Abstract
Isothermal oxidation behavior of the high-temperature Cu–13Al–5Fe (in mass, %) SMA on temperature range of 500–900 °C was studied using thermo-gravimetric method. TG curve showed that the alloy has linear oxidation characteristics. From thermo-gravimetric measurements, it was determined that the alloy shows abnormal oxidation characteristics at 600 °C. As K P values of the alloy at oxidation temperatures of 700 and 800 °C were calculated as 0.00166 mg cm−2 s−1 and 0.0022 mg cm−2 s−1, respectively, K P value at 600 °C was found as 0.0029 mg cm−2 s−1. DTA curve exhibited that reason of the abnormal oxidation behavior at 600 °C is the endothermic reaction which causes dissolution of Fe(Al,Cu) precipitates within matrix at 568.13 °C. XRD patterns of the oxidized samples were found to be compatible with the results obtained from thermo-gravimetric measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shape memory alloys (SMAs) have attracted great attention as smart materials, given their ability to function as sensors and actuators. Their self-transformation ability also owns and makes them attractive as the means to assist in solving various unsolved or unnoticed problems. Therefore, SMAs also become a goal for different scientific disciplines, and martensitic transformations are studied with different approaches by both experimental and theoretical researchers [1]. The general knowledge concerning martensite and martensitic transformations in Cu-based alloys such as Cu–Zn, Cu–Zn–Al or Cu–Al–Ni, among many SMA families, develops constantly, and it is accepted that thermo-elastic characteristic of the martensite in these alloys is generally responsible for the reversible shape change capacities during their transformation [2]. However, the transformation temperatures of the ternary Cu-based and binary equiatomic Ni–Ti alloys, which constitute two of the most successful commercial SMA systems today, being <100 °C, pose limitations in terms of their practical use. Therefore, SMAs having transformation temperatures of >100 °C are required to be developed in order to meet the needs that are necessary in new generation engineering applications in areas such as robotics, automotives and aeronautics. The materials within this class are called as high-temperature shape memory alloys (HTSMAs) [3]. Given the low-cost advantages achieved in recent years, Cu-based alloys, in particular, are again drawing great interest for high-temperature applications, due to the possibility of reaching high martensitic transformation temperatures above 200 °C [4]. Moreover, the ternary Cu–Al–Fe alloys, as have been obtained through the addition of Fe to the binary Cu–Al alloys in recent years, already constitute a new HTSMA group that is open to attention thanks to the high martensitic transformation temperatures they have [5–8].
The purpose of this study was to investigate the isothermal oxidation behavior of high-temperature Cu–13Al–5Fe (in mass, %) SMA at a temperature interval of 500–900 °C. The study and determination of the oxidation behaviors of SMAs, particularly at elevated temperatures, are very significant. Thus, the information about the functional behaviors they exhibit at elevated temperature applications in atmospheric environments could be obtained.
Experimental
Oxidation behavior of the high-temperature Cu–13Al–5Fe (in mass, %) SMA produced with arc melter method [8] was studied using thermo-gravimetric method in the TG/DTA (Perkin Elmer Pyris) system. The alloy samples whose oxidation will be investigated were firstly cut into dimensions of 3 × 3 × 3 mm, and then, their surfaces were polished via grinding and cleaned with acetone, respectively. The cleaned samples were placed in the TG/DTA furnace, and for this study, they were heated to the oxidation temperatures of 500, 600, 700, 800 and 900 °C with a heating rate of 50 °C min−1 within a pure N2 gas environment. After the samples were heated to the determined oxidation temperatures in the TG/DTA, they were kept for 1 h in an oxygen gas atmosphere of 100 mL min−1. Accordingly, time-dependent mass gain data of the samples at relevant oxidation temperatures were obtained. Phase analysis of the oxidized samples was conducted at room temperature by taking the XRD (Rigaku RadB-DMAX II) patterns at the interval of 2θ = 10°–80° using Cu Kα radiation.
Results and discussion
A thermo-gravimetric mass gain curve, which exhibits oxidation behavior of Cu–13Al–5Fe alloy sample in between room temperature and 900 °C, is shown in Fig. 1. It is generally well known that a mass increase in the oxidation behavior of metals or alloys comprises two parts: linear and parabolic [9, 10]. From Fig. 1, it is seen that Cu–13Al–5Fe alloy exhibits linear oxidation characteristics at all temperatures from room temperature up to 900 °C.
