Abstract
This study is focused on mixed/chimeric advanced drug delivery nanosystems and specifically on pH-sensitive liposomes, combining lipids and pH-responsive amphiphilic block copolymers. Chimeric liposomes are composed of hydrogenated soy phosphatidylcholine (HSPC) and two different poly(n-butylacrylate)-b-poly(acrylic acid) (PnBA-b-PAA) block copolymers with 85 and 70% content of PAA, at six different molar ratios. PAA block exhibits pH responsiveness, because of the regulative group of –COOH. Chimeric bilayers are composed of HSPC and PnBA-b-PAA. Experiments are carried out by using differential scanning calorimetry (DSC) in order to investigate their thermotropic properties. DSC indicated disappearance of the pretransition effect in all chimeric lipid bilayers, at both buffers [phosphate buffer saline (PBS) and citrate buffer], and slight changes of the main transition temperature (T m). Contrariwise, the cooperativity (T 1/2) presented alterations between the two different buffers. Chimeric liposomes have been prepared and their physicochemical characteristics have been explored in PBS and citrate buffer by measuring the size, size distribution and ζ-potential. Liposomes are found to retain the mean value of their size during the stability studies. The physicochemical characteristics and the stability assessment of chimeric liposomes are correlated with DSC measurements of mixed bilayers. The incorporation of the appropriate amount of these novel pH-responsive block copolymers affects the cooperativity and the liposomal stabilization and imparts pH responsiveness (functionality), which was confirmed by performing experiments in acidic environment (citrate buffer). In conclusion, the results from DSC measurements provide useful information regarding the quality by design process for rationally preparing mixed/chimeric liposomal platforms to incorporate bioactive molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drug delivery nanosystems (DDnSs) were developed to provide a therapeutic amount of the drug to the proper site in the body. There are many categories of DDnSs. Liposomes are considered to be one of the most extensively investigated drug delivery nanosystems, exhibiting high therapeutic efficiency, low immunogenicity, as well as the ability of minimizing the chances of toxic side effects. Unfortunately, liposomes (which are composed mainly of phospholipids) present some disadvantages, such as their rapid recognition and uptake by reticuloendothelial system (RES) that reduce their plasma half-life, as well as their degradation by lysosomal enzymes, after their endocytosis. As a result, the amount of the drug that is received by the cell is lower and the desired pharmacokinetic profile of the drug is changed. Stimuli-sensitive forms of liposomes have been developed in order to overcome the above disadvantages. pH-sensitive liposomes (PSLs) represent one of the most promising and attractive kind of these forms [1, 2]. The incorporation of different kinds of pH-sensitive polymers, such as poly(acrylic acid)s (PAAs), succinylated PEG and N-isopropylacrylamide (NIPAM) containing copolymers, transforms conventional liposomes to pH-sensitive ones. Such liposomal formulations are characterized as mixed/chimeric liposomal systems because they are composed of two different biomaterials. These formulations had been categorized as advanced drug delivery nanosystems (aDDnSs). PAA is a synthetic polymer, which belongs to the family of poly(carboxylic acid)s, providing pH sensitivity [3]. If the proportion of hydrophobic alkyl acrylate block increases, the membrane of liposomes become more susceptible to destabilization under acidic pH (i.e., inside endosome). Analytically, pH-sensitive liposomes remain stable at physiological pH (7.4), but destabilize and acquire fusogenic properties under acidic pH (~5.3), due to their modulatory materials, containing “ionizable” chemical groups, such as amines, phosphoric acids and carboxylic acids, and changing dramatically their conformation in response to environmental pH alteration [4, 5]. Generally, conventional liposomes consist of phospholipids with positive charged choline as their polar head. In this case of polymer grafted pH-sensitive liposomes, the pH-sensitive polymer can be ionized by basic solution, so COO− groups can largely be produced and be electrostatically adsorbed to conventional liposomes, in order to produce pH-sensitive liposomes. The COO− groups cause electrostatic repulsion and make liposomes be charged, and unable to fuse and, as a result stable at physiological pH. While the pH level reduces to approximately 5.0 (resembling pathological tissue), the COO− groups turn into COOH groups and the electrostatic repulsion disappears. Polymer chains shrink, causing the implanted anchor (hydrophobic block) chains to be drawn out from the bilayers. As a result, liposomes are enforced to release their drug content. The amount of anchor chains plays a crucial role, because small amounts may not generate enough force to tear out bilayers [6]. In physiological environment (pH ~7.4) a different situation predominates, where polymer chains keep extended, because of the expulsion between COO− groups. Polymer chains implant into bilayers via their “anchor.” The grafted anchor could make the drug carriers more stable in a pH 7.4 environment. The stretched conformation of polymer could sustain the integrity of pH-sensitive liposomes, and it is expected to result in less drug release from pH-sensitive liposomes at pH 7.4 than at pH 5.0. There are many pathological states which are associated with different pH profiles from that of normal tissues, such as ischemia, infection, inflammation and tumors. In tumor tissues vasculature is disorganized, leading to hypoxia, production of lactic acid, hydrolysis of ATP, higher glycolysis rates and so increase of proton production, diminishing pH value at ~5.7. Lymphatic drainage is poor, enhancing the creation of acidic microenvironment [7]. Besides antitumor therapy, PSLs may be used as anti-infection therapy, gene therapy for the treatment of genetic and acquired diseases, cancer immunotherapy, immunotherapy with vaccines and oral drug delivery of acid-labile drugs [8, 9]. Moreover, in magnetic resonance imaging (MRI) of tumors infection, paramagnetic PSLs may accumulate in the acidic environment of pathological tissues, and there they could be triggered with local ischemia and release the encapsulated contrast agents.
