Abstract
Thermal properties and structure of bulk glasses of (As2S3)1−x(Sb4S4)x system (x varies from 0 to 60 mol%) were studied by differential scanning calorimetry and Raman spectroscopy. It was found that with increasing Sb content the glasses can be sorted out to the three groups. The structure of glasses with x ≤ 10 is build-up mainly from AsS3/2 pyramidal units and the well-known crystallization resistance of As2S3 can explain the reluctance of these undercooled liquids against crystallization. In glasses with a higher content of antimony, i.e., 10 < x ≤ 30 mol%, the vibration characteristics of As4S4 clusters appear. Undercooled melts of these glasses crystallize forming both β-As4S4 and high-temperature phases of Sb2S3. Structure of glasses with the highest antimony content (x > 30 mol%) is based on SbS3/2 structural units significantly lowering stability of their undercooled melts from which only Sb2S3 crystallizes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chalcogenide glasses of various compositions have been intensively studied for many years due to the wide range of their technological applications. Amorphous selenium is useful in photovoltaic and solar cells, as a rectifier, in xerography and photography, the production of colored glass and enamels. Binary chalcogenide glassy systems such as As–S/Se, Ge–S/Se, Sb–S/Se, can be used as IR detectors, optical switches, and in particular as a rewritable recording medium based on the transformation of amorphous–crystalline phase [1–3].
One of the most extensively studied binary chalcogenide glasses is arsenic trisulfide mainly because of its excellent glass-forming ability and wide infrared transmission. On the other hand, although the arsenic and antimony belonging to the same group of the periodic system, the preparation of glassy Sb2S3 is very difficult and requires high cooling rates [4]. It has been shown that basic structural units of glassy As2S3 and Sb2S3 are the trigonal pyramids MS3/2 (M = As or Sb) bonded to each other by S atom [5–7]. In order to improve or modify appropriately the desired properties of these materials, the ternary-mixed As2S3–Sb2S3 system has been extensively studied during the last decades [8–10].
The proper description of thermal behavior of these glasses is important for understanding of their properties and subsequent applications.
To study the role of elements of V. group on thermal properties and stability, the influence of changing Sb/As molar ratio of glassy system (As2S3)100−x(Sb4S4)x (x = 0, 5, 10, 20, 30, 40, 50, and 60 mol%; Sb/As: 0–2.99) was used.
Thermal properties have been studied using conventional differential scanning calorimetry (DSC) and StepScan DSC, some other information about the structure was obtained by Raman spectroscopy and XRD analysis.
Experimental
The glasses with composition (As2S3)100−x(Sb4S4)x (x = 0, 5, 10, 20, 30, 40, 50, and 60 mol%) were prepared in conventional manner by direct synthesis from the 5 N purity elements. Synthesis was carried out in evacuated quartz ampoules in a horizontal rocking tube furnace at 850 °C. During the synthesis, the melt was homogenized for 24 h. The glass was then obtained by the melt quenching in cold water. Both the homogeneity and the composition were assessed using an X-ray fluorescence (μ-XRF) EAGLE II (Roentgen Messtechnik AG) analyzer, the changes in composition were found less than 1 %, i.e., within experimental error.
The DSC measurements were carried out in the temperature range 150–350 °C using Pyris 1 calorimeter (Perkin-Elmer). Crude-crushed glassy samples (approx. 10 mg) were encapsulated into sealed aluminum pans. Empty pan was used for baseline run. Instrument was carefully both temperature and enthalpy calibrated with the help of In and Zn standards, measurements were carried out in nitrogen atmosphere. For conventional measurement, the heating rate was 10 °C min−1. To obtain values of glass transition temperature, T g, and the change of isobaric specific heat capacity at glass transition, ΔC p , independent on experimental conditions (especially on heating or cooling rate) and thermal history of glass, the StepScan DSC (Perkin-Elmer) was used, for details on StepScan DSC, see, e.g., [11]. The sample sealed in an aluminum crucible was measured in the temperature range 100–250 °C, with temperature step of 1 °C and the heating rate 10 °C min−1 in the temperature step. Maximum permissible differential heat flow at the end of the isotherm was ΔQ = ±0.0001 mW.
Raman spectra were measured on a FT-IR spectrometer IFS 55 with FRA 106 Raman attachment (both Bruker). Nd:YAG laser (1,064 nm) with power 100 mW was used for excitation and Raman scattering were detected bu Ge detector cooled by liquid nitrogen. Spectra were recorded with resolution 2 cm−1 and 250 scans were averaged.
XRD structural analysis of powder samples was performed by D8-Advance (Bruker) diffractometer, 40 kV, 30 mA Cu Kα spectral line, and graphite monochromator.
