Abstract
This contribution deals with the preparation and characterization of Co-doped malayaite pigments. Pigment samples were prepared by solid-state reaction at different firing temperatures (1200–1400 °C). For characterization of thermal behaviour, pigment formation and thermal stability were studied by the methods of thermal analyses. The compounds were evaluated from standpoint of their phase composition and particle size distribution. The XRD analysis of samples prepared at 1,300–1,400 °C indicates the occurrence of two-phase compounds, i.e. the malayaite and the cassiterite. Average value of mean particle size of tested malayaites is moved ~10 μm. The great attention was focused on determination of impact of firing temperature on the pigment-application properties. Because the malayaite compounds belong into ceramic pigments, the pigment-application properties were observed after application into organic matrix in mass tone and middle-temperature glaze in different pigment amounts. Generally, the colour appearance of tested malayaites is dependent on the firing temperature and it moves in different shades of blue colour. From pigmentary point of view, it is possible to recommend 15 % of mass pigment for sufficient colouring of middle-temperature glaze. The higher firing temperature provides the formation of pigment samples with the higher saturation and the higher values of hue angle. For preparation of Co-doped malayaite pigments with the excellent colour properties, the firing temperature 1,350 °C is necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The research of inorganic pigments is in the past decades focused on replacing or other substitution of toxic elements (Pb, Cr6+, Se, Cd, Hg, Sb, etc.) which were included in the pigment composition. Production of these industrial inorganic pigments considering their toxic inappropriateness was discontinued. Therefore, from pigmentary point of view, the search and development on preparation and characterization of new environmentally friendly pigment compounds are concentrated.
Ceramic pigments are formed by a crystalline host lattice containing chromophore cations. Transition metals with unfilled d-orbitals or lanthanides with unfilled f-orbital belong among to typical the chromophores, which are used for preparation of colour ceramic pigments. The incorporation of the chromophore into the host lattice usually results in the formation of a substitution or less often an addition compounds. The colour of the formed pigment is determined by the properties of the created ligand field which surrounds chromophore [1].
The malayaites are stannic substances derived from a natural occurring mineral the Malayaite which has been chemical formula CaSnOSiO4 or more precisely CaSnSiO5. It crystallises in the monoclinic system with space group P21/a and cell parameters: a = 0.7146 nm, b = 0.8887 nm, c = 0.6668 nm and β = 113.35° [2]. A coloured appearance of natural malayaites is different, the colours are moving from colourless, over white, greenish- grey, light yellow up to orange. The colour of these natural malayaites is dependent in part on present admixture or impurity, which has a role as doping agent (chromophore), but also in next part on amount of impurity [1]. Next factors having the impact on final colour of prepared malayaites are firing time and temperature of preparation [3–6], occurrence of secondary phases [3, 6, 7], type and amount of mineralizers [3, 5, 7, 8], coodopants [3, 9, 10] and of course different methods of preparation [3, 8, 11].
The stannic compounds crystallize in different structures (cassiterites, tin sphenes or recently malayaites, perovkites and pyrochlores), and they are studied thanks to their excellent heterogeneous properties. For example, it is system Ca-(Sn,Ti)-Si–O with photoluminescence [12] or also the CaSnO3 and the CaSnSiO5 with excellent electrochemical properties find the utilization as anodes in Li-batteries [13]. Several methods of synthesis of the stannate compounds are studied too. It is the classical method based on solid-state reaction [14], precipitation method [15], polymeric precursor method [16], a sol–gel method [8], spray- and freeze-drying techniques [17].
