to Fig. 7, B5 blend DIFDTA curve presents the lowest oxidation in the liquid phase.
B10 DIFDTA curve indicates that the increase of biodiesel concentration promotes different changes in the mechanisms of volatilization and oxidation in temperature ranges before the occurrence of autoignition. In B10 blend, there was a higher formation of heavier oxidized products in the liquid phase (4.95%), increasing the autoignition point, as shown in the DIFDTA curves of Fig. 7, which maintained the liquid phase at higher temperatures promoting its higher oxidation. This fact is even commented by other researchers who report that when petroleum-derived liquid fuel is subjected to high temperatures, in the presence of air, insoluble oxidation products are formed.
In Fig. 6, the B20 DIFTG curve shows two oxidation steps, clearly indicated by their respective exothermic peaks, which indicate that different oxidation products are being formed in each step. Very probably lighter oxidized products are formed in the first step, which decompose at lower temperatures and heavier ones in the second one, which in turn decompose at much higher temperatures.
Comparing B10 and B15 blend DIFDTA curves, there is an enlargement of the endothermic peak before the autoignition point, indicating a higher thermal instability and consequent higher volatilization of the products formed by interaction between diesel and biodiesel.
Comparing the DIFTG curves of B15 and B20 blends, it can be seen that due to the fact that B20 contains a higher content of biodiesel, the formation of the oxidized products in the liquid phase occurs in two stages. In the first one, there is a small peak at 178 °C generating a temporary maximum of 1.78% accumulated oxidized mass, after which it decreases, indicating that some of the lighter formed products volatilize. Between 200 and 205 °C a second oxidation stage in the liquid phase begins, with a maximum formation rate at 228 °C, with an accumulated oxidized mass of 2.48%. Table 1 shows the different oxidized accumulated masses in each case, as well as the maximum oxidation temperatures in respective liquid phases.
Figure 8 shows how the ignition temperature and the temperature of maximum released heat flux during combustion of the evolved gases are correlated with the biodiesel content in the blend. It is important to note that, while the ignition temperature is a polynomial function of second order of biodiesel content, the temperature of maximum released heat is a polynomial function of third order of BD content, indicating that the interaction between biodiesel and diesel in blend is changing, as BD content changes.
The above data indicate that when heated in air, independently of the evolution of a significant mass of gases by vaporization or by cracking of the heavier components of the blends, oxidized mass is formed in the liquid phase of the diesel/biodiesel blends, part of which remains as a carbonaceous residue.
These residual heavier products may cause diesel engine cycle problems such as filter clogging, hampering the engine burning process [8] and requiring replacement of engine damaged parts at a higher frequency than when the fuel is only diesel.
Activation energy
Activation energy (Eα) values, obtained using the isoconversional method of Ozawa Flyn–Wall with Doyle approximation (OFW) at different conversion degrees (α) in oxidative (air) and inert (nitrogen) ambient, are shown in Table 2. The respective curves, as a function of conversion degree, are shown from Figs. 9–13.
It must be noted that, considering six independent inter-laboratory measurements, activation energies estimated by the isoconversional kinetic method used in the present article may have an estimated deviation of ± 8% of the respective measured value [25].
The activation energy curves of diesel oil (B0) and biodiesel (BD) in air and N2, are shown, respectively, in Figs. 9 and 10. In the latter case, values for diesel are almost the half of those for biodiesel (BD). However, in air, the relative difference between activating energies does not reach 50% for all studied conversion degrees (from 10 to 90%). BD shows higher values for Eα for same levels of B0 conversion in N2, because in BD they happen at higher temperatures, due to the fact that biodiesel components are more stable than the diesel hydrocarbon components at atmospheric pressure, according to Fig. 1.
In Fig. 9, the relative increase of the activation energies in air is due to diesel (B0) oxidation in liquid phase as was quantified in Fig. 6. Thus, a higher energy is needed to break the existing non-saturated bonds, which does not occur in N2 ambient.
In the case of Biodiesel in Fig. 10, besides that at atmospheric pressure it is more stable than diesel oil and for this reason needs higher activation energies for its volatilization and thermal decomposition, as it has oxygen in its composition, which facilitates its oxidation in any ambient, it presents a lower change of the activation energy as a function of conversion degree in air.
In Fig. 10, although in N2, for lower conversion degrees the activation energies for biodiesel are lower than in air because there is no oxidation in liquid phase, for the higher conversion degrees the activation energies are higher than in air, because heavier components are progressively decomposed only by thermal action.
Figures 11–14 show the activation energy curves of the studied B5, B10, B15 and B20 blends. Different behavior occurs in an inert (N2) or oxidant ambient (air).