Results and discussion
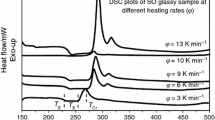
Bulk glasses were checked by XRD and the absence of crystalline phase was confirmed. The basic thermal properties of samples were determined by both conventional and StepScan DSC methods. Conventional DSC scans are shown in Fig. 1. Only one glass transition temperature, T g, was found for each glassy sample therefore one can conclude that the glasses are not phase separated; for the compositional dependence of T g, see Fig. 2.
DSC results sorted out the glasses into three groups: (1) x = 0–10, (2) x = 20–30, and (3) x = 40–60.
The antimony content in glasses of the first group (x = 0–10 mol% Sb4S4; Sb/As: 0–0.222) is relatively low and undercooled melts are stable—no crystallization was found, Fig. 1. Therefore, antimony content influences thermal properties only a little, the glass transition temperature remains practically unchanged and tightly close to this one of As2S3, see Fig. 2. According to the Raman spectra, the structure is mainly based on AsS3/2 units and well-known resistance of As2S3 to crystallization [12] explains excellent thermal stability of these undercooled liquids.
The influence of antimony on the glass structure manifests itself in the second group (20–30 mol% Sb4S4, Sb/As: 0.511–0.857). Glass transition temperature increases with increasing Sb content and both exo- and endothermic changes are also observed on DSC scans, see Fig. 1. Using database PDF-4+, v. 2013, the analysis of XRD patterns, Fig. 3, revealed that the first exothermic DSC peak corresponds to crystallization of high-temperature form of As4S4 (β-realgar, JCPDS no. 00-0510781), which is stable above 266 °C. The following endothermic peak can be explained by the decomposition of β-As4S4 into As2S3 and melt in accordance with the phase diagram of As–S system [13]. The second exothermic peak corresponds to the crystallization of Sb2S3 (stibnite, JCPDS no. 00-042-1393) as it was confirmed by XRD. All crystalline phases were also confirmed by Raman spectroscopy.
T g of glasses belonging to the third group (x = 40–60 mol%, Sb/As: 1.33–2.99) increases furthermore with increasing Sb content. XRD analysis confirmed that only Sb2S3 crystallized from undercooled melts, Fig. 3. X-ray record was compared with the database and two orthorhombic Sb2S3 modifications were identified, differing each to other by cell size—a 1 = 11.31070 Å, b 1 = 3.83630 Å, c 1 = 11.22850 Å (JCPDS no. 01-073-0393); a 2 = 11.23900 Å, b 2 = 11.31300 Å, and c 2 = 3.84110 Å (JCPDS no. 00-042-1393). This finding corresponds well with double-crystallization peak, which can be seen on the DSC.
Basic structural information was obtained by Raman spectroscopy. The characteristic feature of spectra is a wide band in the spectral range 250–400 cm−1, Fig. 4. With increasing antimony content, the maximum of this band shifts from 344 cm−1 typical for As–S vibration in AsS3/2 pyramids [6, 14] to ~300 cm−1 characteristic for Sb–S vibration in SbS3/2 pyramids [15]. Moreover, the sharp compositionally independent bands at 165, 185, 224, 359, and 381 cm−1, indicating the presence of As4S4 molecular clusters (β-realgar) [16, 17], are clearly seen in the spectra of the second group of glasses, but they are partly visible already in the spectra of the first group. The content of As4S4 clusters reached the maximum for x = 20 and the vibrational band at 275 cm−1 simultaneously appears corresponding to the characteristic vibration of the As4S3 molecular clusters [18], its intensity increases and for x = 40 reached the maximum while at same composition β-realgar is not already detectable in the spectrum. In the glasses with the highest content of antimony (x = 50, 60), the relatively broad band at 205 cm−1 appears and its intensity increases with increasing antimony content. From this we can conclude that this band can be associated with the Sb–S vibration.
From the analysis of Raman spectra, it follows that besides basic structural units, i.e., trigonal pyramids AsS3/2 and SbS3/2, molecular clusters β-As4S4 and As4S3 also play significant structural role.
It turned out that in the case of crystallization of undercooled melts, the ratio Sb/As is crucial. Sulfur is probably tightly bound to antimony which leads to a deficiency of sulfur bound to arsenic. This deficit results in the formation of stable As4S4 and As4S3 clusters.
Conclusions
Thermal properties of the (As2S3)100−x(Sb4S4)x bulk glasses (x = 0, 5, 10, 20, 30, 40, 50, and 60 mol%) were studied by differential scanning calorimetry, the structure of glasses was investigated by Raman spectroscopy and products of crystallization of undercooled melts were characterized using XRD.