Substitutional solid solutions of foreign cations are theoretically possible on the tin, silicon and calcium sites and allow to prepare industrial pigments [2, 18]. For example, Cr-doped compounds have been widely investigated as ceramic pigments from reason of their synthesis conditions, Cr ions different oxidation states (II–VI) leading to different colours and variable degrees of stability [19]. Pink chromium-doped malayaite Ca(Sn,Cr)SiO5 is the most important chromium pigments used in the ceramic industry for colouring glazes, and it is catalogued under number 12-25-5 in the CPMA classification [20]. In literature, it is possible to find several works dealing the substitution of Cr cations on different positions of malayaite lattice. But studies of recent years prove that the chromium cations are in octahedral sites replacing the SnIV centres [21, 22]. Regarding Co-doped malayaites, the results from Mossbauer spectra of 119Sn indicate the incorporation of cobalt ions in the lattice of malayaite, which tends to make the tin atom environment more asymmetric [23].
As the chromophore for colouring of the malayaite lattice, cobalt was selected and the Co-doped malayaite pigment Ca(Sn,Co)SiO5-δ was prepared in this work. For characterization of thermal behaviour, formation and stability of pigments, the methods of thermal analysis were used. The great attention was focused on identification of the impact of firing temperature on colour properties of prepared pigments and evaluating of their phase composition and particle size distribution.
Experimental part
Materials and preparation of pigment samples
The tested sample based on the malayaite defined as Ca(Sn,Co)SiO5−δ was prepared from mixtures of oxides and carbonate by the solid-state reaction. As starting materials, precipitated CaCO3 (98.5 % purity, Merck Group KGaA, Germany), SnO2 (99.9 % purity, Alfa Aesar GmgH & KG, Germany), natural micro-milled SiO2 (99.6 % purity, Sklopísek Střeleč, a.s., Czech Republic) and mixed Co3O4 (94.0 % purity, Shepherd Color Company, USA) were used. The reaction mixtures having composition 33.33 mol% CaCO3, 31.67 mol% SnO2, 1.67 mol% Co3O4 and 33,33 mol% SiO2 were hand milled in an agate mortar in the required stoichiometric ratio. The homogenized powder was placed into corundum crucibles for next firing procedure. Calcination was performed in an electric furnace with the temperature rate 10 °C min−1. The heating temperature in the interval 1,200–1,400 °C was maintained for 4 h with the step of 50 °C and then spontaneously cooled to room temperature. The obtained calcined powder pigments were hand milled in an agate mortar by reason homogenization of agglomerates. Thereafter, the samples were washed by hot distilled water, filtered and dried for a reason of elimination of water-soluble substances.
Prepared pigments were dispersed into two binding systems namely glaze P 07410 (transparent middle-temperature leadless colourless lustrous frit glaze, Glazura Roudnice a.s., Czech Republic) in mass ratio 5–25 % and organic matrix (dispersive acrylic paint Parketol, Balacom a.s., Czech Republic) in a mass tone.
Characterization techniques
Determination of colour properties
The main aim of this work was to study the influence of calcination temperature on the colour properties of malayaite pigments. The colour properties of all heat-treated pigments without application, i.e. in powdered form, and samples applied into the two binding systems were measured in the visible region of light (400–700 nm) using ColourQuest XE (HunterLab, USA). The measuring conditions were the following: illuminant D65 (6,500 K), 10° complementary observed, geometry of measurements d/8°. The colour properties were described in terms of CIEL*a*b* system (1976). The value L* represents the lightness or darkness of the colour. In the CIEL*a*b* system, it is described by numbers from zero (black) to hundred (white). It means that in this system L* = 50 correspond to the grey colour. The value a* (the red–green axis) and b* (the yellow–blue axis) indicate colour hue. The next characteristics of the colour were determined too, namely the chroma and the hue angle. The chroma C is calculated according to Eq. 1 and represents a purity of the colour from 0 (grey) to 100 (pure colour), i.e. chroma is the degree of difference between a colour and grey. The hue angle, H°, is expressed in the degrees and ranges from 0° to 360°. It is defined by an angular position in the cylindrical colour space (for the blue H° = 195–285 and for the violet H° = 285–350) [24]. The value of hue is calculated using Eq. 2.