When comparing B5 activation energy curves in air and nitrogen there is almost no difference. In this case, it is important to note that there is a slight and almost same increase of Eα in both ambient as conversion increases, indicating a significant interaction between the raw materials diesel (B0) and biodiesel (BD). The activation energy increases almost linearly with the conversion degree, due to the thermal decomposition of the increasing heavier components as seen in Figs. 2 and 6, which occurs in a practically same way, also indicating a very good compatibility and miscibility of both fuels in this blend.
Figure 12 shows B10 blend activation energy curves. This time it can be noticed that there is a significant difference of Eα for the two kinds of ambient, showing that the major content of biodiesel forms more oxidized products in liquid phase than in B5 case. This can be probably due to the higher biodiesel content interacting with those of diesel, causing a higher oxidizing effect in liquid phase, with a progressive formation of more oxidized and heavier products in liquid phase, as seen in Fig. 6. These oxidation products need higher temperatures for their thermal decomposition and, consequently, higher activation energies at the new higher temperatures as seen in Fig. 11. In B10 case, it must be reminded that a higher oxidation occurs in liquid phase in air ambient, due to also air oxidation action, causing higher activation energies of thermal decomposition in air than in N2, in all conversion degrees, as noted in Fig. 12.
However, for Blend B15, as can be noticed in Fig. 13, there is a higher volatilization of lighter products than in B10, according to the respective higher endothermic peak, as seen in respective DIFDTA curve in Fig. 7. This can be due to an excess of biodiesel lighter components that probably do not interact with those of diesel, being released after oxidized by air to the ambient at lower temperatures, while the higher and also heavier biodiesel components seem to be more interactive with the diesel heavier components, decreasing the DIFTG values at higher temperatures. These facts also explain the higher activation energies in air at lower conversion degrees than in N2, where the higher content of lighter biodiesel components is released, as well as the increasingly similar values of activation energy for higher conversion degrees as seen Fig. 13. Consequently, a smaller formation of heavier oxidized products in liquid phase occurs as seen in DIFTG curve in Fig. 6, indicating the occurrence of changes of interaction, volatilization and decomposition mechanisms, which, due to the apparently lower interaction with diesel require smaller activation energies than in B10, in air or in N2.
As the content of biodiesel is increased in B20, the mechanism changes become more evident, as seen in respective DIF TG curve in Fig. 6, with two stages of formation of oxidized products in liquid phase, probably due to a higher excess of lighter and heavier biodiesel components than in B15 case. The first oxidation step happens up to 175 °C, after which thermal decomposition starts, indicating that lighter components are being released and or decomposed in this step. At a higher temperature range, the second stage of formation of heavier oxidized products in liquid phase begins, increasing up to 225 °C, after which their thermal decomposition and burnout occurs. The two stages of oxidation promote, by the progressive formation of heavier products, an increase of the activation energy in the oxidized ambient as conversion increases.
It is important to note that clogging o fuel pipes of diesel/biodiesel blends has been reported, when the biodiesel content is higher than 15% [26]. As seen in the present results in B20 blend case, this very probably occurs due to the incomplete combustion of the heavier products that can be formed in the second oxidation stage in liquid phase.
Conclusions
-
As biodiesel addition to diesel oil is increased, an excess that does not interact with diesel forms more stable non-volatile components. As a consequence, a lower mass loss occurs in the blend volatilization stages in air than in nitrogen, due to the formation of heavier and non-volatile oxidized products in the liquid phase.
-
DIFTG curves, obtained by the difference between the TG curves in air and nitrogen of a given sample, allow a quantitative analysis of the accumulated content of the oxidized products in liquid phase, as a function of the temperature of analysis, which, depending on the biodiesel content, may happen in two stages.
-
DIFDTA curves, also obtained by the difference of respective DTA curves in air and nitrogen, indicate that the blend ignition temperatures and the temperatures at which maximum heat flux is evolved from the combustion released gases are, respectively, polynomial functions of second and third orders of the biodiesel content of the blend, confirming the change on its interactivity with diesel oil, as its content increases.
-
When heated in air, biodiesel, diesel oil and their blends undergo changes in their component volatilization, with simultaneous occurrence of oxidation in liquid phase, even after their autoignition.
-
Modifications in the mechanisms of blend components volatilization and thermal decomposition, verified by the respective activation energy changes as a function of conversion degree, depend mainly on the biodiesel content added to diesel and to the interaction between the two fuels.
-
For same degrees of conversion, the changes promoted by the air oxidizing action require higher values of activation energy than when heating in an inert ambient, especially for blends B15 and B20, which have higher contents of biodiesel.
-
Soybean biodiesel, which has a higher content of unsaturated compounds than diesel, enhances the process of liquid phase oxidation of the blends, promoting their higher thermal and oxidative instability, which in B15 and B20 blends increases the oxidation stages occurring in liquid phase.
-
In the case of blends above 15% biodiesel addition to diesel, due to the possibility of higher heavy oxidized products formation in the liquid phase, there is a risk of tube clogging, indicating the need of shorter periods of exchange, to not interfere on the performance of diesel cycle engines.
