Abstract
Two novel mononuclear lanthanide complexes, [Ln(2,4-DClBA)3(terpy)(H2O)]·H2O (Ln = Dy(1), Er(2)); 2,4-DClBA: 2,4-dichlorobenzoate; terpy: 2,2′:6′,2″-terpyridine; have been synthesized and characterized by single-crystal and powder X-ray diffraction. The results reveal that complexes 1–2 are isomorphous, and each Ln3+ ion is nine coordinated adopting a distorted monocapped square antiprismatic molecular geometry. Mononuclear complexes 1–2 are stitched together via Cl–π and hydrogen bonding interactions to form the 1D, 2D, 3D supramolecular structures, which the 3D is rarely observed. Thermal decomposition mechanism is determined by TG/DSC-FTIR, and the 3D stacked plots for the FTIR spectra of the evolved gases were recorded. Heat capacities of complexes 1–2 were measured, and the derived thermodynamic functions (H T − H 298.15), (S T − S 298.15) and (G T − G 298.15) of the complexes relative to the standard reference temperature 298.15 K were obtained. The activation energy E values of the first decomposition stage for title complexes were calculated by integral isoconversional nonlinear (NL-INT) and Starink methods. Complex 1 shows the characteristic emission of Dy3+ with strong yellow emission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study of lanthanide complexes combining with aromatic carboxylic acids has attracted extensive attention, according to their diverse coordination types and fascinating properties [1–4]. At 2014, Sun et al. [5] reported that the rare earth complexes show potential antifungal properties, exerting various and selective inhibitions against the plant pathogenic fungi. The lanthanide complexes also had good bacteriostatic activity against Candida albicans, which has been reported by Zong at 2015 [6]. In addition, a wide variety of applications including gas separation and storage [7], nonlinear optics, electroluminescent materials [8], luminescent bioprobes [9], and catalysis [10] have been utilized as functional materials. However, the direct excitation of lanthanide metal centers is limited by small absorption cross sections, low molar absorption coefficients, and the Laporte forbidden f–f transitions [9, 11]. So the suitable ligands are used to stimulate luminescence of lanthanide ions via the effective energy transformation, which is called “antenna effect” [12], but the triplet states energies of organic chromophores should be high or close to lanthanide ions [13].
Aromatic carboxylic acids are usually chosen as the organic ligands since they can blind to the lanthanide cations in various modes: monodentate, chelating, bridging, and bridging–cheating to form supramolecular structures [14]. Terpy has been shown to be an excellent antenna unit for lanthanide luminescence and also performed the good coordination ability as the second ligand [15].
Therefore, we choose 2,4-dichlorobenzoic acid (2,4-DClHBA) as the main ligands, and 2,2′:6′,2″-terpyridine (terpy) as auxiliary ligands to validly sensitize the lanthanide metal ions to assemble two novel hybrid compounds [Ln(2,4-DClBA)3(terpy)(H2O)]·H2O (Ln = Dy(1), Er(2); 2,4-DClBA = 2,4-dichlorobenzoate; terpy = 2,2′:6′,2″-terpyridine). The structures of the title complexes are all different from our previous reports [16–19], and they are all mononuclear molecules, which makes them to form the multidimensional structure. The thermal decomposition processes were studied by the TG/DSC-FTIR technology. The heat capacities of the complexes were studied, which is crucial for designing chemical processes as well as for the progress of thermodynamic theories. Luminescence spectrum for complex 1 and the average activation energy E values of the first decomposition stage for title complexes were also studied.
Experimental
Materials and methods
LnCl3·6H2O was acquired by the reaction of Ln2O3 (Ln = Dy, Er, Beijing Lanthanide Innovation Technology Co., Ltd, 99.9 %) and hydrochloric acid aqueous solution in the condition of water bath heating at a constant temperature of 353.15 K, followed by evaporation of the liquid. The other analytically pure chemicals were purchased and used without further purification.