The simultaneous TG–DTA analysis
The thermal analysis was used for characterisation of thermal behaviour and formation of the tested malayaite. The thermal analysis of starting raw materials (CaCO3, SnO2, Co3O4 and SiO2) and the reaction mixture contained stoichiometric ratio of ingredients were performed by STA 449C Jupiter (Netzsch, Germany). This equipment allows the simultaneous registration of the thermoanalytical curves TG and DTA. The starting raw materials and the reaction mixture were studied by thermal analysis in corundum crucibles in air in temperature region 30–1,200 °C and 30–1,400 °C, respectively, with heating rate 10 °C min−1. Corundum was used as reference inert material.
Thermal stability
A heating microscope with automatic image analysis EM201-12 (Hesse-Instruments, Germany) was used for study of the thermal stability of heat-treated samples. This instrument enables to monitor the thermal stability of powdered materials maximally until 1,600 °C into the furnace, with the guarantee of 100 °C delay on the sample, i.e. maximum 1,500 °C on the sample. The equipment has been calibrated using pure metallic Sn or In. For measurement of heat-treated samples, tablets in shape of cylinders with a diameter of 3 mm and height of 6 mm were prepared. Changes of sample′s areas were determined. The conditions of this study were the following: the temperature rate—10 °C min−1; end temperature—1,600 °C into the furnace, i.e. 1,500 °C on the sample. The sensitivity conditions for taking a new image were area change—5 %; shape factor change—5 %; corner angle change—10 %.
Particle size distribution
Particle size distribution was measured by a Mastersizer 2000/MU (Malvern Instruments, Ltd., GB). It is the highly integrated laser measuring system (He–Ne laser, λ = 633 nm) for analysis of particle size distribution. The equipment uses scattering of the incident light on particles. The signal is evaluated on the basis of Mie theory or Fraunhofer bending.
XRD analysis
The phase composition of the tested powdered samples was studied by X-ray diffraction analysis using an equipment diffractometer D8 Advance (Bruker, GB) in the range 2Θ from 10 to 80°. Cu Kα1 (λ = 0.15418 nm) radiation was used for range 2Θ < 35° and Cu Kα2 (λ = 0.15405 nm) for range 2Θ > 35°. A scintillation detector was used. The identification of individual phases was evaluated in accordance of obtained diffraction patterns with the data contained in the PDF cards [25].
Results and discussion
Results of the thermal analysis
For better evaluation and support of results from thermal analysis of tested the malayaite compound, the studies of thermal analysis of raw materials were performed. The TG/DTA analyses of the used starting raw materials were studied in the temperature range of 30–1,200 °C.
Figure 1a depicts the well-known record of DTA curve of precipitated CaCO3 (mass sample: 159.30 mg). The small exothermic and the dominant endothermic effects were detected on DTA curve. The first exothermic effect with maximum 234 °C is connected with recrystallization of particles of the precipitated CaCO3. The dominant endothermic effect of this record with minimum 900 °C agrees with decomposition of CaCO3 [26]. Total mass loss recorded on TG curve, which is showed in Fig. 1b, is 43.3 % and it is caused by decomposition of CaCO3. The second thermoanalytical record in Fig. 1a represents the DTA curve for Co3O4 with mass sample 183.90 mg. On DTA curve, only the one endothermic effect was detected. This effect with minimum at 930 °C indicates reduction of Co3O4 to CoO [27, 28]. Total mass loss recorded on TG curve (Fig. 1b) is 5.8 % and it is related with reduction of Co3O4 to CoO.