References
Andrade RDA. Calor de combustão de blendas do tipo diesel/biodiesel e diesel/bio-óleo [Calorific power of Diesel/Biodiesel and Diesel/Bio-oil blends]. Master’s Dissertation. Universidade de Brasília, Brasília, Brasil, 2009.
Silva I. The use of combined processes to increase the extraction yied of macaúba oil. Master’s Dissertation. Universidade Federal do Rio de Janeiro Federal, Rio de Janeiro, Brasil, 2009.
Pasin JAB. Mato Grosso State logistics of soybeans export in grains. BNDES Newsp. 2007;14(27):195–212.
Penteado MCPS. Identification of bottlenecks and establishment of an action plan for the Brazilian biodiesel program success. Master’s Dissertation. University of São Paulo, São Paulo, Brazil, 2005.
http://www.anp.gov.br. Accessed 20 Jan 2017.
Oliveira DPJ. Internal combustion engines, mechanic systems II, FATEC – SP. São Paulo: Piracicaba; 1997.
http://www.chevronwithtechron.ca/products/documents/Diesel_Fuel_Tech_Review.pdf. Accessed 17 May 2017.
Knothe G, Gerpen JV, Krahl J, Ramos LP. Biodiesel Hanbook. São Paulo: Translated to Portuguese by Ramos LP. Edgard Blucher; 2006.
List GR, Wang T, Shukla VKS. Storage, handling, and transport of oils and fats. In: Shahidi F, editor. bailey’s industrial oil and fats products. 6th ed. Hoboken: Wiley; 2005.
Lo KM, Cheung PCK. Antioxidant activity of extracts from the fruiting bodies of Agrocybe awgerita var. Alba. Food Chem. 2005;89(4):533–9.
Monyem A, Gerpen JHV. The effect of biodiesel oxidation on engine performance and emissions. Biomass Bioenergy. 2001;20:317–25.
Valle MLM, Leonardo RS, Dweck J. Comparative study of biodiesel oxidation stability using Rancimat, PetroOXY, and low P-DSC. J Therm Anal Calorim. 2014;116:113–8. https://doi.org/10.1007/s10973-014-3706-6.
Knothe G. Some aspects of biodiesel oxidative stability: a review. Fuel Process Technol. 2007;88:669–77.
Kowalski B, Gruczynska E, Maciaszek K. Kinectis of repassed oil oxidation by pressure differential sanning calorimetry measurements. Eur J Lipid Sci Techonol. 2000;102(5):337–41.
Karavalakis G, Stournas S, Karonis D. Evaluation of the oxidation stability of diesel. Biodiesel Blends. 2010;89(9):2483–9. https://doi.org/10.1016/j.fuel.2010.03.041.
Mc Cormick RL, Westbrook SR. Storage stability of biodiesel and blends. Energy Fuels. 2010;24:690–8.
Leonardo RS, Valle MLM, Dweck J. The thermal processing in air of ethylic soybean biodiesel after accelerated aging, with and without antioxidant. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6314-4.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;42(6):639–42.
Leiva CRM, Crnkovic PM, Santos AM. The use of thermogravimetry to determinate the activation energy of combustible oil combustion processes. Quim Nova. 2006;29(5):940–6.
Soares RW, Menezes VJ, Fonseca MVA, Dweck J. Characterization of carbonaceous products by TG and DTA. J Therm Anal. 1997;49:657–61.
Morais LC, Dweck J, Campos V, Rosa AH, Fraceto LF, Büchler PM. Combustion and pyrolysis of a sludge from wastewater treatment plant. Mat Sci Forum. 2010;660–661:1009–14.
Sbirrazzuoli N, Vecchio S, Catalani A. Isoconversional kinetics study of alachlor and metolachlor vaporization by thermal analysis. Int J Chem Kinet. 2004;3:74–80.
Ozawa T. Kinectic analysis of derivate curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Flynn JH, Wall A. General treatment of thermogravimetry of polymers. J Res Nat Bur Stand. 1966;70 A:487–523.
ASTM International, E1641—16 method, Standard Test Method for Decomposition Kinetics by Thermogravimetry Using the Ozawa/Flynn/Wall Method, 2016.
Pereira FMM. Estudo da degradação do óleo lubrificante em motores alimentados com biodiesel B100 [A study on lubricant oil degradation in engines feeded with B100 biodiesel]. Master’s Dissertation.Universidade Tecnológica Federal do Paraná, 2015 (in portuguese).
Acknowledgements
To the Brazilian National Research Council—CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, T.F., Dweck, J. Liquid phase oxidation quantitative analysis of biodiesel/diesel blends by differential TG and DTA. J Therm Anal Calorim 134, 1953–1963 (2018). https://doi.org/10.1007/s10973-018-7298-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7298-4