Abstract
Ceramic pigments based on Y2O3–Al2O3 system doped by cobalt as a colourant agent were synthesized by solid-state reaction at temperatures up to 1,400 °C. The reactivity of initial mixtures of components was improved by the mineralizer LiF and the mechanical activation in a planetary ball mill. The temperature region of the product formation was followed by the method of thermal analysis. The effect of the synthetic method on the phase composition of the products was studied by X-ray diffraction analysis. Studied pigment-application properties of the product include the measurement of optical properties in the visible region of light and particle size distribution. The simple solid-state reaction led to the formation of turquoise samples that contain mainly blue CoAl2O4 spinel and next to it also YAlO3 perovskite and Y3Al5O12 garnet phases. The mineralizer LiF promotes the formation of yttrium aluminium double oxides of sandy-yellow to grey–brown colour hue, although the samples also contain small amount of blue CoAl spinel phase. Intensive milling process did not results in CoAl spinel phase and the samples contain yttrium aluminium perovskite and cobalt oxide. Evaluation of Kubelka–Munk absorption as a function of the pigment concentration was found that hiding is complete by adding of 5 mass% of pigment to the ceramic glaze. Resulting colour hue of all pigment applications into ceramic glaze is blue. The size of particles lies in the range of 7–26 μm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
YAlO3 perovskite (YAP) is one of the three yttrium aluminium double oxides in combination with Y4Al2O9 monoclinic (YAM) and Y3Al5O12 garnet (YAG) structures, which are formed within the pseudobinary system between alumina (Al2O3) and yttria (Y2O3) oxides [1–4]. The yttrium aluminium perovskite exhibits orthorhombic structure with the Pbnm space group and the lattice parameters a = 5.1377 Å, b = 5.2736 Å and c = 7.3085 Å [5].
The properties of YAlO3, such as high refractive index, optical transparency, chemical inertness and high thermal and mechanical resistance, make it suitable as a host material for the preparation of coloured ceramic pigments. Inorganic pigment is a substance consisting of small particles almost insoluble in the applied medium and used for colouration of many materials including glazes and ceramic bodies, protective or magnetic properties. Pigments can be characterised by their chemical composition and optical or physical properties [6]. Ceramic pigments are usually coloured material based on mixed metal oxides, which have high thermal stability to be used at high temperature and are inert to the action of molten glass (frits and sintering acids) [7, 8]. Perovskites represent a small but important part of the range of the inorganic pigments. In the perovskite structure like REAlO3, substitution of Al3+ ions by some other ions can change the initial colourless appearance to a red, brown or green colour, depending on the type and extent of the substitution. The most often cited are red perovskite pigments based on REAlO3 structure which were prepared by substituting of rare earth ions by chromium [9–11]. Ardit et al. [12] synthesised green compounds based on perovskite structure by the ceramic route according to the Y1−yCayCr1−x−yAlxTiyO3 stoichiometry. The main aim of this work was to extent the line of coloured perovskite aluminates by the preparation of blue pigment based on partial substitution of yttrium ions by cobalt.
However, in the synthesis of pure YAlO3, the achievement of single phase YAP represents a hard challenge. Solid-state reaction involving temperatures above 1,400 °C results in desired product only after intensive homogenisation of the reactants in a planetary ball mill in acetone or ethanol medium with employment of mineralizers in the reaction of mixtures [10, 13, 14]. Blosi et al. [15] reported that the aqueous sol–gel combustion process which makes the achievement of pure YAP easier, whilst an innovative two-step route of the microwave-assisted synthesis applied to YAP leads to the formation of YAP, YAM and also YAG phases [16]. Widely used technique, which allows preparing of pure YAP phase at a temperature lower than 1,000 °C, is co-precipitation [11, 17, 18]. Rane et al. [19] described the preparation of single phase cubic LaAlO3 by hydrazine method from LaAl(C2O4)3·N2H4·3H2O at the temperature 1,000 °C.
In this paper, ceramic pigments based on Y2O3–Al2O3 perovskite system doped by cobalt oxide (CoO) as a colourant agent are prepared by solid-state reaction and resulted powders are evaluated with respect to their phase composition, optical properties and particle size distribution.