a DTA curves of the starting raw materials—a full line precipitated CaCO3 (mass sample: 159.30 mg); b dashed line Co3O4 (mass sample: 183.90 mg); (conditions of measurement: atmosphere—air, the heating rate—10 °C min−1). b TG curves of the starting raw materials—a full line precipitated CaCO3 (mass sample: 159.30 mg); b dashed line Co3O4 (mass sample: 183.90 mg); (conditions of measurement: atmosphere—air, the heating rate—10 °C min−1)
The following figures (Fig. 2a,b) show the records of DTA and TG curves for next materials—natural micro-milled SiO2 and SnO2. Figure 2a describes the DTA curve of natural micro-milled SiO2 (mass sample: 174.10 mg), and in the temperature region 30–1,200 °C, the two endothermic and the small one exothermic effects were detected. The first one endothermic effect with minimum 570 °C indicates the reversible phase transition α-quartz to β-quartz [29, 30]. The second endothermic effect with the minimum 777 °C can be probably related with some changes of impurities, which are presented into the natural SiO2. The last one is the exothermic effect with maximum 1,047 °C and it is related with phase transition β-quartz to β-cristobalite [30]. No mass loss was recorded on the TG curve (Fig. 2b). Fig. 2a describes the DTA record for the last one of the starting raw material-SnO2 with the mass sample 183.60 mg; from this record, it is evident that in the temperature region 30–1,200 °C, no effect on DTA curve was detected. No mass loss was recorded on the TG curve (Fig. 2b) in cause of SnO2 too.
a DTA curves of the starting raw materials—a full line natural micro-milled SiO2 (mass sample: 174.10 mg); b dashed line SnO2 (mass sample: 183.60 mg); (conditions of measurement: atmosphere—air, the heating rate—10 °C min−1). b TG curves of the starting raw materials—a full line natural micro-milled SiO2 (mass sample: 174.10 mg); b dashed line SnO2 (mass sample: 183.60 mg); (conditions of measurement: atmosphere—air, the heating rate—10 °C min−1)
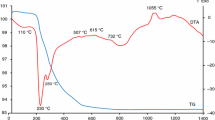
The thermal analysis for study of the synthesis of Co-doped malayaite pigment has been used. Figure 3 depicts the thermoanalytical curves TG and DTA of reaction mixture for synthesis of tested malayaite pigment (mass sample: 252.00 mg),which were monitored in temperature region 30–1,400 °C. From this picture, it is evident that several exothermic and two endothermic effects were detected. The first one is the exothermic effect with maximum 251 °C and it is connected with recrystallization of precipitated CaCO3. The next effect, which has the endothermic character with minimum 571 °C, is related with reversible transformation of quartz from α- to β-form [29, 30]. Next, it is the endothermic effect with minimum at 880 °C and it is connected with decomposition of CaCO3 in conformity with partial reduction of Co3O4 to CoO [26–28]. The exothermic effect with maximum 1,040 °C agrees with next phase change of SiO2 [30]. The last two exothermic effects with maximum at 1,214 and 1,325 °C are connected with the start (effect at 1,214 °C) and the finishing (effect at 1,325 °C) of formation of malayaite pigment. Total mass loss, which is showed on TG curve of this TG–DTA record, is 14 % and it is caused by decomposition of CaCO3 and the partial reduction of Co3O4 to CoO.
Results of the thermal stability
The heat-treated powders (prepared at temperatures 1,200–1,400 °C) pressed into tablets were studied by the heating microscope. The temperature range was held from laboratory temperature to 1,500 °C. The dependence of the area changes of tablets on increasing temperature examined for the heat-treated samples is showed in Fig. 4. From thermal stability point of view, especially pigment prepared at higher temperatures gives good results. The lowest thermal stability was observed for the pigment prepared at 1,200 °C. The decrease of area was started about 1,270 °C and had the fastest character. The change of area for this heat-treated sample was the greatest —25.6 %. The result was probably affected by the phase composition of this sample, i.e. by the occurrence of unreacted components. The degradation of the pigment prepared at 1,250 °C started at almost the same temperature as in previous case (1,275 °C), but ran more slowly. This result was probably affected by the presence of certain mass of incorporated CaSnO3. The sample′s area dropped about 18.7 %. The results for the heat-treated samples prepared at temperatures 1,300–1,350 °C are similar to each other. The decreases of area of the samples prepared at 1,300 and 1,350 °C dropped about 13.8 or 10 % and they were started at almost the same temperature—1,236 and 1,245 °C. The slowest degradation of the pigment was recorded for pigment prepared at 1,400 °C and it was started about at 1,300 °C. The decrease of area was 8.5 %. Because the malayaite pigments belong to the group of ceramic pigments, the thermal stability is one of the most important characteristics, especially in the temperature area of glazing (our cause—1,050 °C). It is possible to pronounce the opinion that the changes in samples area into temperature 1,200 °C are a maximally 3 %. Therefore, pigments prepared at 1,300–1,400 °C have very well the thermal stability.
