Abstract
The purpose of this study was to examine the direct effect of sucrose and sucralose on the main phase transition of dipalmitoyl phosphatidylcholine (DPPC) liposomes with differential scanning calorimetry, for use as potential cryopreservatives for cells. Because cells are typically treated directly with cryopreservatives and not thermally cycled to erase thermal history, we have focused on the impact that these sugars have on the main phase transition during the first heating and cooling cycles. The incorporation of sucrose appears to have very little effect on the phase behavior of DPPC liposomes except a broadening of the main phase transition. In contrast, sucralose leads to a drop in the transition peak temperature from 41.5 to 39.6 °C when there is 50 mol% sucralose present. Further increases in sucralose concentration result in the peak transition temperature gradually increasing again to 41.1 °C at 87 mol% sugar. This change is accompanied by decrease in the width of the transition, during heating, as well as increased hysteresis. This phenomenon may be due to a kosmotropic effect resulting in partial dehydration and interdigitation of the bilayer as the sucralose concentration is increased. These findings indicate that sucralose may be useful for the cryopreservation of cell membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbohydrates are found in a wide variety of organisms known to survive extreme conditions such as freezing, desiccation, and/or oxygen deprivation [1]. Survival of these organisms is directly associated with the synthesis and accumulation of excess amounts of sugar that prevent damage to biological membranes under such extreme conditions. Several studies aimed at understanding this phenomenon have indicated that carbohydrates (mono- and disaccharides) have a significant stabilizing effect on the physical properties of phospholipid membranes [2–4].
The thermal behavior of phospholipid bilayers has been extensively studied, and there are many excellent reviews describing the complex behavior of different lipid systems [5–7].
Throughout these studies, synthetic phospholipid bilayers, particularly that of 1,2-dipalmitoyl phosphatidylcholine (DPPC), have been used as a simple membrane model, since it is the most abundant lipid in most eukaryotic cells and its phase behavior is very well understood and characterized [8]. The main phase transition of this liposome system, gel to the liquid crystalline, occurs due to an increase in the mobility of the fatty acid chains as a result of decreased hydrophobic van der Waals interactions. This transition is highly sensitive to perturbations such as pH, ionic strength, and the introduction of other molecules and is relatively easily probed using differential scanning calorimetry (DSC).
Previous studies, on various types of hydrated DPPC systems, show that, in the presence of specific sugars (e.g., trehalose, sucrose, maltose, and glucose), the membrane phase transition from the gel to liquid crystalline phase can be significantly altered [4]. It has been suggested that certain carbohydrates may act as substitutes for water molecules on the membrane surface, as well as within the bilayer itself; effectively replacing water molecules and stabilizing the membrane during dehydration [9–11]. According to this “water-replacement” hypothesis, the sugar molecules insert themselves between the lipid head groups within membranes, subsequently leading to the sugars interacting with the lipids via hydrogen bonding [9, 10]. These hydrogen bonding interactions between sugar molecules and lipid polar head groups lead to a decrease in the packing efficiency of the hydrocarbon chains and an increase in the fluidity of the bilayer [10]. Additional studies have also shown that protection of the membrane bilayer is closely associated with the ability of certain sugars to vitrify, forming a glassy matrix that inherently prevents leakage and fusion in membrane bilayers [10–13].
In the present study, we focus on investigating the effects of sucrose and its analog sucralose on hydrated DPPC unilamellar vesicles with hopes of better understanding the interaction between carbohydrates and phospholipid membranes.
Materials and methods
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) were purchased from Avanti Polar Lipids (Alabaster, AL) as lyophilized dry powders (purity > 99 %) and were stored at −20 °C. Sucrose was purchased from Fisher Scientific (Hampton, NH) as a crystalline solid (purity: ACS Certified). Sucralose (purity > 98 %) was purchased as an anhydrous powder from Spectrum Chemical Mfg. Co. (New Brunswick, NJ, USA). All reagents were used without further purification.
