Abstract
In this study, a series of the cross-linked polyurethane (PU)/PEG4000 blend was synthesized as novel form-stable phase change materials (FSPCMs) for thermal energy storage through a facile and solvent-free synthetic strategy. In the obtained FSPCM, the PU functioned not only as supporting materials but as phase change substance. The PEG in FSPCM acted as phase change substance. The influence of PEG4000 content on the crystalline properties, phase change properties, thermal reliability and stability was extensively studied by X-ray diffraction (XRD), differential scanning calorimetry (DSC), thermal cycling test and thermogravimetric analysis (TG), respectively. Moreover, the chemical and crystalline structure of obtained PU and FSPCM was confirmed by Fourier transform infrared spectroscopy (FTIR) and polarizing microscope (POM). XRD and POM results indicated that the obtained FSPCM has similar crystalline structure to PEG. DSC results showed that FSPCM appears superior phase change properties with the phase change temperature and the latent heat in the range of 27.73–48.94 °C and 72.19–113.4 J g−1, respectively. The thermal cycling test and TG verified the excellent thermal reliability and stability of FSPCM. The obtained FSPCM exhibited great potential in the field of TES such as the solar energy collection system, exterior wall thermal insulation systems and hot water circulation system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thermal energy storage (TES) has attracted considerable attention due to the deterioration of environment and the depletion of fossil energy [1]. In recent years, the latent heat TES system employing phase change materials (PCMs) has been extensively developed in many areas such as solar energy system [2], sustainable building [3], smart textile [4] and electric industry [5]. PCMs have the reversible ability to store and release the latent heat during the phase change process. PCMs are often divided into solid–solid PCM, solid–liquid PCM, solid–gas PCM and liquid–gas PCM according to the phase transition state [6]. Solid–gas and liquid–gas PCMs are rarely applied in the practical application due to the volume fluctuation [7].
Solid–liquid PCMs have the intrinsic advantage of higher latent heat over the solid–solid PCM [8]. Various substances such as paraffin [9], alcohols [10], fatty acid [11] and polyethylene glycol (PEG) [12, 13] have been studied as solid–liquid PCMs for TES. However, solid–liquid PCMs must be encapsulated or sealed to prevent the leakage in the practical application [14]. In order to address this problem, the study of form-stable PCMs (FSPCMs) for latent heat TES have become a hot issue in the last decades [15]. The FSPCM will keep their state form when the ambient temperature is higher than the melting temperature of phase change functional substance [16]. Generally speaking, FSPCMs can be obtained through blending solid–liquid PCMs with supporting materials [17]. The commonly used supporting materials include inorganic porous material (vermiculite [18], expanded perlite [19], silicon dioxide [20], diatomite [21, 22]), cross-linked polymer (poly(acrylonitrile-co-itaconate) [23], poly(polyethylene glycol diacrylate) [24], epoxy resin [25]), polymer with high melting point (polymethyl methacrylate [26], polyethylene [27] and ethylene–vinyl acetate [28]).
PEG is acknowledged as a promising solid–liquid PCM for TES because of its relatively high phase change heat, non-toxic, non-corrosiveness, eco-friendliness, biodegradability and low cost [6]. Zhang et al. [29] prepared polymethyl methacrylate/PEG/aluminum composites as FSPCMs for TES. As long as the mass fraction of PEG was below 70%, the prepared composite FSPCMs remained solid without leakage. Tang and his coworkers [30] reported a FSPCM composing of PEG, SiO2 and Al2O3, the phase change enthalpy of obtained FSPCM reached 124 J g−1, and the thermal conductivity was improved by 12.8%. Fang et al. [25] developed the PEG/epoxy resin composite as FSPCM through casting molding method, and the epoxy resin with three-dimensional network structure provided structural strength and prevented the leakage of the melted PEG. Tang and his group [31] studied the multiwall carbon nanotubes/PEG/SiO2 composites as an efficient full-band sunlight conversion and storage device. The obtained composite exhibited rapid and broadband visible light-harvesting characteristics, light–thermal conversion, thermal energy storage ability, form-stable effects and high thermal conductivity. From previous studies, the supporting material could ensure the shape stabilization of melting PEG in the FSPCMs.