Equipment and conditions of the experiment
Analyses for C, H, N in the corresponding complexes were carried out via Vario-EL II element analyzer, and the percentage of the lanthanide cations were obtained by EDTA titrimetric analysis. The data of single-crystal X-ray diffraction were collected on a Smart-1000 diffractometer with graphite–monochromatic Mo Kα (λ = 0.71073 Å) at the temperature of 298(2) K. The structures were solved using the SHELXS-97 program (direct methods) and refined by the full-matrix least squares on F 2 using the SHELXL-97 program. Powder X-ray diffraction identification was carried out by a Bruker D8 ADVANCE X-ray diffractometer in a scanning range of 5°–50° (2θ) with graphite–monochromatic Cu Kα (λ = 1.54178 Å) at 298 (2) K. TG/DSC-FTIR analyses were performed with a heating rate of 10 K min−1 using a NETZSCH STA 449 F3 instrument with a Bruker TENSOR27 Fourier transform infrared spectrometer. The luminescence spectra were measured on an F-4600 Hitachi Spectrophotometer. Heat capacities of the prepared complexes were carried out on a NETZSCH DSC 200 F3 (nitrogen atmosphere) in the temperature of 263.15–337.15 K using an indirect measurement method. Non-isothermal kinetics of the first decomposition stage for title complexes were determined with the NETZSCH STA 449 F3 thermal analyzer under simulated air atmospheres by the linear heating rate of 10, 12, 15, 20 K min−1.
Synthesis
Dissolve 2,4-DClHBA (0.6 mmol) and terpy (0.2 mmol) in ethanol (95 %) and adjust the solution at the pH of 5–7 with the prepared NaOH solution (1 mol L−1). Add the mixed ligands solution to LnCl3·6H2O (0.2 mmol) aqueous solution under stirring and deposit it for 12 h. The powders of target complexes were obtained by filtration, and the colorless, pink platy single crystals were acquired through the method of solvent extraction at room temperature. Element analysis: calcd (%) for C36H24Cl6N3O8Dy: C, 43.16; H, 2.41; N, 4.19; Dy, 16.22. Found: C, 42.84; H, 2.43; N, 4.10; Dy, 16.15. calcd (%) for C36H24Cl6N3O8Er: C, 42.96; H, 2.40; N, 4.17; Er, 16.62. Found: C, 42.53; H, 2.36; N, 4.04; Er, 16.48.
Results and discussion
Crystal structure
Single-crystal X-ray diffraction reveals that complexes 1–2 are isomorphous, and crystallize in the triclinic space group Pī (Table 1). As a consequence, the crystal structure of complex 1 will be described here in detail.
Complex 1 is mononuclear, which is different from the most synthesized binuclear complexes [20, 21]. The mononuclear unit contains one Dy3+ cation, three 2,4-DClBA acid ligands, one tridentate terpy ligand, a coordinated water molecule, and an uncoordinated water molecule (Fig. 1a). Each Dy3+ cation is coordinated with nine atoms. There are five oxygen atoms from 2,4-DClBA acid ligands, which coordinate to Dy3+ by bidentate chelating, monodentate coordination modes. One oxygen atom comes from the coordinated water, and three nitrogen atoms supported by the tridentate terpy (Fig. 1b). The Dy–O bonds range from 2.290(5) to 2.632(8) Å and average Dy–N bond distances for the terpy molecule are 2.544 Å, which is obviously to found that the distance of Ln–N is reducing almost with the decrease in lanthanide radius, which is also according with the process of thermal decomposition (Table 2).
As shown in Fig. 2a, the mononuclear molecular structure of complex 1 is assembled into a supramolecular 1D chain by two independent and moderate Cl–π interactions [22] along the z-axis direction at the distance 3.368 and 3.560 Å. The chains are further packed into a 2D sheet in the xz plane (Fig. 2b) through two kinds of different hydrogen bonding interactions (Table 3). One is between carboxylic oxygen from monodentate 2,4-DClBA acid ligands and C–H of the adjacent terpy ligands unit at a distance of 2.820 Å along x-axis, and anther C–H···O hydrogen bonds [23] exists between uncoordinated water molecules and C–H of the neighboring terpy unit along the line of angle from z-axis and negative x-axis at the distance of 2.530 Å. There exist O–H···C hydrogen bonds between carbon from 2,4-DClBA ligands unit and O–H of the adjacent uncoordinated water unit at the distance of 2.890 Å to form 3D supramolecular network (Figure 2c).