Preparation of unilamellar vesicles for DSC
Unilamellar vesicles were prepared by hydrating 40 mg of dry lipid powder in 1 mL of ultrapure deionized water. Each sample was hydrated above the main phase transition temperature for 60 min with frequent vortexing to ensure homogenous mixing. After hydration, each sample was subjected to three freeze/thaw cycles using liquid nitrogen and a dry heating block and then extruded above the Tm using an Avanti Polar Lipids mini-extruder (Alabaster, AL) through a 100 nm polycarbonate membrane (Whatman Nucleopore; Clifton, NJ, USA) for a total of 15 passes across the membrane. Following extrusion, each sample was refrigerated overnight (4 °C) prior to the addition of sugar.
Stock sucrose and sucralose solutions were prepared by dissolving an appropriate amount of sugar in high-purity deionized water. Various quantities of stock sugar were added to 100 μL portions of previously prepared unilamellar liposomes, in 2.5 μL additions, approximately every 5 min. Additional 2.5 μL aliquots of high-purity deionized water was added to each sample to guarantee the same final volume for each liposome/sugar solution, as well as to ensure the desired mole percent of sugar was obtained (0.0–87 %). Each liposome/sugar sample was again refrigerated overnight (4 °C) prior to analysis.
Differential scanning calorimetry (DSC)
The thermotropic phase behavior of liposomes in the absence and presence of sugar was examined using a TA Instruments DSC Q2000 calorimeter (New Castle, DE, USA). Each sample (10 μL) was hermetically sealed in T-zero aluminum DSC pans. Samples were equilibrated at 30 °C for 5 min prior to analysis. Data were acquired every 0.20 s at a 2.0 °C min−1 heating and cooling rates over a temperature range of 30–60 °C. All DSC curves were obtained versus a reference pan containing 10 μL of ultrapure deionized water. The onset of the main phase transition, the peak temperature, transition width at half height, and change in enthalpy were directly determined and recorded for both the first heating and cooling cycles, with TA Universal Analysis 2000 software. Reported values and standard error on the mean are for analysis of three independent samples.
Results and discussion
DPPC–sucrose
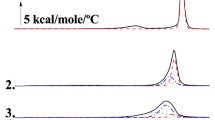
Upon the addition of sucrose, the main phase transition temperature for DPPC changes very little for both the first heating (Fig. 1) and cooling cycles (Fig. 2), indicating that sucrose exerts no substantial destabilizing or stabilizing effect on either the gel or liquid crystalline phase of DPPC liposomes. However, it is possible that the addition of a molecule such as sucrose could have little or no effect on the transition temperature but could still perturb the environment in or around the fatty acid chains. This perturbation, while leaving the main phase transition temperature unchanged, can alter the temperature range over which the transition occurs. In a highly ordered system, one would expect a very narrow temperature range associated with this melting event and that the temperature range might increase as disorder within the fatty acid chains increases. In order to indirectly determine the relative change in the order or disorder of the fatty acid chains, we obtain qualitative estimate of the transition cooperativity by measuring the peak width at half peak height of the gel to liquid crystalline main phase transition [14, 15]. As seen from the DSC curves of the first heating and cooling cycles, the peak width at half peak height gradually increases from 0.4 °C to a maximum of 0.8 °C for the heating cycle and from 0.4 to 2.6 °C for the cooling cycle as sucrose concentrations increase (Tables 1, 2).
As previously mentioned, the peak width at half height may be used to determine the cooperativity of the melting process and an increase in this leads us to believe that we have perturbed the environment around the fatty acid chains of the lipid molecules to such an extent that they are now substantially more disordered, resulting in the main phase transition occurring over a broader temperature range. The induced perturbation may be due to reorganization of lipid molecules within the bilayer as a result of the presence of the sugar near the lipid headgroup. The likelihood, of such a hydrophilic molecule having a more localized affect on the fatty acid chains, is very small. If this were the case, we speculate that the sugar would likely displace water from the headgroup region of the liposome, leading to an increase in the transition temperature, which is typically seen for a kosmotropic effect [16, 17].