XRD characterisation
The XRD patterns in Fig. 5 depict an impact of firing temperature on the phase composition of Co-doped malayaite compounds. From this figure, it is evident that synthesis of Co-doped pigments at the all tested firing temperatures did not bring the formation on a single-phase malayaite system. The XRD pattern for the lowest used temperature (1,200 °C) contains the diffraction lines of malayaite as a major phase and calcium stannate, cassiterite, cristobalite and wollastonite phases as unreacted or additional components as minor compounds and it is evident that this temperature is not sufficient for good production of the malayaite compound. The increasing heating temperature 1,250 °C produces the three-phase product having compositions the malayaite, calcium stannate and cassiterite, i.e. this temperature 1,250 °C is still not sufficient for preparation of products with good pigmentary properties. But similar results were obtained during the firing temperatures during 1,300–1,400 °C. For these XRD patterns, the diffractive lines for the two-phase compounds were indicated. The malayaite and the cassiterite structure were identified. Contribution published by Piña [23], concerning by preparation of Co-doped malayaite pigment, on the occurrence of cassiterite phase in Co-doped malayaite compound points out too. Therefore, it is possible to suppose than cobalt is incorporated in malayaite structure in our case too. But for good react of used starting raw materials by solid-state reaction, temperature minimally 1,300 °C is necessary. From pigmentary point of view, it is not necessary to prepare pigment with single-phase composition, but the most important are the conditions of synthesis, which have to be appropriate for the industrial utilization. Therefore, the method of synthesis of tested malayaite compounds was not improved, and pigmentary-application properties of the samples were also measured. The identified phase composition, an adequate structure and JPDF numbers are summarized in Table 1.
Results of particle size distribution
All prepared pigments were subjected to measurement of particle size distribution in dependence on heating temperature. The most important value, which characterizes particle size, is the value d 50. From Table 2, it is evident that with the increase of firing temperature, the main particle size grows too. A pigment prepared by the calcination of reaction mixture at 1,200 °C has value of particles about 7.00 μm. The temperature increase by 50 or 100 °C caused a gradual augmentation in the mean particle size about 1.6 or 3 μm. The similar results were obtained for firing temperatures 1,350 and 1,400 °C, and the values of particles mean were about 13.3 μm. So, the results of particles size analysis showed that hand grinding of the samples in the agate mortar after the firing process was sufficient for the potential incorporation of the pigments into an organic matrix or ceramic glaze. For these applications, the value of mean particle size should not exceed 15 μm.
The colour properties
The great attention of this work was devoted on study of the effect of firing temperature on colour properties of tested Co-doped malayaite pigments. Although the results from XRD analysis for sample prepared at 1,200 °C showed the occurrence of unreacted or additional components as minor compounds, this calcining temperature (1,200 °C) in the study of the impact of the firing temperature on the colour properties was included. The results from DTA-TG analysis were also the reason of this decision, because these results detected that the beginning of formation of Co-doped pigment at the temperature 1,214 °C probably was. The colour properties of pigments without application (in powder form) and after application in the organic matrix in mass tone and transparent glaze P 07410 in mass ratio 5–25 % were studied.