The aim of this study was to study the PnBA-b-PAA grafted lipid bilayers, primarily using DSC, and continue to a rational design and development of pH-sensitive liposomes. The main goal of this investigation was to prepare mixed/chimeric liposomes composed of hydrogenated soy phosphatidylcholine (HSPC) and two different block copolymers poly(n-butylacrylate)-b-poly(acrylic acid) (PnBA-b-PAA) with 85 and 70% content of PAA at six different molar ratios, and to study their physicochemical characteristics.
Materials and methods
Materials
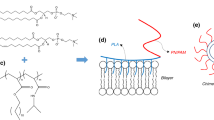
The phospholipid which was used for producing bilayer and liposomal formulations was the hydrogenated soy phosphatidylcholine (HSPC) (Fig. 1a). It was purchased from Avanti Polar Lipids Inc., (Albaster, AL, USA) and used without further purification. Chloroform and all other reagents used were of analytical grade and purchased from Sigma–Aldrich Chemical Co. The PnBA-b-PAA block copolymers were synthesized by RAFT polymerization using 2-dodecylsulfanylthiocarbonylsulfanyl-2-methylpropionic acid as the chain transfer agent in the presence of AIBN as the radical initiator and using 1,4-dioxane as the polymerization solvent. The polymerization occurred at 65 °C for 24 h. The molecular weights of the synthesized copolymers PnBA-b-PAA 30/70 and 15/85 were 10,000 g mol−1 and 20,000 g mol−1, while the content of PAA segments was 70 and 85% by weight, respectively (Fig. 1b).
Methods
Preparation of HSPC:PnBA-b-PAA 15/85 and HSPC:PnBA-b-PAA 30/70 mixed/chimeric bilayers
Pure lipid and mixed/chimeric bilayers were prepared by mixing the appropriate amounts of HSPC PnBA-b-PAA 15/85 and 30/70 in chloroform/methanol (1:1 v/v) solutions and the subsequent evaporation of the solvents under vacuum and heat. Briefly, stock solutions were prepared by dissolving the copolymers PnBA-PAA 30/70 and 15/85 in chloroform/methanol (1:1 v/v). Appropriate amounts of these solutions were added to HSPC, weighted into PBS vials, in order to obtain the desirable molar ratios (9:0.0, 9:0.1, 9:0.5, 9:1.0, 9:2.0 and 9:3.0), where necessary chloroform/methanol (1:1 v/v) solution was added. Then, the vials were transferred to a vacuum machine (Techne Dri-Block DB3Thermostat Teche Sample Concentrator). Mixed/chimeric phospholipids/block copolymer films were formed by removing the solvent at 50 °C. The films were maintained under vacuum for 2 h and then in a desiccators for at least 24 h in order to remove traces of solvent. The obtained laminated bilayers were hydrated into the appropriate aqueous medium and then were studied by differential scanning calorimetry.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) experiments were carried out using an 822eMettler-Toledo (Schwerzenbach, Switzerland) calorimeter calibrated with pure indium (T m = 156.6 °C). Sealed aluminum 40 μL crucibles were used as sample holders. Chimeric HSPC:PnBA-b-PAA 15/85 and HSPC:PnBA-b-PAA 30/70 (9:0.1, 9:0.5, 9:1.0, 9:2.0 and 9:3.0 molar ratios) fully hydrated bilayers were investigated. Thirty minutes prior to measurements each mixture, after it was placed in the crucible, was hydrated using the appropriate medium (PBS and citrate buffer). Then, the crucible was sealed. Two and a half heating–cooling cycles were performed in order to ensure good reproducibility of the data. The temperature range was from 20 to 60 °C, and the scanning rate 5 °C min−1. Before each cycle, the samples were subjected to a constant temperature of 20 °C to ensure the equilibration, while an empty aluminum crucible was used as reference. The second heating and cooling runs were taken into account, and the calorimetric data obtained (characteristic transition temperatures T m/s, enthalpy changes ΔΗ m/s, the width at half-height of the C p profiles ΔT 1/2m/s) were analyzed using Mettler-Toledo STARe software.
Preparation of pH-sensitive liposomes
Conventional and mixed/chimeric liposomal drug nanocarriers were prepared by the thin-film hydration method. Briefly, appropriate amounts of HSPC:PnBA-b-PAA 15/85 and HSPC:PnBA-b-PAA 30/70 (9:0.0, 9:0.1, 9:0.5, 9:1.0, 9:2.0 and 9:3.0 molar ratios) mixtures were dissolved in chloroform and then transferred into a round flask connected to a rotary evaporator. Vacuum was applied, and the mixed phospholipids/copolymer thin film was formed by slow removal of the solvent at 50 °C. The mixed film was maintained under vacuum for at least 24 h in a desiccator to remove traces of solvent and subsequently it was hydrated in PBS. The HSPC:PnBA-b-PAA 15/85 and HSPC:PnBA-b-PAA 30/70 mixed/chimeric lipid films were hydrated in HPLC-grade water by slowly stirring for 1 h, in a water bath above the phase transition of lipids (41 °C). The resultant multilamellar vesicles (MLVs) were subjected to two, 5-min sonication cycles (amplitude 70, cycle 0,7) interrupted by a 5-min resting period, in water bath, using a probe sonicator (UP 200S, dr. hielsher GmbH, Berlin, Germany). The resultant small unilamellar vesicles (SUVs) were allowed to anneal for 30 min.