However, the traditional FSPCMs exhibit inferior thermal reliability because of migration behavior of the phase change working substance after repeated use [23], and the supporting materials inevitably degraded the thermal storage density of the FSPCMs. Therefore, it is essential and significant to improve the interface bonding strength between the phase change substance and supporting materials in FSPCM; meanwhile, the negative effect of the supporting materials on the phase change latent heat of FSPCMs should be minimized.
In this study, a series of FSPCM including PU and PEG4000 is prepared via facile and solvent-free synthetic strategy for TES. The cross-linking structure of PU in FSPCM will ensure the stable macroscopic shape and prevent the leakage of PEG4000 during phase change process. The PU was synthesized with MDI and xylitol as skeleton and PEG4000 as phase change functional chains, and it was selected as the supporting materials in FSPCMs for the first time. In the obtained FSPCM, solid–liquid phase transition of PEG and solid–solid phase transition of PU will occur simultaneously, which will obviously improve the phase change enthalpies. Moreover, the PEG chain in PU favors to the interface bonding strength between PU and PEG, which will prevent the migration of the PEG after repeat use. The obtained FSPCMs were extensively studied by the Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), polarizing microscope (POM), differential scanning calorimetry (DSC), thermal cycling test and thermogravimetric analysis (TG).
Experimental
Materials
4, 4′-Diphenylmethane diisocyanate (MDI) was provided by Yantai Wanhua Polyurethane Co., Ltd. (Shandong, China). Polyethylene glycol (PEG, M w = 4000 g mol−1) and xylitol were obtained from Kelong Chemical Reagent (Chengdu, China). The reagents above are used as received.
Preparation of the FSPCMs
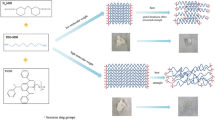
The detailed synthetic route of FSPCM is showed in Fig. 1. Firstly, PEG4000 and MDI (molar ratio of PEG4000 to MDI was 1/2) were loaded into a three-necked flask, and the prepolyurethane was obtained after stirring 3 h under 80 °C. Then, the melted xylitol was (molar ratio of xylitol to MDI was 1/5) added into above prepolyurethane and stirred for 2 h at 100 °C. Thirdly, the extra PEG4000 under different content (Table 1) was poured into the above mixture. The thermal curing was performed in an oven for 15 h at 105 °C, after that the FSPCMs with different PEG content were obtained. The obtained cross-linked polyurethane without extra PEG4000 was named as PU.
Characterization
FTIR
The FTIR was used to study the chemical structure of PEG4000, polyurethane (PU) and prepared FSPCMs, and measurements were taken on the Nicolet 560 (Nicolette Co., USA) in the scanning range of 4000 and 400 cm−1 with a resolution setting of 4 cm−1.
SEM
The morphology of pure PU and prepared FSPCM was detected on JEOLJSM-5900LV (Japan) at an accelerated voltage of 20 kV, and the fracture surface of sample was putter-coated with gold powder after wetting-off in liquid nitrogen.
XRD
The crystalline properties of PEG4000, PU and FSPCMs were studied by XRD, and the testing was conducted on Phillips X’Pert Pro MPD diffractometer in Bragg–Brentano geometry. The measurement data were collected in a range of 2θ = 5° – 50° by a scanning rate of 0.04° min−1 at room temperature.
POM
POM was employed to crystalline morphology of PEG4000 and FSPCMs, and the POM images were detected on the XPR-500D microscope (China) equipped with a video camera.
DSC
Phase change properties (phase change temperature and latent heat) of PEG4000 and FSPCMs were investigated using the thermal analysis instrument DSC 204 (NETZSCH, Germany). About 8 mg specimen was putted into the aluminum crucible and sealed. The samples were heated from ambient temperature to 90 °C to erase prior heat history of the samples; then, the specimen was cooled to 0 °C to collect crystalline data. The specimen was lastly heated to 80 °C to record the melting data. Both heating and cooling temperature scans were carried out at the rate of 10 °C min−1 under a dry nitrogen atmosphere.
Accelerated thermal cycling testing
Accelerated thermal cycling testing was performed with 100 consecutive heating/cooling processes with a heating rate of 3 °C min−1 (in the temperature interval of 20–100 °C) in a high–low-temperature chamber to evaluate the thermal reliability of the prepared FSPCMs. Then, the phase change temperature, latent heat and chemical structure of thermal treated samples were assessed by DSC and FTIR, respectively.