It was found that with increasing antimony content the studied glasses can be divided into three groups differing in their thermal properties and structure.
It is worthy to point out that the glassy system under study is sulfur understoichiometric related to As(III) and Sb(III) stable oxidation state. It was found that contrary to wider spectrum of possible arsenic compounds, antimony is able to form only SbS3/2 pyramids and so understoichiometry of sulfur has to be compensated by stable arsenic molecular clusters As4S4 and As4S3. This in turn reduces homogeneity of glasses and thermal stability of their undercooled melts for approx. x > 20. Thus, the Sb/As ratio is the key feature, and it was found that glasses are homogeneous and stable only for Sb/As less than 0.5, i.e., for x < 20.
References
Zakery A, Elliot SR. Optical properties and applications of chalcogenide glasses. J Non Cryst Solids. 2003;330:1–13.
Elliot SR. Glasses and amorphous materials. In: Zarzycki J, editor. Material science and technology. Weinheim: VCH; 1991. p. 375–454.
Bureau B, Zhang XH, Smektala F, Adam J-L, Troles J, Ma H, Boussard-Pledel C, Lucas J, Lucas P, Le Coq D, Riley MR, Simmons JH. Recent advances in chalcogenide glasses. J Non Cryst Solids. 2004;345&346:276–83.
Červinka L, Hrubý A. Structure of amorphous and glassy Sb2S3 and its connection with the structure of As2X3 arsenic chalcogenide glasses. J Non Cryst Solids. 1982;48:231–364.
Kamitsos EI, Kapoutsis JA, Couleac IP, Iovu MS. Structure and bonding in As–Sb–S chalcogenide glasses by infrared reflectance. J Phys Chem B. 1997;107:11061–7.
Li W, Seal S, Rivero C, Polez C, Richardson K, Pope A, Schulte A, Jain H, Antoine K, Miller AC. Role of S/Se ratio in chemical bonding of As–S–Se glasses investigated by Raman, X-ray photoelectron, and extended X-ray absorption fine structure spectroscopies. J Appl Phys. 2005;98:053503/1–11.
Dalba G, Fornasini P, Grunta G, Burattini E. XRD and EXAfs study of the local structure in some non-crystalline Sb–S compounds. J Non Cryst Solids. 1989;107:261–9.
El Idrissi Raghni MA, Lippens PE, Olivier-Foucarde J, Jumas JC. Local structure of glasses in As2S3–Sb2S3 system. J Non Cryst Solids. 1995;192(93):191–4.
White K, Crane RL, Snide JA. Crystallization kinetics of As–Sb–S in bulk glass and thin film form. J Non Cryst Solids. 1988;103:210–20.
Černošková E, Černošek Z, Holubová J. Thermoanalytical study and structure of stoichiometric As–Sb–S glasses. J Therm Anal Calorim. 2014. doi:10.1007/s10973-013-3298-6.
Holubová J, Černošek Z, Černošková E. StepScan DSC—the useful tool for the study of the glass transition phenomenon. J Therm Anal Calorim. 2013;111:1633–8.
Černošek Z, Černošková E, Beneš L. Crystalline arsenic trisulfide: preparation, differential scanning calorimetry and Raman scattering measurements. Mater Lett. 1999;38:336–40.
ASM International. In: Massalski TB, et al., editors. Binary alloy phase diagram, 2nd ed. Materials Park: ASM International; 1990.
Lucovsky G, Martin RM. A molecular model for the vibrational modes in chalcogenide glasses. J Non Cryst Solids. 1972;185:8–10.
Koudelka L, Frumar M, Pisárčik M. Raman spektra of Ge–Sb–S systém in the S-rich region. J Non Cryst Solids. 1980;41:171–8.
Muniz-Miranda M, Sbrana G, Bonazzi P, Menchetti S, Pratesi G. Spectroscopic investigation and normal mode analysis of As4S4 polymorphs. Spectrochim Acta A. 1996;52:1391–401.
Banerjee A, Jensen JO, Jensen JL. A theoretical study of As4S4: bonding, vibrational analysis and infrared and raman spektra. J Mol Struct. 2003;626:63–75.
Němec P, Jedelský J, Frumar M, Černošek Z, Vlček M. Structure of pulsed-laser deposited arsenic-rich As–S amorphous thin films, and effect of light and temperature. J Non Cryst Solids. 2005;351:3497–502.
Acknowledgements
This work was supported under the Project MSM 7AMB12SK056.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holubová, J., Černošek, Z. & Černošková, E. Thermal properties and structure of (As2S3)100−x(Sb4S4)x glassy system. J Therm Anal Calorim 116, 699–702 (2014). https://doi.org/10.1007/s10973-014-3761-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3761-z