Experimental
Synthesis and application of the powders to a binder
The compounds Y2O3 (Indian Rare Earth Ltd., India), Al(OH)3 (ZSNP a.s., Slovakia), CoCO3.Co(OH)2 (Shepherd Colour Comp., USA) and the mineralizer LiF (Spolechemie, a.s., CR) were used as starting materials for the preparation of blue ceramic pigments according to Y2O3–(CoO)x–(Al2O3)1−x stoichiometry, where x = 0.05; 0.2 and 0.5. The mineralizer LiF was added in a fixed amount of 5 mass%. The application of mechanical solid-state reaction with a high-energy ball milling process was examined. The mixtures of the reagents were thoroughly grinded in an agate mortar with a pestle and placed into agate mill jars. The high-energy milling process was carried out in a planetary ball mill Pulverisette 5 (Fritsch, GmbH, Germany) for 4 h with a rotation speed of 200 rpm. The reaction mixtures were milled together with agate balls (Ø 10 mm) in a ball-to-powder mass ratio of 20:1 with ethanol. The activated reaction mixtures in alumina crucibles were heated in the air atmosphere at 1,100, 1,200, 1,300 and 1,400 °C for 4 h at each heating step. The products were gradually cooled to room temperature. The obtained pigments were further hand milled in an agate mortar to homogenise agglomerates. The mineralizer was removed from the final products by decantation in 400 mL of hot distilled water.
The second group of Y2O3–(CoO)x–(Al2O3)1−x powders was prepared by simple solid-state reaction, i.e. without a high-energy milling process. The mixtures of reagents and the mineralizer LiF (5 mass%) were hand milled in an agate mortar with a pestle. Then, the homogenous mixtures were heated in an electric furnace at high temperatures by the same way, like it was mentioned above.
The third group of powders was also synthesised by simple solid-state reaction, but in that case, without the mineralizer.
For testing in ceramic glaze, aqueous suspensions containing 1–10 mass% of the pigment and 90–99 mass% of the frit lustrous colourless glaze with value of the thermal expansivity coefficient a 20–500 °C = 56.8 × 10−7 K−1 and recommended firing temperature 1,050 °C (Glazura, a.s., CR) were prepared by hand milling. These slurries were deposited on the wall tile bodies and further fired at the temperature 1,050 °C for 15 min. For testing in organic matrix, suspensions containing 1 g of the pigment and 1.5 cm3 of a binder were homogenised. Colour paints were prepared by deposition of the slurries on the white not absorb paper. Wet film thickness was 100 μm.
Simultaneous TG–DTA analysis
Thermal analysis was used for studying of thermal behaviour of the reaction powder mixtures before and after intensive milling process. The formation of YAlO3 perovskite pigments was followed by a STA 449C Jupiter (NETZSCH, Germany), which allows evaluation of data with simultaneous registration of the thermoanalytical curves TG and DTA. Thermal behaviour of the reaction mixture containing Al(OH)3 Y2O3 and CoCO3 was studied by thermal analysis at the temperature region from 30 to 1,300 °C. Heating rate was 10 °C min−1. The analysis was carried out in a ceramic crucible under air atmosphere and α-Al2O3 was used as a reference material.
X-ray diffraction analysis
The phase composition of the pigments was studied by X-ray diffraction analysis. Diffractograms of the samples were obtained using an equipment diffractometer D8 (Bruker, GB) with a goniometer of 17 cm in the range 2Θ of 10–80°. Cu Kα1 (λ = 0.15418 nm) radiation was used for the angular range of 2Θ < 35° and Cu Kα2 (λ = 0.15405 nm) for the range of 2Θ > 35. A scintillation detector was used.
Measurement of the colour properties of applied pigments
The colour properties of all prepared pigments were objectively evaluated by measuring of spectral reflectance with a spectrophotometer ColorQuerst XE (HunterLab, USA). The measurement conditions were the following: an illuminant D65, 10° complementary observer and measuring geometry d/8°. For description of colour, the colour space CIE L*a*b* was used. The L*a*b* colour space (also referred as CIELAB) is presently one of the most popular colour space for description of object colour and it is widely used virtually in all fields of applications. In this colour space, L* indicates the lightness, and a* and b* are the chromaticity coordinates. In a*b* diagram, a* and b* indicate colour directions: +a* is the red direction, −a* is the green direction, +b* is the yellow direction and −b* is the blue direction. The centre is achromatic; as a* and b* values increase, the saturation of the colour also increases. The value L* is related to the neutral grey scale. In the L*a*b* system, it is described by numbers from zero (black) to one hundred (white). The value C (Chroma) represents saturation of the colour and it is calculated according to the Eq. 1. The colour hue of pigments is also possible to express as a hue angle H° (Eq. 2). Hue angle H° is defined as starting at the +a* axis and expressed in degrees; 0° is red (+a*), 90° is yellow (+b*), 180° is green (−a*) and 270° is blue (−b*) [20].