Mean particle size and size distribution
The effect of block copolymers on the liposomal lipid bilayers was evaluated by measuring the size (mean hydrodynamic diameter) and size polydispersity index (PDI) of liposomes. The mean hydrodynamic diameter (D h), polydispersity index (PDI) and ζ-potential of the particles were used for the characterization of the liposomal dispersions immediately after preparation (t = 0 days), as well as for the monitoring of their physical stability over time (t = 30 days). 100 μl aliquots were 30-fold diluted in HPLC-grade water. Measurements were performed at a detection angle of 90° and at 25 °C in a photon correlation spectrometer (Zetasizer HSA, Malvern, UK) and analyzed by CONTIN method (MALVERN software).
Results and discussion
The thermotropic behavior of HSPC:PnBA-b-PAA mixed/chimeric bilayers
Differential scanning calorimetry (DSC) profiles obtained for pure HSPC lipid membrane and HSPC:PnBA-b-PAA 15/85 and 30/70 mixed lipid membranes are shown in Figs. 2 and 3, while changes of the calorimetric parameters are summarized in Tables 1 and 2. The pure HSPC bilayers hydrated in phosphate-buffered saline (PBS, pH 7.4) undergo two endothermic phase transitions with increasing temperature in the range examined: a broad very low enthalpy pretransition from gel (Lβ′) to rippled (Pβ′) phase centered at 50.0 °C and a clear major transition from Pβ′ to liquid crystal phase (La), centered at 54.3 °C [10, 11]. The pure HSPC bilayers hydrated in citrate buffer (pH 4.0) undergo the two endothermic phase transitions in lower temperatures (slight hysteresis). The decrease of the main transition temperature means decrease in the stability of the membrane, and subsequently, preleakage of the possible encapsulated drug [12]. The pretransition is centered at 48.2 °C, whereas the sharp main transition at 53.8 °C. The calorimetric parameters of the main transition (Table 1) are close to the literature [13], in which the main transition temperature of HSPC lipid in HPLC-grade water is at 52 °C.
DSC heating scans of a HSPC:PnBA-b-PAA 15/85 and b HSPC:PnBA-b-PAA 30/70 fully hydrated mixed/chimeric bilayers in phosphate-buffered saline (PBS), pH 7.4 at (i) 9:0.0, (ii) 9:0.1, (iii) 9:0.5, (iv) 9:1.0, (v) 9:2.0 and (vi) 9:3.0 molar ratios. The limits for the calculation of thermotropic parameters are from 25 to 60 °C
DSC heating scans of a HSPC:PnBA-b-PAA 15/85 and b HSPC:PnBA-b-PAA 30/70 fully hydrated mixed/chimeric bilayers in citrate buffer, pH 4.0 at (i) 9:0.0, (ii) 9:0.1, (iii) 9:0.5, (iv) 9:1.0, (v) 9:2.0 and (vi) 9:3.0 molar ratios. The limits for the calculation of thermotropic parameters are from 25 to 60 °C
The slight pretransition peak referred to as the lamellar gel (Lβ′) to rippled phase (Pβ′) transition of HSPC was strongly affected by the presence of both copolymers. Specifically, the presence of the polymeric guest caused the disappearance of the pretransition peak, in both buffers (Figs. 2, 3; Tables 1, 2). The disappearance of the pretransition may be attributed to the induction of an Lβ-like molecular orientation by the presence of the block copolymer, as it happens in the case of the incorporation of cholesterol into the lipid bilayer, because the amphiphilic character of copolymers may be compared to the amphiphilic behavior of cholesterol. It is possible that the HSPC molecules are arranged almost parallel to the normal bilayer [12]. The presence of the block copolymers disrupts the packing of the gel state, and the pretransition disappears [14]. Another possible explanation for the pretransition’s disappearance is the penetration, at least to some extent, of the block copolymer into the lipid bilayers, and as a consequence of this interaction, the polar head groups of bilayers changed their mobility and consequently their ordering, resulting disappearance of the pretransition event [15, 16]. Moreover, complete suppression of the pretransition is induced even from the smallest polymer molar ratios, probably indicating the polymer location near the bilayer interface [17]. The polymer may be close to the more polar choline moiety by the setting up of strong polar interactions between the –COO− groups and the –N(CH3)3 groups of the HSPC phospholipid at the almost neutral pH 7.4 of PBS. More specifically, hydrogen bonds are formed between PAA and lipids of the membrane of liposomes. –COO− group of PAA interacts with the –N(CH3)3 group of the more polar choline moiety of the HSPC phospholipid, at the almost neutral pH 7.4 of PBS. In the citrate buffer, non-ionized carboxyl groups of PAA interact with polar phosphate head groups of HSPC [18]. That is because the hydrophobic block of PnBA, as mentioned above, penetrates the bilayer, while the hydrophilic PAA stretches out of the bilayer surface, due to its expanded hydrophilic form, at the almost neutral pH 7.4 of PBS. As far as the situation in the citrate buffer is concerned, the interaction between PAA blocks and HSPC lipids occurs on the membrane surface and the driving force may be the formation of hydrogen bonds between non-ionized polymer carboxyl groups and the lipids polar head phosphate groups [19], explaining the disappearance of the pretransition.