Previously, the binuclear molecular complex [Dy(2,4-DClBA)3phen]2 has been reported [20]. Each Dy3+ cation is surrounded by eight atoms, which is described as a distorted square antiprism. The structure of the mononuclear [Dy(2,4-DClBA)3(terpy)(H2O)]·H2O is totally different from the complex [Dy(2,4-DClBA)3phen]2, and the water molecule has coordinated with the Dy3+ at the molecular complex [Dy(2,4-DClBA)3(terpy)(H2O)]·H2O structure. As shown in Table 2, the average distance of the Dy–N for complex 1 is 2.544 Å and is shorter than the complex [Dy(2,4-DClBA)3phen]2 with the distance of 2.547 Å, indicating that the terpy has a stronger blinding affinity for the Ln3+ ions than the phen ligand.
Powder X-ray diffraction
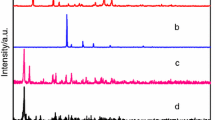
The powder X-ray diffraction (PXRD) patterns [24, 25] of complexes 1–2 and two ligands have been carried out at room temperature. As shown in Fig. 3, the curves of the title complexes are different from the two ligands, indicating the two new complexes have been produced. The curves are almost similar with each other, indicating they are isostructural. As shown in Fig. 4, the diffraction peaks are almost in agreement with simulated data, showing that the structures of the powder complexes 1–2 are similar with the pure crystal.
Thermal decomposition processes
The thermal decomposition processes of complexes 1–2 were determined from 298.15 to 1173.15 K at a heating rate of 10 K min−1 under a simulated air atmosphere. The data of thermal decomposition of the title complexes are listed in Table 4, and the IR spectra of the evolved gases at different temperature are shown in Figs. 4 and 5. Thermal decomposition process of complex 1 is different from complex 2, according to the TG curves. Complex 1 and 2 would be described here in detail.
There are at least five thermal decomposition processes for complex 1 according to the DTG curve, which was shown in Fig. 4a, and it is consistent with the FTIR spectra of the evolved gases (Fig. 4b). The first stage between 373.15 and 402.15 K belongs to the release of one uncoordinated water molecule with a mass loss of 1.77 % (Calcd: 1.80 %), and at the same time, there is a small endothermic peak (T p = 389.45 K; ΔH = 84.15 kJ mol−1). As shown in Fig. 6a, the IR spectra at 393.15 K appear weak absorption peak at 3560–3875 and 1376–1853 cm−1, indicating the production of water at first stage. The second stage at temperature of 402.15–514.15 K accounts for the loss of one coordinated water and part terpy with the mass loss of 8.78 % and absorbs little quality of heat (T p = 494.45 K; ΔH = 19.15 kJ mol−1). In the IR spectra at 494.05 K, we can find the weak absorption peak of water at 3571–3879, 1372–1847 cm−1 and the peak of CO2 at 2367–2319 cm−1. In the third step, there is a mass loss of 16.29 % at the temperature of 514.15–684.15 K, belonging to the decomposition of the rest terpy, and one small endothermic peak (T p = 527.85 K; ΔH = 24.93 kJ mol−1) on the DSC curve can be observed. The thermal decomposition processes also generate CO2 (2365–2317 cm−1), H2O (3571–3879 cm−1) and appear the characteristic peaks of ν C=C, νC=N at 1597, 1489 cm−1, according to the IR spectra. In the fourth and fifth stages, the 2,4-DClBA occurs to decompose, which shows in the TG/DTG-DSC curve at the temperature of 684.15–793.15 and 793.15–1045.15 K with the mass loss of 39.85 and 14.58 %. Different from the former stages, the two stages appear one big exothermic peak at DSC curve (T p = 819.35 K; ΔH = −2830.17 kJ mol−1), and the complex 1 is completely degraded into Dy2O3 with a mass loss of 81.27 % (Calcd: 81.40 %). In Fig. 6a, there is the same absorption at temperature of 771.15 and 527.85 K, except that the absorption peak of CO2 is much stronger. But at 819.35 K, the peak of H2O disappears and only emerges the CO2 absorption at 2364–2316,679 cm−1.