TG
TG was used to assess the thermal stability of PEG4000, PU and FSPCMs, and the testing were performed on thermogravimetry–differential thermal analysis instrument SDT-Q600 (TA, America) in the temperature range of 30–600 °C at a heating rate of 10 °C min−1 under nitrogen atmosphere.
Results and discussion
Chemical structure
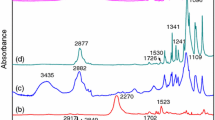
FTIR measurements are employed to study the chemical structure of PEG4000, PU and prepared FSPCM, and the FTIR spectra are presented in Fig. 2a–c, respectively. As for pure PEG4000, shown in Fig. 2a, the broad absorption peak at 3490 cm−1 is ascribed to stretching vibration of –O–H and the distinct peak at 1113 cm−1 is attributed to the C–O–C symmetrical stretching vibration. Moreover, the characteristic absorption peaks at 2886, 1464, 1349, 1276, 956 and 847 cm−1 correspond to C–H vibration. In Fig. 2b, the PU exhibits same peak position with pure PEG4000 at 2886, 1464, 1349, 1276, 956 and 847 cm−1, which are caused by the vibration of PEG segments in PU. The new characteristic peaks at 1722 and 1539 cm−1 are detected in Fig. 2b, which are derived from the –C=O– and –N–H– stretching vibration of urethane group. In addition, the broad and blunt peaks in the range of 3666–3172 cm−1 are attributed to the N–H belong to the urethane groups. The disappearance of absorption peak at 3440 cm−1 coupling with the detection at 1722 and 1539 cm−1 proves the reaction occurs between –OH and NCO. As can be seen from Fig. 2c, the FSPCM-2 has the same absorption peaks position with PU indicating the same chemical structure of them. However, the absorption peak intensity of PU and FSPCM are different, especially at 1722, 1539 and 1113 cm−1. The FSPCM has stronger absorption at 1113 cm−1 and weaker absorption at 1722 and 1539 cm−1, which must be caused by the addition of extra PEG4000 in PU.
Morphology of FSPCM fracture surface
SEM is conducted to detect the morphology difference between PU and FSPCM-2, and the SEM images of samples are demonstrated in Fig. 3. As shown in Fig. 3, pure PU exhibits relative flat and dark fracture surface. Compared PU, FSPCM-2 exhibits obvious multilayered structure; the dark gray area and brighter rugged structure in the image are corresponding to PU and PEG4000, respectively. The brighter areas are emerged into the PU matrix, which means that the phase separation does not occur in FSPCM-2. In addition, the cross-linked network structure and interface bonding strength between PU and PEG will prevent the leakage of melted PEG from FSPCM when the ambient temperature above the melting point of LA.
Crystalline properties
XRD
The phase change performance of FSPCMs is positive, depending on their crystalline properties. XRD measurements are taken to investigate the crystalline properties, and diffraction spectra of PU, PEG4000 and FSPCMs are presented in Fig. 4. As shown in Fig. 4, all samples exhibit similar and strong diffraction peaks, indicating the existence of crystalline structure. PEG4000 exhibits distinct and sharp diffraction peaks at 19.16°, 23.28°, 26.62° and 35.98°, which are caused by the crystal plane of (120) (112) (210) and (212). The strong and sharp diffraction peaks at 19.16° and 23.28° are also detected in PU diffraction spectrum; however, diffraction peaks at 26.62° and 35.98° are weak and almost invisible. This phenomenon must result from that the movement of PEG segments in PU is restrained by the cross-linked structure. FSPCMs demonstrate diffraction peaks at 19.16°, 23.28°, 26.62° and 35.98°, respectively, corresponding to (120), (112), (210) and (212) crystal plane. Moreover, the diffraction peak intensity is between that of PEG4000 and PU, and increase from FSPCM-1 to FSPCM-3. As for FSPCM-1, the diffraction peaks at 26.62° and 35.98° are obscure, which means that the crystalline ability of additive PEG4000 and PEG segments are also affected by the cross-linked structure. The diffraction peaks at 26.62° and 35.98° become distinct with the PEG content increase from FSPCM-1 to FSPCM-3, which means the restriction of cross-linked structure on the crystalline ability of PEG4000 decreases. Therefore, FSPCM-3 exhibits more clear diffraction peaks at 26.62° and 35.98°.