Figure 2 shows the time-dependent mass gain curves of the samples for which isothermal oxidation experiments were conducted at 500, 600, 700, 800 and 900 °C. Because the oxidation characteristic of the alloy is linear, mass gains per second on unit surface areas of the samples were calculated based on the following relationship [11]:
where ΔW change of mass; A sample surface area; K P oxidation rate constant; and t oxidation time. Due to the oxidation characteristic of Cu–13Al–5Fe alloy in Fig. 1, the K P value is calculated from the slope of the linear section of the \( \Delta W/A - t \) curve. From here, the calculated K P values of the samples oxidized at 500, 600, 700, 800 and 900 °C are 0.00153, 0.0029, 0.00166, 0.0022 and 0.0033 mg cm−2 s−1, respectively. The results thus obtained are very interesting: It is seen that the K P value of the samples oxidized at 600 °C is dramatically higher than the K P values of the samples oxidized at 700 and 800 °C (Fig. 3). Indeed, a high K P value at 600 °C could be also identified by looking at the mass gain curves given in Fig. 2. When Fig. 2 is studied carefully, it can be seen that both the slope of the linear increase region of the mass gain curve at 600 °C is steeper than the ones oxidized at 700 and 800 °C and that mass gain saturation value of the samples oxidized at 600 °C is significantly higher than the ones oxidized at 700 °C, being nearly equal to the saturation values of the samples oxidized at 800 °C. Thus, the oxidation of Cu–13Al–5Fe alloy at 600 °C was completed with an abnormal mass gain. This oxidation behavior leads one to assume the occurrence of a phase transformation in Cu–13Al–5Fe alloy at approximately 600 °C. In order to verify this assumption, between room temperature and 900 °C, a differential thermal analysis (DTA) curve of the alloy shown in Fig. 4 was taken. The DTA curve comprises three endothermic peaks, which occur at 424.44, 533.55 and 568.13 °C. The first endothermic peak is the reverse martensite transformation peak. The martensite → austenite transformation temperatures (A s, A f) of the same alloy had already been determined [8], and although it was not given there, the reverse martensite transformation temperature of this alloy is 352.9 °C, based on the differential scanning calorimeter (DSC) curve. This circumstance can be explained by looking at the DSC curves belonging to Cu–13Al–5Fe alloy in Fig. 5: While the reverse martensite transformation temperature in the first cycle is around 440 °C, it is seen that the reverse martensite transformation temperatures in the second and third cycles are approximately 350 °C and that peaks in the heating direction overlap from this point forward. From here, it is seen that, at the first stage, the alloy is thermally unstable and that thermal stability occurs with an increasing number of thermal cycles. The second endothermic peak at 533.55 °C belongs to the transformation of β 1 → β (B2), which is high-temperature phase transformation. It is thought that the third peak at 568.13 °C on the DTA curve is the dissolution peak of the precipitates in matrix [12–15]. Here, the peak upon which we must focus is the third endothermic peak, because it is known that the microstructure of Cu–13Al–5Fe alloy consists of Fe(Al,Cu) precipitate phase [8]. In conclusion, it is thought that Fe(Al,Cu) precipitates within the alloy were dissolved in the matrix as a result of the endothermic reaction at 568.13 °C and that this dissolution reaction was directly responsible for the abnormal mass gain during oxidation at 600 °C. During the dissolution of Fe(Al,Cu) precipitates, initially the Fe, Cu and Al atoms dissociate, while the Al atoms, whose affinity is the highest among these atoms released at that moment, from Ellingham diagram [16], form the Al2O3 oxide phase by quickly capturing the oxygen atoms as they are released to the environment. Because the Al2O3 oxide layer that covers the sample surface will form a shield against the oxidation of Fe and Cu atoms, the dominant oxide phase contained by the samples will be Al2O3, as can be understood from the XRD results we will discuss later. Accordingly, since there will be a greater quantity of Al atoms on the sample surface at nearly 600 °C with respect to the oxidation temperatures of 700 and 800 °C, the mass gain value and oxidation speed constant value of the sample at this temperature are greater than those at 700 and 800 °C.
DSC thermal cycle curves of Cu–13Al–5Fe alloy (third cycle obtained from Ref. [8])
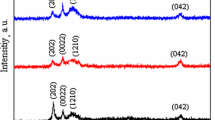
The changes that occurred (depending on oxidation temperatures) in the structural properties of the oxidized Cu–13Al–5Fe alloy samples were studied by carrying out XRD analysis. The XRD patterns of the samples whose oxidations occurred at the temperature interval of 500–800 °C are shown in Fig. 6. (However, although XRD analysis of the sample oxidized at 900 °C was conducted twice, the XRD patterns were not shown because the operator could not obtain a healthy result.) The XRD pattern of the sample oxidized at 500 °C consists of diffraction peaks belonging to three different phases: martensite structure peaks belonging to matrix, the Fe(Al,Cu) precipitate peak and the Al2O3 oxide phase peak. Because the oxidation of the alloy is very low at this temperature, the intensity of the Al2O3 peak is very weak. Apparently, abnormal changes took place in the phase components and phase quantities of the alloy samples oxidized with increasing oxidation temperatures, thus being compatible with the DTA curve of the alloy (Fig. 4). As seen from the XRD pattern of the sample as a result of oxidation at 600 °C, there was a dramatic increase in the intensity of the Al2O3 peak, while the Fe(Al,Cu) peak disappeared. However, it is seen that the intensities of the Al2O3 peaks in the XRD patterns of the samples oxidized at 700 and 800 °C were substantially lower than the intensities of the same phase in the XRD pattern at 600 °C. These results, particularly when evaluated with the results obtained from Fig. 2, demonstrate that Cu–13Al–5Fe alloy has an abnormal oxidation behavior due to its phase characteristics.