The incorporation of both block copolymers has caused changes in the thermotropic behavior of HSPC lipid bilayers affecting extensively the specific enthalpy ΔH m of the main transition in PBS (Fig. 2) and in citrate buffer (Fig. 3). While the amount of the block copolymer was increased at all molar ratios, in both buffers, the specific enthalpy ΔH m of the main transition was decreased significantly. This phenomenon happens because, as the molar ratio of the block copolymer increases, the mobility around carbon–carbon bond is increased. As a result, we assume that the conformations are more disordered and fluid like, which leads to less prerequisite energy added as heat (which is translated as enthalpy) to overcome the van der Waals forces between HSPC molecules (or intersegmental forces within HSPC molecules) [20, 21]. Especially, for HSPC:PnBA-b-PAA 15/85 lipid bilayers at the two highest molar ratios the main peak disappeared leading to a possible solution-like model of the mixed/chimeric system. This may be attributed to the fact that the hydrophobic PnBA block of the polymer interpolated between the lipids causes hydrophobic defects, forcing the bilayer to change its structure in order to eliminate these defects (membrane disruption happens eventually), decreasing dramatically the observed ΔΗ m.
The temperature at which the thermal event starts (T onset,m) remained almost the same at all molar ratios of the mixed lipid membranes of both polymers, in both buffers. The only exception to that was at HSPC:PnBA-b-PAA 30/70 9:3.0 molar ratio system, where the decrease of T onset,m was larger in citrate buffer compared to the one in PBS.
The main transition temperature (T m temperature at which the change of the heat capacity (ΔC p) at constant pressure, is maximum) remained more or less unaffected (the PBS-hydrated bilayers seem more rigid) for both mixed/chimeric lipid bilayers, in both buffers (Figs. 2, 3; Tables 1, 2), in contrast to the dramatic decrease of ΔΗ m. This fact indicates that the interactions of the PAA polymeric segment with HSPC lipids affect the mobility of the polar head groups of lipids (dramatic decrease of ΔΗ m) much more than the mobility of the acyl chains of phospholipids [palmitic (16C) and stearic (18C) acyl chains presented in HSPC structure] (slight alteration of T m) [22]. Moreover, the PnBA block acts as a spacer within the liposomal membranes, separating the phosphatidylcholine head groups and decreasing the van der Waals forces between the hydrocarbon chains [23].
Concerning the changes of the cooperativity events, increasing the molar ratio of the polymeric guests, a significant decrease in cooperativity has been observed (Tables 1, 2), because the ΔΤ 1/2 values increased significantly (Tables 1, 2). This may be comparable to the decrease in cooperativity that happens while incorporating cholesterol into DPPC bilayers [20]. The only ratio, which slightly sidetracks the trend is HSPC: PnBA-b-PAA 30/70 9:3.0 molar ratio system, which has better cooperativity (lower ΔΤ 1/2 value) compared with the right away previous molar ratio (9:2.0). Between the two buffers (i.e., PBS vs citrate buffer), in citrate buffer the increase of ΔΤ 1/2 values are larger than those in PBS; that means lower or worse cooperativity of the HSPC lipids and PnBA-b-PAA 15/85 and 30/70 in citrate buffer. Using citrate buffer, where the pH is 4.0, the –COO− of PAA block is protonated to –COOH and PAA chain loses its expanded form. The polymer chain shrinkage will cause the implanted hydrophobic chains to be drawn out from the bilayers, destabilizing the membrane, decreasing thus the cooperativity of the systems. A small amount of polymer maybe cannot generate enough force to tear out bilayers inside, so the ΔΤ 1/2 values can explain the slight differences among the different molar ratios [6].
According to the literature, the effect of PAA is pH-dependent and occurs in an acidic environment. PAA does not change the lamellar structure of phosphatidylcholines, but makes it more fragile and permeable. As carboxylic groups are protonated, the polymer becomes more hydrophobic and surface adsorption increases. This may lead to increased lateral compression of the bilayer and disruption of the membrane [24]. Destabilization of the membrane can lead to the appearance of one or more lateral phases with increased gel-to-liquid transition temperature and lower cooperativity. The fraction of these new phases increases as the concentration of PAA in the system increases [25,26,27].
Moreover, the lack of sharp transitions indicates poor cooperativity [28]. It is obvious that by increasing the block copolymer content in the mixed/chimeric bilayers, shoulders appear which are attributed to the fact that the copolymers are not uniformly distributed in the HSPC bilayers, suggesting lateral phase separation in the HSPC bilayers into polymer-rich and polymer-poor nanodomains (Figs. 2, 3). Generally, broad transitions indicate that the distribution of components in the mixtures is heterogeneous the polymer-poor regions are characterized by high T m, exhibiting a sharp transition in the curve, whereas polymer-rich regions are characterized by a broad transition [29]. The main transition peaks at 9:1.0, 9:2.0 and 9:3.0 molar ratios were broadened, which may be related to bilayers fluidization effects (solution-like model) [18, 22]. In the presence of foreign substances, just as the block copolymer, able to penetrate into the bilayer giving a “solution-like” system, a modification in the shape and the size distribution of the nanoclusters takes place and their number increases noticeably. At the same time, the clusters become smaller and their surface more ramified. Consequently, the cooperativity of the melting process decreases and the curves broaden noticeably [15].