The colour appearance of powder pigments is blue, and the blue shades were in dependence on the firing temperature. The colour properties of samples without application (in powdered form) and samples applied into organic matrix together with the values of mean particle size are summarized in Table 2. The obtained results of both studied samples showed that temperature increase affected the values of lightness (L*), but not significantly. They are moved from 56 to 64 for powders and from 52 to 69 for the samples applied into organic matrix. The shift of colour of powder pigments from pale blue to bright blue is caused by increasing temperature from 1,200 to 1,400 °C. This is clearly visible from the values of colour coordinates b*, because the coordinates b* have the decreasing character with the increasing firing temperature. The highest value of b* coordinate was −16.57, and it was assigned to temperature 1,400 °C. The hue angle is closely connected with the colour coordinate b* in this case. The values of H° moved from 181.59 to 275.52 for powders and from 181.65 to 279.85 for the samples applied into organic matrix, and they indicated the shift of colour from the blue to the very near field of the violet. The values of colour coordinate a* had very slowly upward trend with growing firing temperature. Therefore, the impact of heating temperature is insignificant in this case. The values of saturation (C) calculated for powdered pigments lie in range from 5.77 to 16.63. The pigment prepared at 1,400 °C is characterized by the highest saturation (C). Very similar results were obtained for pigment samples which were applied into the organic binder in mass tone including individual evaluated trends. The highest value of coordinate b* was −46.18 and it was assigned to temperature 1,400 °C too. For temperature 1,400 °C, it was calculated the biggest value of saturation (C) too. The shifts to the higher values of a* and the lower values of b* in comparison with the colour coordinates for powdered pigments were caused by the presence of used organic binder.
The second studied binding system was transparent glaze P 07410 with the glazing temperature 1,050 °C. Because the tested malayaites can belong into group of the high temperature ceramic pigments, the transparent glaze with the middle-temperature of glazing for study of colour properties was selected. From reason recommendation of appropriate mass of pigment, the impact of used mass of pigment on the colour properties was investigated too.
The surface of studied tiles was without visible crack and bubbles. The colour appearances of tiles with applied tested malayaites moved in different interesting pastel shades of blue colour in dependence of firing temperature and used mass of pigment. The colour properties of compounds applied into glaze P 07410 are in Table 3. The obtained results showed that temperature increase not significantly affected the value of lightness (L*). The decrease of L* values with the growing temperatures was very small. This value (L*) was visibly affected by the mass of used pigment. For the higher values of mass of pigments, the decreasing of the L* values were obtained. The shift of colour of the pigments applied into glaze from pastel pale blue to blue was caused by augmentation the temperature and pigment mass too. It is again clearly visible from the values of colour coordinates b*. The smallest changes of b* values for 5 % pigment mass were detected, but the next addition of pigment mass on 10 % brings significant decrease of b* values. Although following the growing of mass of pigment samples cause the decrease of coordinates b*, it is this the decrease insignificant. These changes are very small and gradual. From Table 3, it is evident that the changes in the values of hue angels are very small, and they are similar to each other. The values of H° moved in the narrow range, i.e. from 276.85 to 285.47, and it is shifted from the blue to the violet colour space. The gradual increasing changes in the values of a* coordinates in dependence on the augmentation firing temperature and pigment mass were registered. The highest saturation C was calculated for temperature 1,400 °C and the pigment mass 25 % (28.19). But it is evident that the changes of saturation values for mass of pigments 15–25 % in dependence on the firing temperature are very similar. Therefore, from technological and industrial point of view (low operating and material costs), it is possible to state that 15 % of pigment mass is suitable and adequate the mass for colouring of middle-temperature transparent glaze P 07410. The results of both colour characteristics (C and H°) determined for individual mass of pigments in dependence on firing temperature suggest about stable behaviour of Co-doped malayaite in molten glaze. For preparation of Co-doped malayaite pigments with the excellent colour properties, the firing temperature 1,350 °C is necessary.