Conclusions
TG analysis showed that high-temperature Cu–13Al–5Fe SMA exhibits linear oxidation behavior. From the oxidation experiments conducted at isothermal heat treatment temperatures increasing within the range of 500–900 °C, it was seen that the alloy demonstrates abnormal oxidation behavior at 600 °C. It was determined that the oxidation rate constant of the alloy at this temperature is remarkably higher by comparison with the oxidation rate constants of the samples oxidized at 700 and 800 °C. The DTA curve revealed that the reason for the abnormal oxidation behavior of the alloy at 600 °C is the endothermic reaction at 568.13 °C, which leads to the dissolution of the Fe(Al,Cu) precipitate phase within the matrix. From XRD patterns of the oxidized alloy samples, it was seen that the amount of oxide phase is substantially high in the sample oxidized at 600 °C, as expected.
References
Otsuka K, Kakeshita T. Science and technology of shape-memory alloys: new developments. MRS Bull. 2002;27:91–100.
Stoiber J, Gotthardt R, Van Humbeeck J. Hysteresis effects during martensitic transformation in Cu–Zn–Al shape memory alloys. In: Hornbogen E, Jost N, editors. 1st European Symposium on Martensitic Transformations in Science and Technology. Bochum, Germany; 1989. p. 297–304.
Ma J, Karaman I, Noebe RD. High temperature shape memory alloys. Int Mater Rev. 2010;55:257–315.
Ma YQ, Jiang JB, Xu HB. Martensitic transformation and thermal stability in Cu–Al–Co and Cu–Al–Zr alloys. Acta Metallurgica Sinica (English Letters). 2003;16:445–8.
Raju TN, Sampath V. Effect of ternary addition of iron on shape memory characteristics of Cu–Al alloys. J Mater Eng Perform. 2011;20:767–70.
Raju TN, Sampath V. Influence of aluminium and iron contents on the transformation temperatures of Cu–Al–Fe shape memory alloys. Trans Indian Inst Met. 2011;64:165–8.
Yang S, Su Y, Wang C, Liu X. Microstructure and properties of Cu–Al–Fe high-temperature shape memory alloys. Mater Sci Eng B. 2014;185:67–73.
Yildiz K, Kök M, Dağdelen F. Cobalt addition effects on martensitic transformation and microstructural properties of high-temperature Cu–Al–Fe shape-memory alloys. J Therm Anal Calorim. 2015;120:1227–32.
Kök M, Pirge G, Aydoğdu Y. Isothermal oxidation study on NiMnGa ferromagnetic shape memory alloy at 600–1000 °C. Appl Surf Sci. 2013;268:136–40.
Kök M, Yildiz K. Oxidation parameters determination of Cu–Al–Ni–Fe shape-memory alloy at high temperatures. Appl Phys A. 2014;116:2045–50.
Li Z, Qian S, Wang W. Characterization and oxidation behavior of NiCoCrAlY coating fabricated by electrophoretic deposition and vacuum heat treatment. Appl Surf Sci. 2011;257:4616–20.
Adorno AT, Guerreiro MR, Benedetti AV. Thermal behavior of Cu–Al alloys near the α-Cu–Al solubility limit. J Therm Anal Calorim. 2001;65:221–9.
Adorno AT, Guerreiro MR, Ribeiro CA, Guerreiro CTR. Influence of silver additions on the thermal behavior of the Cu-8 mass% Al alloy. J Therm Anal Calorim. 2001;64:1141–6.
Silva RAG, Machado ES, Adorno AT, Magdalena AG, Carvalho TM. Completeness of β-phase decomposition reaction in Cu–Al–Ag alloys. J Therm Anal Calorim. 2012;109:927–31.
Recarte V, Perez-Landazabal JI, Ibarra A, No ML, Juan JS. High temperature β phase decomposition process in a Cu–Al–Ni shape memory alloy. Mater Sci Eng A. 2004;378:238–42.
Gaskell DR. Metallurgical Thermodynamics. In: Cahn RW, Haasen P, editors. Physical Metallurgy, Fourth, Revised and Enhanced edition. North-Holland (Printed in The Netherlands); 1996. p. 431.
Acknowledgements
The thermo-gravimetric analysis of samples was done by E. Ercan (Bitlis Eren University).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yildiz, K. Oxidation of high-temperature Cu–Al–Fe shape memory alloy. J Therm Anal Calorim 123, 409–412 (2016). https://doi.org/10.1007/s10973-015-4912-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4912-6