Referring to DSC cooling curves and experimental data, small hysteresis was observed, for HSPC:PnBA-b-PAA 15/85 at all molar ratios, in both buffers. Taking into consideration the amphiphilic character of the block copolymer, a suggestion on the formation of interdigitated phase could be that it is made as an effort of compensating the destabilization from the insertion of hydrophobic PnBA nanodomains, because interdigitation is created as an effort of the lipid bilayer to minimize free energy and remain thermodynamically stable. So, in all the molar ratios, the incorporation of the PnBA-b-PAA 15/85 probably caused the creation of a new phase, maybe an interdigitated phase [23, 30].
On the other hand, we have to point out some differences between HSPC:PnBA-b-PAA 30/70 different molar ratio bilayers. At all molar ratios, the bilayers undergo one exothermic phase transition, while reducing the temperature in the range examined (the curves are sharper in citrate buffer, than in PBS). As far as the 9:0.1 and 9:0.5 molar ratios are concerned, the temperature of crystallization remained almost the same with the pure HSPC bilayers, in both buffers. However, at 9:1.0 molar ratio there has been observed a significant increase of 3.6 °C in PBS, whereas a decrease of 2.0 °C in citrate buffer occurred, compared to pure HSPC bilayers. However, the temperature of crystallization at 9:2.0 molar ratio showed a decrease of almost 1 °C in PBS, but an increase of 1.9 °C in citrate buffer. Finally, the temperature of crystallization at 9:3.0 molar ratio slightly increased (~1 °C) in both buffers. Regarding both the DSC heating and cooling scans of HSPC:PnBA-b-PAA 30/70 different molar ratio bilayers, we have to point out that the 9:0.1, 9:0.5 and 9:1.0 molar ratios in citrate buffer present actually two peaks at the melting temperature and the crystallization temperature regions. These bimodal distributions, which are translated as asymmetries of the heat capacity profile, may be attributed to the creation of a new phase (coexisting with liquid crystalline phase). This new phase may interact differently with the acidic environment (citrate buffer, pH 4.0), causing differentiation of the melting temperature and crystallization temperature. pH absolutely has a key role to that phenomenon because, contrary to citrate buffer, in PBS DSC heating scans there is no bimodal peak and in DSC cooling scans there are very slightly observed bimodals, in 9:0.1, 9:0.5 and 9:1.0 molar ratios.
The physicochemical characteristics of HSPC:PnBA-b-PAA mixed/chimeric liposomes
At the preparation day (t = 0 days), the mean hydrodynamic diameter (D h) of all the liposomes incorporating the block copolymer PnBA-b-PAA 15/85 was larger than the D h of the HSPC conventional liposomes (Fig. 4a; Table 3). As the molar ratio of the block copolymer was increased, the D h was decreased, except for the 9:2.0 molar ratio. The 9:1.0 molar ratio shows the lowest D h value, while the 9:0.1 shows the highest one. It is remarkable that D h of 9:0.5, 9:1.0 and 9:3.0 molar ratios is close to the D h of the conventional liposomes (9:0.0). Regarding the polydispersity index (PDI), all chimeric molar ratios have higher PDI values than the conventional one. The only exception is the 9:0.1 molar ratio, showing the lowest value. The PDI presented an irregular variation, when the amount of the block copolymer was increased.
After the incorporation of PnBA-b-PAA 30/70 at the lowest molar ratio, there was a small decrease of mean hydrodynamic diameter (D h), which was about the same with the decrease at the 9:0.5 molar ratio, compared with conventional HSPC liposomes. Further increase of PnBA-b-PAA 30/70 content caused increase of D h value reaching 123.2 nm at 9:1.0 molar ratio, whereas 9:2.0 and 9:3.0 acquired smaller D h s (108.7 nm and 102.7 nm, respectively) (Fig. 4a). All of these colloidal systems were of more or less low size polydispersity (PDI ranging from 0.228 to 0.380). That indicates that liposome population was rather homogeneous in size. The only exception to that was the PDI value of the 9:2.0 systems, which almost reached 0.5 (Table 4).
We also investigated the ζ-potential values of the prepared mixed/chimeric pH-responsive liposomes. ζ-potential presented an irregular variation too. By the incorporation of PnBA-b-PAA 15/85 into HSPC liposomes, the values of ζ- potential turned from positive to negative, except for the 9:0.1 molar ratio. The 9:0.1 shows also the highest absolute value of ζ-potential, while the 9:0.5 the lowest one. As a result, 9:1.0 molar ratio exhibited the highest absolute value of ζ-potential and the lowest PDI value, being the most stable electrostatically and the most homogenous system, respectively. Generally, the increase of the polymer fraction at the molar ratio caused an acute increase at the absolute ζ-potential values, followed by a gradual decrease. Concerning the HSPC:PnBA-b-PAA 30/70 liposomes, as we increase the amount of PnBA-b-PAA 30/70 in these PBS-hydrated systems, the absolute values of ζ-potential were higher in comparison with conventional liposomes. Only the 9:1.0 molar ratio system did not follow the trend and showed lower ζ-potential value than the previous ratio. From physicochemical point of view, only 9:2.0 and 9:3.0 colloidal dispersions can be characterized as stable systems, because the absolute values of their ζ-potential were between 40 and 80 mV.