For complex 2, the thermal decomposition processes divide into four steps, according to the DTG curve (Fig. 5a). One uncoordinated water decomposes between 374.15 and 401.15 K in the first step with a mass loss of 1.80 % (Calcd. 1.79 %) and appears a small endothermic peak at the DSC curve (T p = 386.45 K; ΔH = 78.99 kJ mol−1). There is no IR absorption in the first stage and can be explained that the signal of gas is too weak to be detected. In the second step, the terpy ligand was decomposed at 401.15–696.15 K with a mass loss of 25.24 % (Calcd 25.17 %), which absorbs the heat of 8.63 kJ mol−1. In the IR spectra (Fig. 6b) at 605.01 K, there are the characteristic bands of CO2 (2367–2315 cm−1) and ν C=C, ν C=N and γ =C–H (1596, 1487 and 795 cm−1), assigning to the decomposition product. The third and fourth step could be seen one big process, which is the decomposition of 2,4-DClBA with the mass loss of 54.87 % (Calcd 54.37 %). According to the DSC, there is a big exothermic peak, indicating the variation of the energy of the process. In the IR spectra, we can find the absorptions of CO2 (2368–2314, 675 cm−1), ν C=C, ν C=N and γ =C–H (1596, 1487 and 795 cm−1) at 772.34 K, and the stronger absorption of CO2 (2368–2314, 675 cm−1), the peak of CO (2183, 2114 cm−1) at 814.92 K. At last, the complex 2 is completely degraded into Ho2O3 with a mass loss of 81.91 % (Calcd 81.33 %).
Kinetics of the first decomposition stage
The non-isothermal multiple scan method has been widely used to obtain the reliable value of the average activation energy E, in which the kinetic model function is not involved. Therefore, the average activation energy E [26] of the first decomposition for complexes 1–2 was been calculated via the integral isoconversional nonlinear (NL-INT) method [27, 28]. As shown in Fig. 7, it is obviously to observe that the activation energy E of the title complexes varies with conversion rate (α), respectively, indicating that the first thermal decomposition stage with losing H2O for complexes 1–2 is complex actions [29].
Heat capacities of the complexes
The molar heat capacities of the complexes 1–2 were measured via DSC from the temperature of 263.15–347.15 K at a heating rate of 10 K min−1 under the nitrogen atmosphere. The average molar heat capacities of title complexes are listed in Table S1 and plotted in Fig. 8. As shown in Fig. 8 the molar heat capacities of complexes gradually increase with the elevation of temperature. The heat capacities of the title complexes were fitted to the following polynomial equations by the method of least squares [19, 30, 31] at reduced temperature (x). The equations are given in following:
The reduced temperature (x) is calculated from the formula x = [T − (T max + T min)/2]/[(T max − T min)/2] (T max = the highest temperature, T min = the lowest temperature). R 2 is the correlation coefficient, and SD is the standard deviation. The smoothed heat capacities, enthalpy, entropy, and Gibbs free energy relative to the reference temperature 298.15 K of the title complexes with an interval of 3 K which is shown in Table S2-S3 were calculated according to the fitted polynomial equations and the following thermodynamic equations:
Luminescent behavior
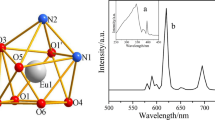
The solid-state luminescent behavior of complex 1 has been investigated in detail at room temperature. The emission spectrum of complex 1 was obtained with excitation wavelength at 279 nm, and the emission spectrum of Dy3+ cation in the range of 450–620 nm shows two different characteristic peaks (Fig. 9) at 483, 580 nm, respectively, which are associated with the 4f–4f transitions of 4F9/2 → 6H15/2, 4F9/2 → 4H13/2. The hypersensitive transition (4F9/2 → 4H13/2) at 580 nm dominates the main emission spectra, resulting in the characteristic yellow luminescence of arrowhead complex. This indicates that organic chromophore terpy with triplet states energies is in the appropriate for sensitizing the Dy3+ cation.
Conclusions
In summary, the synthesis and characterization of the title complexes have been successfully reported. These complexes are isomorphous with nine coordinated atoms, and the crystal structures of them have performed the mononuclear, which is totally different from the previous complexes. Complexes 1–2 show the 1D, 2D, 3D supramolecular structures. According to the study of TG/DSC-FTIR, thermal decomposition mechanisms of the title complexes are obtained and the uncoordinated water all lost firstly. The average molar heat capacity value of the complexes gradually increased with the elevation of temperature, and their heat capacities fitted to a polynomial equation. The derived thermodynamic functions (H T − H298.15), (S T − S298.15), and (G T − G298.15) of the complexes relative to the standard reference temperature 298.15 K were also obtained. Complex 1 shows the characteristic emission of Dy3+ with strong yellow emission.