Conclusions
The results of this contribution can be summarized in the following pronouncement. The main aim of this study was to synthesize Co-doped malayaite pigments. The pigments were prepared using classical ceramic method, i.e. solid-state reaction. The calcining procedure was performed at 1,200–1,400 °C/4 h. First, all the methods of thermal analyses were used for determination of the optimal temperature of pigment formation and for assessment of the thermal stability of prepared heat-treated compounds. It was showed that the formation of Co-doped pigments with malayaite structure starts at 1,214 °C and finishes at 1,325 °C. The Co-doped malayaite pigments prepared at different firing temperature are the thermal stable, namely the samples prepared at 1,300–1,400 °C. The X-ray analysis informed that single-phase malayaite system did not succeed to prepare. For good react of initial mixture, the temperature 1,300 °C is necessary. In XRD patterns, for heat-treated samples prepared at 1,300–1,400 °C, the diffractive lines of the malayaite as the major and the cassiterite as minor phase were identified. Mean particle size moved in range 7.00–13.39 μm in dependence on firing temperature (increasing character with increasing temperature), and this particle size is sufficient for the potential incorporation of the pigments into the organic matrix and ceramic glaze. The great attention was devoted on measuring of pigment-application properties. The colour appearance of powder pigments is blue, and the blue shade on the firing temperature is dependent. Generally, the higher firing temperature allowed formation of pigments with the higher saturation (C) and the higher values oh hue angles (H°). For colouring of the middle-temperature P 07410, it is possible to recommend 15 % of pigment mass, and for preparation pigment compounds with excellent the colour properties minimally 1,350 °C is necessary.
References
Swiler DR. Inorganic pigments, Kirk-Othmer encyclopedia of Chem Technol. 5th ed. New York: Wiley and Sons Inc; 2006.
Ou-benmmou I, Ahamdane H, El idrissi Raghni MA, Bensamka F, Mosset A, El idrissi Moubtasim ML, Jumas JC. Tin sphene micron-sized powders. J Eur Ceram Soc. 2000;20:2159–63.
Cruciani G, Dondi M, Ardit M, Lyubenova TS, Carda JB, Mateucci F, Costa AL. Malayaite ceramic pigments: a combined optical spectroscopy and neutron/X-ray diffraction study. Mater Res Bull. 2009;44:1778–85.
Harisanov V, Pavlov RS, Marinova IT, Kozhukharov V, Carda JB. Influence of crystallinity on chromatic parameters of enamels coloured with malayaite pink pigments. J Eur Ceram Soc. 2003;23:429–35.
Sanghani DV, Abrams GR, Smith PJ. A structural investigation of same tin-based coloured ceramic pigments. Trans J Br Ceram Soc. 1981;80:210–4.
Faurel X, Vanderperre A, Colomban P. Pink pigment optimization by resonance Raman spectroscopy. J Raman Spectrosc. 2003;34:290–4.
Stefani R, Longo E, Escribano P, Cordoncillo E, Carda JB. Developing a pink pigment for glaze. Am Ceram Soc Bull. 1997;76:61–4.
Cordoncillo E, Escribano P, Monrós G, Tena MA, Orera V, Carda J. The preparation of CdS particles in silica glasses by a sol-gel. J Solid State Chem. 1995;118:1–5.
Eppler RA. Selecting ceramic pigments. Am Ceram Soc Bull. 1987;66:1600–2.
Alacron J, Gargallo JJ, Escribano P. Cr–CaO–SiO2 based ceramic pigments. Trans J Br Ceram Soc. 1984;83:81–3.
Heyns AM, Harden PM. Evidence for the existence of Cr(IV) in chromium-doped malayaite Cr4+:CaSnOSiO4: a resonance Raman Study1. J Phys Chem Solids. 1999;60:277–84.
Abe S, Yamane H, Yoshida H. Synthesis and photoluminescence of Ca–(Sn, Ti)–Si–O compounds. Mat Res Bull. 2010;45:367–72.