Stability assessment of HSPC:PnBA-b-PAA chimeric liposomes
Concerning the stability of the nanosystems over a period of 30 days, HSPC:PnBA-b-PAA 15/85 mixed/chimeric liposomes at all molar ratios were found to retain their original physicochemical characteristics (size and size distribution) at least for the time period that they were studied. Regarding D h, at all molar ratios, there are slight variations (increases and decreases), but the difference between the maximum and minimum value is nowhere up to 30 nm at each molar ratio, except from one measurement at 9:3.0 molar ratio (the difference is almost 50 nm, but it is restored by the end of the month). The 9:0.1 molar ratio presents the highest values of D h, while the 9:0.5 the lowest one. Except from 9:0.1, all the other molar ratios show similar values (91.3–176.7 nm). So, the size of mixed/chimeric liposomes at all molar ratios remained more or less stable during the stability studies (Fig. 5a). Regarding the PDI, we can observe similar variations. At all molar ratios, there are slight variations, but the difference between the maximum and minimum value is nowhere up to 0.2 at each molar ratio. So we conclude that the PDI of all molar ratios remained stable during the stability studies. Except 9:0.1, all the other molar ratios present similar values (0.412–0.734). The 9:0.1 molar ratio is remarkable, because is the most stable over time (there is almost gradual decrease at the values, with a maximum difference being only 0.031). Furthermore, 9:0.1 presents the lowest values PDI (<0.117), so 9:0.1 can be characterized the most homogenous and the most stable system, showing nevertheless the highest values of D h. High quantities of copolymer, namely at the 9:2.0 and 9:3.0 molar ratios, are found to be very stable, quite homogenous and showing quite large sizes. On the other hand, medium quantities of polymer, namely the 9:0.5 and 9:1.0 molar ratios, are found to be stable, quite homogenous and containing also the lowest values of D h over 30 days.
Concerning the stability assessment of HSPC:PnBA-b-PAA 30/70, to begin with the 9:0.1 and 9:3.0 molar ratios, we observed a slight increase of almost 10% in their D h (compared with the value of t = 0 days) during the stability assessment of 30 days (88.1–99.3 and 102.7–117.9 nm, respectively). Furthermore, the D h at 9:0.5 molar ratio remained almost stable (approximately 87 nm) throughout the period of 1-month stability assessment. The size of HSPC:PnBA-b-PAA 30/70 9:1.0 and 9:2.0 molar ratios presented the following paradox; their D h were significant smaller the t = 1 days compared with their size the t = 0 days. This means that those systems require more than 30 min of resting period before size measurement, to balance the amount of energy that they received through probe sonication. Besides that paradox, these systems remained almost the same, as it concerns their size, after 1 month of measurements (~97 and 95 nm, respectively). The PDI value for 9:0.1, 9:0.5 and 9:1.0 systems was found to be 0.2–0.3, indicating good dispersion of uniformly sized mixed/chimeric liposomal vesicles. On the other hand, 9:2.0 and 9:3.0 molar ratios exhibited more heterogeneous size distribution since their PDI values fluctuated between 0.35 and 0.45 (Fig. 5b). An increase in size with block copolymer content is observed and retained also in the stability studies.
All these mixed/chimeric liposomes own their stability to both PAA and PnBA blocks. PAA block is a weak polyelectrolyte (pKa of the first ionizable repeat unit being about 4.2), which possesses a hydrophilic and pH-sensitive character. Our dispersions were prepared by adding PBS that exhibits pH ~7.4. At this pH value, the block of PAA was ionized and its chains were expanded. The negative charge of carboxylic groups (COO−), which PAA block exhibits at neutral pH, made liposomes repulsive between each other, preventing fusion and colloidal destabilization. The PAA block also protected liposomes from aggregation processes, by stretching out of the liposomal surface due to its expanded, three-dimensional conformation that acquires at neutral or basic pH [5, 6, 24]. Moreover, the water molecules on the polymeric chains, due to the entropic effect taking place, reduced the conformational ability of each chain, providing steric stabilization, and increased the osmotic pressure among liposomes, leading eventually to liposomes repulsion. On the other hand, the PnBA block acted as a spacer within the liposomal membrane that decreased the van der Waals forces between the hydrophobic HSPC lipid alkyl chains, perturbed the bilayer structures and reduced the membrane tension of the liposomes, thus preventing liposomes from fusion [23]. Low packing density reduces the membrane interfacial tension, which is one of the most important factors causing liposomal fusion. Length mismatch between the PnBA block and the lipid chains may lead also to entanglement and to hairpin conformations of the hydrophobic block of PnBA in the hybrid bilayer, that makes the membrane assume more energetically favorable structures, reducing furthermore the membrane tension [31, 32].
Acidic protocol
We examined the physicochemical characteristics of HSPC:PnBA-b-PAA 15/85 and 30/70 liposomes in citrate buffer (pH 4.0). Changing the pH is one of the ways (besides temperature and solvent) that may disrupt the weak forces, such as intra- and intermolecular hydrogen bonds and van der Waals interactions taking place in these systems, and subsequently cause alterations in structure of the bilayer, leading to alterations of its function [20]. All HSPC:PnBA-b-PAA 15/85 liposomal systems present lower values of D h at the acidic pH than in PBS (Fig. 5b). HSPC:PnBA-b-PAA 30/70 nanosystems did not follow the same trend. Analytically, 9:0.1 molar ratio system presented a tremendous increase of D h, reaching 14.5 μm. We have to point out that this increase was obvious macroscopically, since the dispersion became frosty and misty, and gradually we observed precipitation of white flocculates. The D h of 9:0.5 molar ratio system was increased from 88.1 nm to 143.7 nm. For the rest chimeric liposomal systems, acidic environment caused a decrease of their D h, compared with their D h at the 20th day from preparation.