The enthalpy of the transition was directly measured via DSC based on the quantity of lipid used, prior to hydration and extrusion. Although variations in concentration are likely, the enthalpy of the first heating cycle remained relatively unchanged, only varying from 30.0 kJ mol−1 to a maximum of 34.7 kJ mol−1 (Table 1). Larger changes are observed for the enthalpy in the cooling direction (Table 2), and the measured enthalpies are larger than those for heating. Because we were primarily interested in examining the immediate effect of adding sugars on model membranes, samples were equilibrated at 30 °C for 5 min prior to analysis, and so, thermal history may not be completely erased which could explain the large differences in the measured enthalpies.
One noticeable feature observed in both heating (Fig. 1) and cooling (Fig. 2) DSC curves is the appearance of shoulders that become more pronounced at higher sugar concentrations. This observation may be indicative of partial phase separation of the membrane bilayer, as this effect has been noted with previously studied molecules [18–21] although typically this is accompanied by a decrease in the transition temperature [22]. A similar effect was noticed for membranes containing cationic dimethyldioctadecylammonium and trehalose 6,6′-dibehenate, although the authors were also unable to sufficiently explain this behavior [23].
DPPC–sucralose
Sucralose would be expected to interact with model membranes in a subtly different manner due to a slight increase in hydrophobicity which should allow it to penetrate further into the membrane bilayer, subsequently causing specific alterations in the thermotropic phase behavior associated with the gel to liquid crystalline main phase transition. As seen in the DSC curves (Fig. 3), a small but significant shift in the main phase transition temperature is observed upon the addition of sucralose, in which the transition temperature decreases from an initial value of 41.5–39.6 °C at 50 mol% sucralose (Table 3).
Although the overall change is small, it was reproducible from sample to sample and the same trend was seen in the onset temperature, a parameter considered to be less dependent on sample mass [24]. This decrease in the transition temperature is not accompanied by any significant changes in enthalpy which would suggest that the lipids are still ordered in the gel phase even with the addition of the sugar and that the liquid crystalline phase is being stabilized. It should be noted however that the transition does become less cooperative (Table 2), indicating some changes in the packing of the fatty acid chains within the DPPC bilayer. At high concentrations of sugar, we observe unusual behavior, during the heating cycle, as we see the main phase transition temperature begin to increase, almost returning to the initial value obtained in the absence of sucralose (Fig. 3).
This increase in the main phase transition temperature is accompanied by a simultaneous decrease in the peak width at half peak height, indicating a more cooperative transition from the gel to liquid crystalline phase. More specifically, when 87 mol% sucralose has been added to DPPC liposomes, the peak width at half peak height is only 0.1 °C (Table 3), a significantly narrower and sharper peak than that of aqueous DPPC liposomes. This behavior is very unusual and may be in part due to a biphasic effect exhibited by sucralose in the presence of DPPC liposomes, as an initial decrease followed by an increase in the main phase transition temperature has been previously reported for DPPC bilayers in the presence of ethanol [25–28]. At low sugar concentrations during the cooling cycle, a similar trend is observed, although in this case the temperature of the transition continues to decrease (Fig. 4; Table 4), leading to increased hysteresis, as sugar concentration is increased. This temperature decrease is accompanied by an increase in the peak width at half height, indicating a change in the environment of the fatty acid chains. This combination of decreasing transition temperature followed by an increase accompanied by decreasing peak width and increased hysteresis has been taken as evidence of the formation of an interdigitated phase in some cases.
The magnitude of the thermal hysteresis, determined from peak temperatures, changes from an initial value of 0.5 °C, for aqueous DPPC, to 4.2 °C when 87 mol% sucralose has been added. This trend of increasing hysteresis further confirmed when using onset temperatures for the heating and cooling cycles (Tables 3, 4). When both the hysteresis and the possible biphasic behavior are taken into account, there is evidence strongly indicating that sucralose is able to partition into the membrane bilayer and interact with the fatty acid chains of the lipid molecules, resulting in chemically induced interdigitation of DPPC bilayers by favorably decreasing the orientation of the hydrocarbon chains [28]. This interdigitation of the membrane bilayer subsequently corresponds to a decrease in reversibility of the phase transition that ultimately results in the compressing of the DPPC bilayer and concomitant dehydration [25, 28].