Supporting information
The numbers of the three complexes (CCDC 1459059 (1), 1459061 (2)) contain the supplementary crystallographic data for this paper, which can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Experimental molar heat capacities of complexes 1–2 have been performed in Table S1, and smoothed molar heat capacities and thermodynamic functions of complexes 1–2 were shown in Table S2-S3, respectively.
References
Li RF, Liu XF, Zhang T, Wang LY, Ma LF, Feng X. Two unique lanthanide–organic frameworks based on biphenyl-2,3,3′,5′-tetracarboxylic acid: syntheses, crystal structures and luminescence properties. Polyhedron. 2015;99:238–43.
Zhang S, Duan E, Han Z, Li L, Cheng P. Lanthanide coordination polymers with 4,4′-azobenzoic acid: enhanced stability and magnetocaloric effect by removing guest solvents. Inorg Chem. 2015;54:6498–503.
Maity M, Majee MC, Kundu S, Samanta SK, Sanudo EC, Ghosh S, Chaudhury M. Pentanuclear 3d–4f heterometal complexes of M(II)3Ln(III)2 (M = Ni, Cu, Zn and Ln = Nd, Gd, Tb) combinations: syntheses, structures, magnetism, and photoluminescence properties. Inorg Chem. 2015;54:9715–26.
Ay B, Karaca S, Yildiz E, Lopez V, Nanao MH, Zubieta J. In situ hydrothermal syntheses, structures and photoluminescent properties of four novel metal-organic frameworks constructed by lanthanide (Ln = Ce(III), Pr(III), Eu(III)) and Cu(I) metals with flexible dicarboxylate acids and piperazine-based ligands. J Solid State Chem. 2016;233:415–21.
Sun X, Jin X, Pan W, Wang J. Syntheses of new rare earth complexes with carboxymethylated polysaccharides and evaluation of their in vitro antifungal activities. Carbohydr Polym. 2014;113:194–9.
Zong GC, Huo JX, Ren N, Zhang JJ, Qi XX, Gao J, Geng LN, Wang SP, Shi SK. Preparation, characterization and properties of four new trivalent lanthanide complexes constructed using 2-bromine-5-methoxybenzoic acid and 1,10-phenanthroline. Dalton Trans. 2015;44:14877–86.
Gu JZ, Wu J, Lv DY, Tang Y, Zhu K, Wu J. Lanthanide coordination polymers based on 5-(2′-carboxylphenyl) nicotinate: syntheses, structure diversity, dehydration/hydration, luminescence and magnetic properties. Dalton Trans. 2013;42:4822–30.
Yang L, Liu L, Wu L, Xu Z, Wang L. Isomorphous and isostructural lanthanide coordination polymers based on 2-(4-chlorobenzoyl)benzoic acid: synthesis, structure, characterization, and luminescent properties. Dyes Pigments. 2014;111:176–84.
Oliveira CK, de Souza VP, da Luz LL, de Menezes Vicenti JR, Burrow RA, Severino A, Longo RL, Malvestiti I. Synthesis, crystal structure and luminescent properties of lanthanide extended structure with asymmetrical dinuclear units based on 2-(methylthio)benzoic acid. J Lumin. 2016;170:528–37.
Du M, Wang X, Chen M, Li CP, Tian JY, Wang ZW, Liu CS. Ligand symmetry modulation for designing a mesoporous metal-organic framework: dual reactivity to transition and lanthanide metals for enhanced functionalization. Chemistry. 2015;21:9713–20.
Xu C, Kirillov AM, Shu Y, Liu Y, Guo L, Yang L, Dou W, Liu W, Chen C, Huang X, Zhang J, Liu W. Photoluminescence enhancement induced by a halide anion encapsulation in a series of novel lanthanide(iii) coordination polymers. CrystEngComm. 2016;18:1190–9.
Ay B, Yildiz E, Kani İ. Novel heteroleptic lanthanide organic frameworks containing pyridine-2,5-dicarboxylic acid and in situ generated piperazine-2,5-dicarboxylic acid from piperazine: hydrothermal synthesis and luminescent properties. J Solid State Chem. 2016;233:44–51.
Marques LF, Cuin A, de Carvalho GSG, dos Santos MV, Ribeiro SJL, Machado FC. Energy transfer process in highly photoluminescent binuclear hydrocinnamate of europium, terbium and gadolinium containing 1,10-phenanthroline as ancillary ligand. Inorg Chim Acta. 2016;441:67–77.