Measuring of particle size distribution
The particle size distribution of the samples was measured using a Mastersizer 2000/MU (Malvern Instruments, UK). The equipment employs a scattering of incident light on particles. The pigments were ultrasonically homogenised in Na4P2O7 solution (c = 0.15 mol dm−3) for 90 s and then measured. The signal was evaluated on the basis of Fraunhofer bending. Before the measurements, the samples were gently ground in an agate mortar.
Results
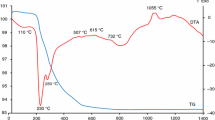
Thermal behaviour of reaction mixtures containing Y2O3, Al(OH)3 and CoCO3.Co(OH)2 before and after high-energy milling process was followed by methods of thermal analysis. In Fig. 1 the TG and DTA curves of the reaction mixture used for the preparation of the pigment Y2O3–(CoO)0.5–(Al2O3)0.5 before high-energy milling process are presented (solid state reaction). The gibbsite and also the cobalt carbonate decomposition take place in several steps. On the TG curve the first mass loss in the range of 30–225 °C was recorded (Table 1). This mass loss (−0.3 %) is connected with loss of water content of initial Co precursor CoCO3.Co(OH)2 and formation of CoCO3.CoO. At the temperature slightly above 200 °C, a weak endothermic peak at 263 °C, which appears as a shoulder, is connected with thermal decomposition of gibbsite and therefore, the formation of boehmite (AlOOH) takes place. A next sharp endothermic peak (328 °C) observed on the DTA curve is associated with the partial decomposition of CoCO3.CoO and formation of Co3O4. In the range of 225–400 °C mass loss is observed (9.85 %) (Table 1). This is connected with decarbonation as well as with dehydration of gibbsite. Most of the water becomes trapped inside the coarse gibbsite grains under a relatively high overpressure. As the temperature increases, the overpressure inside the gibbsite grains could favour the boehmite formation [21]. An endothermic peak with minima at 530 °C is due to the decomposition of boehmite and formation of Al2O3. As the temperature increases above ≈800 °C, the decomposition of cobalt precursor continues. A weak endothermic peak recorded at the DTA curve at 870 °C is connected with the formation of CoO from Co3O4 [22]. In the temperature range of 1,000–1,300 °C, three exothermic peaks with maxima at 1,044, 1,084 and 1,235 °C were detected on the DTA curve. These peaks correspond to the formation of resulting products i.e. yttrium aluminium double oxides (1,044 and 1,235 °C) and spinel compound CoAl2O4 (1,084 °C). This assumption was confirmed by phase analysis of the sample calcinated at the temperature 1,100 and 1,300 °C.
In Fig. 2 the TG and DTA curves of the reaction mixture used for the preparation of the pigment Y2O3–(CoO)0.5–(Al2O3)0.5 after high-energy milling process are presented (mechanical solid state reaction). The mechanical treatment did not induce any significant changes in the reaction mixture. A wide endothermic peak around the temperature 162 °C takes place on the DTA curve. The effect is connected with mass loss on the TG curve compatible to the loss of the water from CoCO3.Co(OH)2. An intensive milling process caused the unification of all endothermic peaks in the temperature range 200–400 °C to the one with minimum at 325 °C (Table 2). This peak corresponds to the decomposition of initial Co and Al compounds, the same as next two endothermic peaks at 523 and 788 °C. In the temperature range of 1,000–1,300 °C, only two exothermic peaks with maximum at 1,038 and 1,073 °C were detected on the DTA curve. These peaks are connected with the formation of resulting products i.e. yttrium aluminium double oxides. The results of thermal analysis indicate that intensive milling process of initial components helps to prepare the yttrium aluminium double oxide without the formation of spinel compound as a secondary product, which was confirmed by results of XRD analysis, described below.