Generally, the above decreases were somehow expected due to the pH-dependent properties of PAA block (pKa ~ 4.2). At high pH, there was no complexation between PAA and liposomal surface (only PnBA block perturbs the membrane), whereas a decrease in pH resulted in a greater binding of PAA block to the membrane. This shift may be attributed to hydrogen bonding between PAA and lipids of the membrane of liposomes [24]. So, at neutral pH (PBS), the block of PAA is ionized (approx. 0.1% of the carboxyl groups remain protonated and 99.9% are ionized; pKa ~ 4.2) and its chains are expanded, while at citrate buffer conditions, where the pH is 4.0, the –COO− of PAA block is protonated to –COOH, and PAA loses its expanded form and the polymer chains shrink [24]. As carboxylic groups are protonated, the polymer becomes also more hydrophobic and surface adsorption to the mixed membrane increases [25, 27, 33,34,35,38].
Conclusions
The block copolymer PnBA-b-PAA is a pH-responsive polymer which transforms HSPC conventional liposomes to advanced drug delivery nanosystems. The above study was performed in two buffers, first at PBS of pH ~7.4, as well as in citrate buffer with pH ~4.0. Under the presence of PnBA-b-PAA at both buffers, the pretransition peak of HSPC disappeared in DSC experiments. For all molar ratios of HSPC:PnBA-b-PAA 15/85 and 30/70 bilayers, T onset,m and T m do not change significantly. This phenomenon may be explained by the decrease of cooperativity between HSPC and polymeric guest, especially in citrate buffer, as the percentage of the copolymer increases (the ΔΤ 1/2 values are increased). In citrate buffer, the carboxyl groups of PAA block are protonated, causing shrinkage of polymer chains and yet destabilization of the membrane. We also concluded that the appearing shoulders in the transition peaks suggest the presence of polymer-rich and polymer-poor domains (nanoclusters). DSC cooling curves of HSPC:PnBA-b-PAA 15/85 bilayers have shown small hysteresis at all molar ratios in both buffers, because of the possible formation of an interdigitated phase. For HSPC:PnBA-b-PAA 30/70 bilayers, bimodal distributions (creation of a new phase) were observed at 9:0.1, 9:0.5 and 9:1.0 molar ratios, especially in DSC heating and cooling scans in citrate buffer. Regarding all HSPC:PnBA-b-PAA liposomal systems in PBS buffer solutions, they have shown constant hydrodynamic diameter values for a long period of 30 days. The hydrophobic PnBA block as a spacer decreases the membrane tension and prevents liposomal fusion, while –COO− groups of PAA block provide electrostatic repulsion and induce liposomal stability. In order to confirm the pH responsiveness of the systems, we finally examined the physicochemical characteristics of HSPC:PnBA-b-PAA 15/85 and 30/70 liposomes in citrate buffer. All systems presented lower values of D h in citrate buffer (the only exception was HSPC:PnBA-b-PAA 30/70 9:0.1 molar ratio system). The incorporation of pH-responsive block copolymers into lipid membranes changes the thermal and physicochemical stability of both bilayers and liposomes. This characteristic can be further exploited in order to deliver drugs exactly to the pathological tissues, achieve prompt effect and then maintain the derived therapeutic drug concentration. Moreover, thermodynamics is the fundamental scientific tool that could efficiently be used for studying and analyzing the behavior of artificial biological membranes, could be correlated with biological networks and further could create scientific platforms for the system therapeutics concept.
References
Liu X, Huang G. Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J Pharm Sci. 2013;8:319–28.
Simões S, Moreira JN, Fonseca C, Düzgüneş N, de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–65.
Fujiwara M, Baldeschwieler JD, Grubbs RH. Receptor-mediated endocytosis of poly(acrylic acid)-conjugated liposomes by macrophages. Biochim Biophys Acta. 1996;1278:59–67.
Lin YL, Jiang G, Birrell LK, El-Sayed MEH. Degradable, pH-sensitive, membrane-destabilizing, comb-like polymers for intracellular delivery of nucleic acids. Biomaterials. 2010;31:7150–66.
Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, Liang XJ. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710.
Wang L, Geng D, Su H. Safe and efficient pH sensitive tumor targeting modified liposomes with minimal cytotoxicity. Colloids Surf B Biointerfaces. 2014;123:395–402.
Felber AE, Dufresne MH, Leroux JC. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv Drug Deliv Rev. 2014;64:979–92.
Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposomal-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2014;34:3042–52.
Hruby M, Filippov SK, Štěpanek P. Smart polymers in drug delivery systems on crossroads: which way deserves following? Eur Polym J. 2015;65:82–97.
Kitayama H, Takechi Y, Tamai N, Matsuki H, Yomota C, Saito H. Thermotropic phase behavior of hydrogenated soybean phosphatidylcholine-cholesterol binary liposome membrane. Chem Pharm Bull. 2014;62(1):58–63.
Koynova R, Caffrey M. Phases and phase transitions of phosphatidylcholines. Biochim Biophys Acta. 1998;1376:91–145.
Chen J, Cheng D, Li J, Wang Y, Guo J, Chen Z, Cai B, Yang T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug Dev Ind Pharm. 2013;39(2):197–204.
Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, Deng Y. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10:81–98.
Benesch MG, McElhaney RN. A comparative differential scanning calorimetry study of the effects of cholesterol and various oxysterols on the thermotropic phase behavior of dipalmitoylphosphatidylcholine bilayer membranes. Chem Phys Lipids. 2016;195:21–33.