POM
To further study the crystalline properties of pure PEG4000 and prepared FSPCMs, the POM test is performed and the POM images of PEG4000 and FSPCMs at 25 °C are presented in Fig. 5. As can be seen from Fig. 5, all samples have obvious cross-extinction pattern, which means that prepared FSPCMs have the same spherulitic structure as the pure PEG4000. As can be seen from Fig. 5a, one spherocrystal is full of whole view indicating the perfect crystalline structure in PEG4000. In Fig. 5b–d, prepared FSPCMs have much more spherocrystal number in view, and the spherocrystal size of FSPCMs is smaller than that of PEG4000. This phenomenon must be due to the fact that the movement of PEG chains is confined by the cross-linked structure. In addition, the spherocrystal size increases with the decreasing of degree of cross-linking from FSPCM-1 to FSPCM-3, which means that the crystalline properties of FSPCM with higher PEG content are less affected by cross-linked structure. These results are in good agreement with XRD results.
Phase change properties
Phase change properties of the PCMs include the phase change temperature (melting temperature: T m; crystallization temperature: T c) and latent heat (melting latent heat: ΔH m; crystallization latent heat: ΔH c). The phase change temperature defines the application range, and latent heat determines the practical application value. Therefore, phase change properties are extremely significant parameters for PCMs. DSC curves of PEG4000 and prepared FSPCMs are shown in Fig. 6, and the corresponding parameters are summarized in Table 2. As shown in Fig. 6, all samples demonstrate obvious melting and crystallization peaks in each heating and cooling process further prove the existence of crystalline structure. PEG4000 exhibits distinct and strong endothermic and exothermic peaks in the temperature range of 20–70 °C, and ΔH m and ΔH c are 166.1 and 162.7 J g−1, respectively, at T m of 51.7 °C and T c of 38.27 °C. The high latent heat resulting from the perfect crystallization structure indicates that PEG4000 has high thermal storage and release capability during phase change process. Meanwhile, prepared FSPCMs exhibit similar solo endothermic and exothermic peaks in temperature range of 10–65 °C, which implies that prepared FSPCMs have similar reversible thermal storage and release ability as PEG4000. Table 2 shows that solid–liquid phase transition of PEG and the solid–solid phase transition of PU simultaneously occur in the similar temperature range of 24–52 °C, indicating that FSPCMs composed of PU and PEG have the property of synergistic phase change. FSPCM-3 has high latent heat with ΔH m of 113.4 J g−1 and ΔH c− of 108.9 J g−1, indicating that FSPCMs is valuable in TES. Compared with pure PEG4000, FSPCMs have relative lower latent heat and phase change temperature; and the latent heat value increases with the increase if PEG content, which is consistent with the variation tendency of spherocrystal size detected in POM images. This fact must be caused by confined crystalline structure in FSPCMs.
The comparison of latent heat theoretical and experimental value for FSPCMs is employed to further study the effect of cross-linked structure on the latent heat value. ΔH m−t/ΔH c−t is the theoretical latent heat of prepared FSPCMs, which is calculated by the following formula:
where ΔH m−PU(ΔH c−PU) and ΔH m−PEG(ΔH c−PEG) are latent heat of PU and PEG in the melting (crystallization) process, respectively, ωPU and ωPEG are PU and PEG content in the FSPCMs, respectively.
As shown in Fig. 7, the latent heat is lower than theoretical value calculated by formula (1), which further prove the cross-linked PU as the polymeric matrix in the FSPCMs has adverse effect on the crystalline property of PEG4000. In other words, the experimental latent heat for PEG-based FSPCMs deviates from the corresponding theoretical data to some extent, which has been reported in previous research [32]. Table 3 summarizes the differential ratio (χ) between the ΔH m (ΔH c) and ΔH m−t (ΔH c−t). From Table 3, the χ of FSPCM with higher PEG content is less than that of FSPCM with lower PEG content, which means that the FSPCM with higher PEG content is less affected by cross-linked structure. These results are in good agreement with XRD and POM results.
Thermal reliability
The DSC curves of FSPCM-1, FSPCM-2 and FSPCM-3 before and after 100 thermal cycling are shown in Fig. 8, and the corresponding data are listed in Table 4. In Fig. 8, the DSC curves of FSPCMs have no obvious variation compared with that of corresponding origin samples, indicating that the crystalline structure of FSPCMs are slightly affected by thermal cycling. From Table 4, the latent heat and phase change temperatures of FSPCMs are slightly varied after 100 thermal cycling, which will not affect the application of thermal energy storage. In addition, the FTIR spectra of FSPCM-2 before and after thermal cycling are presented in Fig. 9. Figure 9 shows that the FTIR spectrum of FSPCM-2 after thermal cycling is almost the same as that of the original samples, which indicates that the FSPCM-2 does not undergo the thermal degradation during thermal cycling. Based on Table 4 and Fig. 9, it can be concluded that prepared FSPCMs have good thermal reliability and chemical stability in terms of phase change properties and FTIR spectra.