Marques LF, Cantaruti AAB, Correa CC, Lahoud MG, da Silva RR, Ribeiro SJL, Machado FC. First crystal structures of lanthanide-hydrocinnamate complexes: hydrothermal synthesis and photophysical studies. J Photochem Photobiol A Chem. 2013;252:69–76.
Carter KP, Pope SJA, Cahill CL. A series of Ln-p-chlorobenzoic acid–terpyridine complexes: lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm. 2014;16:1873.
Zhang YY, Ren N, Xu SL, Zhang JJ, Zhang DH. A series of binuclear lanthanide(III) complexes: crystallography, antimicrobial activity and thermochemistry properties studies. J Mol Strut. 2015;1081:413–25.
Zhang YY, Ren SH, Ren N, Zhang JJ, Geng LN, Wang SP, Shi SK. Crystal structures, spectroscopic, and thermal properties of dysprosium(III) and europium(III) complexes with 3-chloro-4-methoxybenzoic and 1,10-phenanthroline. J Therm Anal Calorim. 2015;119:1803–10.
Qi XX, Wu JC, Ren N, Zhao CL, Zhang JJ, Zong GC, Gao J. Novel lanthanide complexes constructed from 3, 4-dimethoxybenzoic acid: crystal structures, spectrum and thermochemical properties. Thermochim Acta. 2015;615:1–7.
Wang MH, Tan ZC, Sun XH, Zhang HT, Liu BP, Sun LX, Zhang T. Determination of heat capacities and thermodynamic properties of 2-(chloromethylthio)benzothiazole by an adiabatic calorimeter. Chem Eng Data. 2005;50:270–3.
Liu JY, Ren N, Zhang JJ, He SM, Wang SP. Crystal structures, thermal properties, and biological activities of a series of lanthanide compounds with 2,4-dichlorobenzoic acid and 1,10-phenanthroline. Ind Eng Chem Res. 2013;52:6156–63.
Huo JX, Wang Y, Zhang DH, Ren N, Zhang JJ. Syntheses, characterization, luminescence, and thermal decomposition mechanism of four lanthanide complexes with 4-ethylbenzoic acid and terpyridine. J Therm Anal Calorim. 2016;124:1575–85.
Carter KP, Zulato CHF, Cahill CL. Exploring supramolecular assembly and luminescent behavior in a series of RE-p-chlorobenzoic acid-1,10-phenanthroline complexes. CrystEngComm. 2014;16:10189–202.
Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins. Nature. 1993;366:529–31.
Zhou JM, Shi W, Xu N, Cheng P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg Chem. 2013;52:8082–90.
Onoda M, Yamamoto A, Takayama-Muromachi E, Takekawa S. Assignment of the powder X-ray diffraction pattern of superconductor Bi2(Sr,Ca)3–xCu2Oy. Jpn J Appl Phys. 1988;27:L833–6.
Ye HM, Ren N, Zhang JJ, Sun SJ, Wang JF. Crystal structures, luminescent and thermal properties of a new series of lanthanide complexes with 4-ethylbenzoic acid. New J Chem. 2010;34:533.
Vyazovkin S, Dollimore D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Mode. 1996;36:42–5.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Therm Acta. 2003;404:163–76.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Qi XX, Ren N, Zhang DH, Zhang JJ. Synthesis, spectroscopic, thermochemical properties of lanthanide complexes with 3,4-diethoxybenzoic acid and 1,10-phenanthroline. Chem Res Chin Univ. 2015;31:1039–45.
Jin CW, Wang Y, Xu SL, Zhang JJ. Synthesis, crystal structures and thermochemical properties of ternary rare earth complexes based on 3,4-diethoxybenzoic acid and 2,2′-bipyridine. Acta Phys Chim Sin. 2016. doi:10.3866/PKU.WHXB201605263.
Acknowledgements
The research work was supported by the National Natura Science Foundation of China (No. 21473049) and the Natural Science Foundation of Hebei Province (No. B2016205207).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhao, QQ., Ren, N. et al. Crystal structures, thermal properties, and luminescent properties of two novel mononuclear lanthanide complexes with 2,4-dichlorobenzoic acid and 2,2′:6′,2″-terpyridine. J Therm Anal Calorim 126, 1703–1712 (2016). https://doi.org/10.1007/s10973-016-5728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5728-8