Bonora S, Torreggianib A, Finia G. DSC and Raman study on the interaction between polychlorinated biphenyls (PCB) and phospholipid liposomes. Thermochim Acta. 2003;408(1–2):55–65.
Berényi S, Mihály J, Kristyán S, Naszályi NL, Telegdi J, Bóta A. Thermotropic and structural effects of poly(malic acid) on fully hydrated multilamellar DPPC-water systems. Biochim Biophys Acta. 2013;1828(2):661–9.
Matsingou C, Demetzos C. Calorimetric study on the induction of interdigitated phase in hydrated DPPC bilayers by bioactive labdanes and correlation to their liposome stability. The role of chemical structure. Chem Phys Lipids. 2007;145:45–62.
Di Foggia M, Bonora S, Tinti A, Tugnoli V. DSC and Raman study of DMPC liposomes in presence of Ibuprofen at different pH. J Therm Anal Calorim. 2016. doi:10.1007/s10973-016-5408-8.
Tirrell DA. pH-Dependent complexation of poly(acrylic acid) derivatives with phospholipid vesicle membranes. Macromolecules. 1984;17(9):1692–8.
Ceckler TL, Cunningham BE. Transition state thermodynamics of lipid bilayers characterized by differential scanning calorimetry. Chem Educ. 1997;2(6):1–17.
Huang C, Li S. Calorimetric and molecular mechanics studies of the thermotropic phase behavior of membrane phospholipids. Biochim Biophys Acta. 1999;1422:273–307.
Pippa N, Gardikis K, Pispas S, Demetzos C. The physicochemical/thermodynamic balance of advanced drug liposomal delivery systems. J Therm Anal Calorim. 2014;116:99–105.
Pippa N, Chountoulesi M, Kyrili A, Meristoudi A, Pispas S, Demetzos C. Calorimetric study of pH-responsive block copolymer grafted lipid bilayers: rational design and development of liposomes. J Liposome Res. 2016;26(3):211–20.
Drummond DC, Zignani M, Leroux JC. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39:409–60.
Munavirov BV, Filippov AV, Rudakova MA, Antzutkin ON. Polyacrylic acid modifies local and lateral mobilities in lipid membranes. J Dispers Sci Technol. 2014. doi:10.1080/01932691.2013.823096.
Filippov A, Munavirov B, Sparrman T, Ishmuhametova V, Rudakova M, Shriram P, Tavelin S. Interaction of a poly(acrylic acid) oligomer with dimyristoylphosphatidylcholine bilayers. Langmuir. 2011;27:3754–61.
Fujiwara M, Grups RH, Baldeschwieler JD. Characterization of pH-dependent poly(acrylic acid) complexation with phospholipid vesicles. J Colloid Interface Sci. 1997;185:210–6.
Chen J, He CQ, Lin AH, Xu F, Wang F, Zhao B, Liu X, Chen ZP, Cai BC. Brucine-loaded liposomes composed of HSPC and DPPC at different ratios: in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2014;40(2):244–51.
Ohtakee S, Schebor C, Palecek SP, de Pablo JJ. Phase behavior of freeze-dried phospholipid-cholesterol mixtures stabilized with trehalose. Biochim Biophys Acta. 2015;1713:57–64.
Demetzos C. Differential scanning calorimetry (DSC): a tool to study the thermal behavior of lipid bilayers and liposomal stability. J Liposome Res. 2008;18(3):159–73.
Amado E, Kressler J. Interactions of amphiphilic block copolymers with lipid model membranes. Curr Opin Colloid Interface Sci. 2011;16:491–8.
Shen W, Hu J, Hu X. Impact of amphiphilic triblock copolymers on stability and permeability of phospholipid/polymer hybrid vesicles. Chem Phys Lett. 2014;600:56–61.
Pearson RT, Warren NJ, Lewis AL, Armes SP, Battaglia G. Effect of pH and temperature on PMPC–PDPA copolymer self-assembly. Macromolecules. 2013;46:1400–7.
Felber AE, Dufresne M-H, Leroux J-C. pH-sensitive vesicles, polymeric micelles and nanospheres prepared with polycarboxylates. Adv Drug Deliv Rev. 2014;64:979–92.
Hao W, Han X, Shang Y, Xu S, Liu H. Insertion of pH-sensitive bola-type copolymer into liposome as a “stability anchor” for control of drug release. Colloids Surf B Biointerfaces. 2015;136:809–16.
Xia T, Hao W, Shang Y, Xu S, Liu H. Incorporation of amphipathic diblock copolymer in lipid bilayer for improving pH responsiveness. Int J Polym Sci. 2016. doi:10.1155/2016/5879428.
Goldberg R, Schroeder A, Barenholz Y, Klein J. Interactions between absorbed hydrogenated soy phosphatidylcholine (HSPC) vesicles at physiologically high pressures and salt concentrations. Biophys J. 2011;100:2403–11.
Demetzos C. Biophysics and thermodynamics. The scientific building-blocks of bioinspired drug delivery systems. AAPS PharmSciTech. 2015;16(3):491–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Aimilia Kyrili and Maria Chountoulesi have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kyrili, A., Chountoulesi, M., Pippa, N. et al. Design and development of pH-sensitive liposomes by evaluating the thermotropic behavior of their chimeric bilayers. J Therm Anal Calorim 127, 1381–1392 (2017). https://doi.org/10.1007/s10973-016-6069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6069-3