Thermal stability
Thermal stability is significant for practical application of prepared FSPCMs; Fig. 10 shows the TG curves of PU, FSPCMs and pure PEG4000. As can be seen from Fig. 10, all samples exhibit a single degradation stage implying one-step degradation mechanism. PEG4000 has excellent thermal stability with the onset degradation temperature is higher than 350 °C; meanwhile, PU exhibits relative lower onset degradation temperature but still exceed 300 °C. The onset degradation temperatures of FSPCM-1, FSPCM-2 and FSPCM-3 are 315.9, 333.6 and 340.5 °C, respectively, which means that prepared FSPCMs are thermal stable until 315 °C. In conclusion, the prepared FSPCMs have good thermal stability and can be applied in the condition under 300 °C.
Conclusions
This study focuses on the preparation and characterization of novel FSPCM composing PEG and cross-linked PU for the TES. Both of PU and PEG functioned as the phase change substance and PU was also served as the supporting materials in the obtained FSPCM. The results showed that the PU can prevent the leakage of melting PEG when the PEG content is below 50%. The FTIR curves confirmed the chemical structure of PU and FSPCM. XRD and POM results proved that the FSPCMs have the similar spherulitic structure with PEG4000 while the crystal size of FSPCMs is smaller than that of PEG4000. DSC test showed that the FSPCM has superior phase change properties with the phase change temperature and the latent heat in the range of 27.73–48.94 °C and 72.19–113.4 J g−1. TG and thermal cycling test verified the thermal stability and reliability of FSPCM. It can be concluded that the PU/PEG blend can be employed as promising FSPCM in the fields of TES such as the solar energy collection system, exterior wall thermal insulation systems and hot water circulation system.
References
Pielichowskaa K, Pielichowski K. Phase change materials for thermal energy storage. Prog Mater Sci. 2014;65:67–123.
Kenisarin M, Mahkamov K. Solar energy storage using phase change materials. Renew Sustain Energy Rev. 2007;11:1913–65.
Xu T, Chen Q, Zhang Z. Investigation on the properties of a new type of concrete blocks incorporated with PEG/SiO2 composite phase change material. Build Environ. 2016;104:172–7.
Mondal S. Phase change materials for smart textiles—an overview. Appl Therm Eng. 2008;28(11–12):1536–50.
Khateeb SA, Farid MM, Selman JR, Al-Hallaj S. Design and simulation of a lithium-ion battery with a phase change material thermal management system for an electric scooter. J Power Sour. 2004;128(2):292–307.
Weibo Kong XFZL. A facile synthesis of solid–solid phase change material for thermal energy storage. Appl Therm Eng. 2017;117:622–8.
Li Y, Wang S, Liu H, Meng F, Ma H, Zheng W. Preparation and characterization of melamine/formaldehyde/polyethylene glycol crosslinking copolymers as solid–solid phase change materials. Sol Energy Mater Sol C. 2014;127:92–7.
Wu D, Ni B, Liu Y, Chen S, Zhang H. Preparation and characterization of side-chain liquid crystal polymer/paraffin composites as form-stable phase change materials. J Mater Chem A. 2015;3(18):9645–57.
Karaipekli A, Biçer A, Sarı A. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers Manag. 2017;134:338–73.
Memon SA, Lo TY, Shi X, Barbhuiya S, Cui H. Preparation, characterization and thermal properties of Lauryl alcohol/Kaolin as novel form-stable composite phase change material for thermal energy storage in buildings. Appl Therm Eng. 2013;59(1–2):336–47.
Fu X, Liu Z, Wu B, Wang J, Lei J. Preparation and thermal properties of stearic acid/diatomite composites as form-stable phase change materials for thermal energy storage via direct impregnation method. J Therm Anal Calorim. 2016;123(2):1173–81.
Fu X, Xiao Y, Hu K, Wang J, Lei J, Zhou C. Thermosetting solid–solid phase change materials composed of poly (ethylene glycol)-based two components: flexible application for thermal energy storage. Chem Eng J. 2016;291:138–48.