As mentioned earlier, shoulders appear on the main phase transition of DPPC–sucrose curves as well as DPPC–sucralose DSC curves, which can be suggestive of phase separation. Previous studies investigating the addition of trehalose to hydrated DPPC liposome bilayers suggest that trehalose demonstrates obvious differences in the way the sugar interacts with DPPC depending on which side of the liposome bilayer trehalose is present in, which could possibly lead to a “double” phase transition or the appearance of a shoulder [4, 17]. In order to investigate this possibility, we prepared our liposomes in an aqueous solution of the sugar in order to ensure encapsulation of the sugar within the membrane, which should lead to an equal amount of sugar on the inside and outside of the liposome bilayer. In each case, the DSC curves from liposomes encapsulated in sugar were almost indistinguishable from those obtained when sugar was added after extrusion (data not shown). Therefore, the observed shoulders appearing on the main phase transition peaks cannot be attributed to the possible phase separation of the membrane bilayer due to the presence of sugar, although they could be due to the reorganization of the lipids during the phase transition.
Conclusions
An examination of the phase behavior of model membranes in the presence of either sucrose or sucralose revealed some interesting details. Sucrose as expected induced very small changes in the main phase transition behavior, except for a significant increase in the peak width at half peak height. This would appear to suggest that sucrose does not interact strongly with model membranes although it can perturb the environment of the fatty acid chains decreasing cooperativity and altering the lipid packing. The addition of sucralose however resulted in an initial decrease in the phase transition temperature, followed by a rise in transition temperature as the sugar concentration was further increased. This rise in the transition temperature was accompanied by a decrease in the peak width at half peak height and an increase in hysteresis. The combination of these three phenomena may be indicative of interdigitation of the bilayer accompanied by partial dehydration of the membrane through the disruption of ordered water structures near the membrane surface [23]. Although the pre-transition could provide additional valuable information regarding environmental changes near the lipid headgroups, potentially supporting our hypothesis, this transition was not observed during these studies. Additional studies on more concentrated liposome systems are planned which will hopefully allow us to observe the pre-transition.
In addition to potential use as a cryopreservative, sucralose may be a suitable candidate for lyophilization of liposome drug-based systems. Several previous studies have examined the feasibility of using sugars for this purpose, and sucrose has been shown to offer some protection to liposome complexes [29, 30], despite limited interactions with the fatty acid chains, as shown in this study. The enhanced interactions between sucralose and liposomes may improve the recovery, storage lifetime, and effectiveness of lyophilized liposome drug complexes, and this area is worth further investigation.
References
Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–99.
Chowdhry BZ, Lipka G, Sturtevant JM. Thermodynamics of phospholipid-sucrose interactions. Biophys J. 1984;46:419–21.
Tsvetkov TD, Tsonev LI, Tsvetkova NM, Koynova RD, Tenchov BG. Effect of trehalose on the phase properties of hydrated and lyophilized dipalmitoylphosphatidylcholine multilayers. Cryobiology. 1989;26:162–9.
Nagase H, Ueda H, Nakagaki M. Effect of water on lamellar structure of DPPC/sugar systems. Biochim Biophys Acta. 1997;1328:197–206.
Demetzos C. Differential scanning calorimetry (DSC): a tool to study the thermal behavior of lipid bilayers and liposomal stability. J Liposome Res. 2008;18:159–73.
Taylor KMG, Morris RM. Thermal analysis of phase transition behaviour in liposomes. Therm Acta. 1995;248:289–301.
McElhaney RN. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem Phys Lipids. 1982;30:229–59.
McKee T, McKee JR. Biochemistry: the molecular basis of life. 5th ed. Oxford: Oxford University Press; 2012.
Nakagaki M, Nagase H, Ueda H. Stabilization of the lamellar structure of phosphatidylcholine by complex formation with trehalose. J Membr Sci. 1992;73:173–80.
Cacela C, Hincha DK. Low amounts of sucrose are sufficient to depress the phase transition temperature of dry phosphatidylcholine, but not for lyoprotection of liposomes. Biophys J. 2006;90:2831–42.
Golovina EA, Golovin A, Hoekstra FA, Faller R. Water replacement hypothesis in atomic details: effect of trehalose on the structure of single dehydrated POPC bilayers. Langmuir. 2010;26:11118–26.
Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophys J. 1996;71:2087–93.
Hasegawa T, Kawato H, Toudou M, Nishijo J. Thermally hydrated DPPC langmuir film: a trial application to the analysis of interaction of sucrose with DPPC liposome. J Phys Chem B. 1997;101:6701–6.
Ivanova VP, Heimburg T. Histogram method to obtain heat capacities in lipid monolayers, curved bilayers, and membranes containing peptides. Phys Rev E. 2001;63:041914.
Pruchnik H, Bonarska-Kujawa D, Kleszczyńska H. Effect of chlorogenic acid on the phase transition in phospholipid and phospholipid/cholesterol membranes. J Therm Anal Calorim. 2014;118:943–50.
Koynova R, Brankov J, Tenchov B. Modulation of lipid phase behavior by kosmotropic and chaotropic solutes. Eur Biophys J. 1997;25:261–74.
Ohtake S, Schebor C, de Pablo JJ. Effects of trehalose on the phase behavior of DPPC–cholesterol unilamellar vesicles. Biochim Biophys Acta. 2006;1758:65–73.
Jacobson K, Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975;14:152–61.
Tsuchida E, Seki N, Ohno H. Phase separation in mixed liposomes of dipalmitoyl phosphatidylcholine and di-2,4-octadecadiene phosphatidylcholine. Die Makromol Chem. 1986;187:1351–8.
Shaikh SR, Dumaual AC, Jenski LJ, Stillwell W. Lipid phase separation in phospholipid bilayers and monolayers modeling the plasma membrane. Biochim Biophys Acta. 2001;1512:317–28.
Mannock DA, Lewis RNAH, McElhaney RN. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys J. 2006;91:3327–40.
Christensen D, Foged C, Rosenkrands I, Nielsen HM, Andersen P, Agger EM. Trehalose preserves DDA/TDB liposomes and their adjuvant effect during freeze-drying. Biochim Biophys Acta. 2007;1768:2120–9.
Bonora S, Markarian SA, Trinchero A, Grigorian KR. DSC study on the effect of dimethysulfoxide (DMSO) and diethylsulfoxide (DESO) on phospholipid liposomes. Therm Acta. 2005;433:19–26.
Bender J, Michaelis W, Schubert R. Morphological and thermal properties of vesicular phospholipid gels studied by DSC, rheometry and electron microscopy. J Therm Anal Calorim. 2002;68:603–12.
Rowe ES. Lipid chain length and temperature dependence of ethanol-phosphatidylcholine interactions. Biochemistry. 1983;22:3299–305.
Simon SA, McIntosh TJ. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim Biophys Acta. 1984;773:169–72.
Rosser MFN, Lu HM, Dea P. Effects of alcohols on lipid bilayers with and without cholesterol: the dipalmitoylphosphatidylcholine system. Biophys Chem. 1999;81:33–44.
Smith EA, Dea PK. Differential scanning calorimetry studies of phospholipid membranes: the interdigitated gel phase. In: Elkordy AA, editor. Applications of calorimetry in a wide context—differential scanning calorimetry, isothermal titration calorimetry and microcalorimetry. Rijeka: InTech; 2013. p. 407–44.
Maitani Y, Aso Y, Yamada A, Yoshioka S. Effect of sugars on storage stability of lyophilized liposome/DNA complexes with high transfection efficiency. Int J Pharm. 2008;356:69–75.
Wolkers WF, Oldenhof H, Tablin F, Crowe JH. Preservation of dried liposomes in the presence of sugar and phosphate. Biochim Biophys Acta. 2004;1661:125–34.
Acknowledgements
The authors wish to acknowledge financial support for this work from East Carolina University Office of Research and Graduate studies. We also acknowledge the support provided by undergraduate research awards to Chad Day and James Parker through The Office of Undergraduate Research at East Carolina University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pennington, E.R., Day, C., Parker, J.M. et al. Thermodynamics of interaction between carbohydrates and unilamellar dipalmitoyl phosphatidylcholine membranes. J Therm Anal Calorim 123, 2611–2617 (2016). https://doi.org/10.1007/s10973-016-5288-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5288-y