Mouyane M, Womes M, Jumas JC, Olivier-Foucade J, Lippens PE. Original electrochemical mechanism of CaSnO3 and CaSnSiO5 as anode material for Li-ion batteries. J Solid State Chem. 2011;184:2877–86.
Mesíková Ž, Šulcová P, Trojan M. Synthesis and description of SrSn0,6Ln0,4O3 perovskite pigments. J Therm Anal Calorim. 2008;91:163–6.
Pfaff G, Hildenbrand VD, Fuess H. Spectroscopic study of amorphous precursors for alkaline-earth titanates and stannates. J Mater Sci Lett. 1998;17:1983–5.
Alves MCF, Sousa AC, Lima SJG, Longo E, Souza AG, Santos IMG. Influence of precursor salts in the synthesis of CaSnO3 by the polymeric precursor method. J Therm Anal Calorim. 2007;87:763–6.
Lyubenova TS, Matteucci F, Costa A, Dondi M, Carda J. Ceramic pigments with sphene structure obtained by both spray- and freeze-drying techniques. Powder Technol. 2009;193:1–5.
Carda J, Escribano P, Monrós G, Rodrigo MD, Alarcon J. Co–SnO2–CaO–SiO2 based ceramic pigments. Interceram. 1990;39:22–4.
Costa G, Ribeiro MJ, Labrincha JA, Dondi M, Matteucci F, Cruciani G. Malayaite ceramic pigments prepared with galvanic sludge. Dyes Pigm. 2008;78:157–64.
CPMA, Classification and chemicals descriptions of the complex inorganic color pigments. 4th ed. Alexandria, Virginia: Color Pigments Manufacturers Association, Inc; 2010. p. 24.
Lopez-Navarrete E, Caballero A, Orera VM, Lázaro FJ, Ocaña M. Oxidation state and localization of chromium ions in Cr-doped cassiterite and Cr-doped malayaite. Acta Mater. 2003;51:2371–81.
Doménech A, Torres FJ, Ruiz de Sola E, Alarcón J. Electrochemical detection of high oxidation states of chromium (IV and V) in chromium-doped cassiterite and Tin-sphene ceramic pigmenting system. Eur J Inorg Chem. doi:10.1002/ejic.200500775.
Piña C, Arriola H, Nava N. Study of malayaite and malayaite cobalt pigment. Hyperfine Interact. 2005;161:93–7.
Šulcová P. Properties of inorganic pigments and methods of their evaluation. Pardubice: University of Pardubice; 2008 (in Czech). pp. 15–40.
The International Centre for Diffraction Data. Newtown Square. USA: Pennsylvania; 2012.
Sinkó K, Pöppl L, Gábor M, Migály B. Study of the binary CaCO3-SiO2 system by quantatitative DTA. J Therm Anal. 1988;33:1003–12.
Huang JF, Hung A, Wang CHB, Yeh ChT. Geneses of cobaltic oxide. J Chin Chem Soc. 2002;49:819–24.
Hongbo T, Kai K, Baoguo M, Jun X. Solid solution mechanism of Co2O3 during C3S formation. Open Mater Sci J. 2011;5:118–22.
Heaney PJ. Structure and chemistry of the low-pressure polymorphs. Silica: physical behavior, geochemistry and material applications. Rev Miner. 1994;29:1–32.
Lakshtanov DL, Sinogeikin SV, Bass JD. High-temperature phase transition and elasticity of silica polymorphs. Phys Chem Miner. 2007;34:11–22.
Acknowledgements
This work was financially supported by the IGA University of Pardubice within of the Project SGSFChT_2014002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luxová, J., Šulcová, P., Trojan, M. et al. Study of thermal behaviour and stability of Co-doped malayaite ceramic pigments. J Therm Anal Calorim 116, 571–580 (2014). https://doi.org/10.1007/s10973-014-3760-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3760-0