Kong W, Yang Y, Zhou C, Lei J. Novel thermosetting phase change materials with polycarbonatediol based curing agent as supporting skeleton for thermal energy storage. Energy Build. 2017;146:12–8.
Ke H, Ghulam MUH, Li Y, Wang J, Peng B, Cai Y, Wei Q. Ag-coated polyurethane fibers membranes absorbed with quinary fatty acid eutectics solid–liquid phase change materials for storage and retrieval of thermal energy. Renew Energy. 2016;99:1–9.
Li L, Wang G, Guo C. Influence of intumescent flame retardant on thermal and flame retardancy of eutectic mixed paraffin/polypropylene form-stable phase change materials. Appl Energy. 2016;162:428–34.
Lv P, Liu C, Rao Z. Review on clay mineral-based form-stable phase change materials: preparation, characterization and applications. Renew Sust Energy Rev. 2017;68(1):707–26.
Shang L, Lipeng H, Shaolei X, Yongzhong J, Jinhe S, Yan J, Quanyou Z. A novel medium-temperature form-stable phase change material based on dicarboxylic acid eutectic mixture/expanded graphite composites. Sol Energy. 2017;143:22–30.
Xu B, Ma H, Lu Z, Li Z. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl Energy. 2015;160:358–67.
Ramakrishnan S, Sanjayan J, Wang X, Alam M, Wilson J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl Energy. 2015;157:85–94.
Wang W, Yang X, Fang Y, Ding J. Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid–liquid phase change materials. Appl Energy. 2009;86(2SI):170–4.
Lia M, Wu Z, Kao H. Study on preparation and thermal properties of binary fatty acid/diatomite shape-stabilized phase change materials. Sol Energy Mater Sol Cells. 2011;95:2412–6.
Karaman S, Karaipekli A, Sarı A, Biçer A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol Energy Mater Sol C. 2011;95(7):1647–53.
Mu S, Guo J, Zhang S, An Q, Wang D, Liu Y, Guan F. Preparation and thermal properties of cross-linked poly(acrylonitrile-co-itaconate)/polyethylene glycol as novel form-stable phase change material for thermal energy storage. Mater Lett. 2016;171:23–6.
Liu Z, Zhang Y, Hu K, Xiao Y, Wang J, Zhou C, Lei J. Preparation and properties of polyethylene glycol based semi-interpenetrating polymer network as novel form-stable phase change materials for thermal energy storage. Energy Build. 2016;127:327–36.
Fang Y, Kang H, Wang W, Liu H, Gao X. Study on polyethylene glycol/epoxy resin composite as a form-stable phase change material. Energy Convers Manag. 2010;51(12):2757–61.
Wang L, Meng D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl Energy. 2010;87(8):2660–5.
Chen F, Wolcott M. Polyethylene/paraffin binary composites for phase change material energy storage in building: a morphology, thermal properties, and paraffin leakage study. Sol Energy Mater Sol Cells. 2015;137:79–85.
Tian B, Yang W, Luo L, Wang J, Zhang K, Fan J, Wu J, Xing T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol Energy. 2016;127:48–55.
Zhang L, Zhu J, Zhou W, Wang J, Wang Y. Characterization of polymethyl methacrylate/polyethylene glycol/aluminum nitride composite as form-stable phase change material prepared by in situ polymerization method. Thermochim Acta. 2011;524(1–2):128–34.
Tang B, Wu C, Qiu M. PEG/SiO2–Al2O3 hybrid form-stable phase change materials with enhanced thermal conductivity. Mater Chem Phys. 2014;144:162–7.
Tang B, Wang Y, Qiu M, Zhang S. A full-band sunlight-driven carbon nanotube/PEG/SiO2 composites for solar energy storage. Sol Energy Mater Sol C. 2014;123:7–12.
Chen C, Liu W, Wang Z, Peng K, Pan W, Xie Q. Novel form stable phase change materials based on the composites of polyethylene glycol/polymeric solid–solid phase change material. Sol Energy Mater Sol C. 2015;134:80–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, W., Lei, Y., Jiang, Y. et al. Preparation and thermal performance of polyurethane/PEG as novel form-stable phase change materials for thermal energy storage. J Therm Anal Calorim 130, 1011–1019 (2017). https://doi.org/10.1007/s10973-017-6467-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6467-1