The results of XRD analysis showed that in the calcining range of 1,100–1,300 °C does not provide single phase Y–Al–Co system. The phase composition of powder Y2O3–(CoO)0.5–(Al2O3)0.5 strongly depends on the method of preparation and mineralizer application (Table 3). The simple solid-state reaction without intensive activation of the reagents led to the formation of blue spinel compound CoAl2O4 next to yttrium aluminium oxide YAM at the 1,100 °C, YAG phase is formed at the temperature 1,300 °C and the YAP phase at 1,400 °C, whilst addition of the mineralizer LiF brought about the emergence of YAP, YAG and spinel phase at 1,100 °C. The mechanical solid-state reaction inclusive the energy ball milling process of the reagents caused the formation of YAP and YAM phase and also free cobalt and yttrium oxides at the lowest calcining temperature, i.e. 1,100 °C. The calcination at the temperature 1,300 °C led to the formation only one type of yttrium aluminium oxide which is YAP and next to it unreacted CoO and yttrium oxide were identified on XRD pattern.
This results showed that perovskite aluminium yttrium oxide with orthorhombic structure (JCPDS card 04-014-3537) and lattice parameters a = 5.18027 Å; b = 5.32951 Å and c = 7.37059 Å with primitive Pbnm (62) space group can be prepared only by solid-state reaction in the case of increase of the solid-state reactivity of reagents by mineralizer and mechanochemical power of ball milling. The mineralizer LiF is a fluxing agent which decreases the temperature of synthesis of the final product and promotes the formation of a fluid phase. Generally, the results of XRD analysis of samples that are not single phase, usually lead to exchange of synthesis method or conditions. In the case of solid-state reaction it is usually longer time period of heating and higher calcining temperature, or higher amount of mineralizer. However, in the pigmentary field of application, multiphase products are commonly used and much more important are appropriate composition and synthesis conditions for the industrial employment, which provide the most intensive colouration of the powders. That was the reason, why the method of synthesis was not improve and the application properties of the samples into the organic binder and ceramic glaze were tested.
The optical properties of the powder applications were measured after each calcination step to find the most appropriate calcination schedule leading to the formation of intensive colour shades in a ceramic glaze and in an organic binder. The pigment colour is uniquely described by its spectral reflectance curve. In the industrial practise it is better to measure of L*, a* and b* parameters, obtained by the analysis of the reflectance curves provided by spectrophotometer. The problem is, that there isn′t a systematic relation between the values of L*, a* and b* parameters and the concentration of a pigment added into a binder. With the model developed by Kubelka–Munk [23] it is possible to relate systematically the colour with the quantity of pigment added. This model relates the reflectance R for every wavelength to the absorption K and scattering S of light of pigments particles. This means that for every frequency of the visible spectrum, every component of a formulation possesses a coefficient of absorption K and the coefficient of scatter S. In particular, when the layer of the absorbing scattering material is so thick that no light penetrates through the layer (opaque surface), the relationship described the Kubelka–Munk theory, taking into account also the Saunderson correction, is given by Eq. 3 [24].
where r λ is the decimal fractional reflectance (0 < r < 1) measured at the wavelength λ with specular component excluded. From Eq. 1 and with the reflectance curves of the pigment Y2O3–(CoO)0.5–(Al2O3)0.5 (simple solid state reaction; 1,300 °C) applied into the ceramic glaze were possible to calculate the values of the K/S at each wavelength of visible region (Fig. 3). The Kubelka–Munk absorption spectra shown on Fig. 3 present the characteristic spectrum of Co2+ ion in tetrahedral coordination, with a triple absorption peak in the visible region around 545 nm (green region), 585 nm (yellow–orange region) and 630 nm (red region), and with a multiple reflection valley between 400 and 450 nm (blue region), which gives rise to the blue colour [25].
For the industrial practise, it is useful to set the optimal concentration of the pigment added to the glaze to find out when hiding is complete by the Kubelka–Munk absorption as a function of the pigment concentration. The K/S as a function of Y2O3–(CoO)0.5–(Al2O3)0.5 concentration in the glaze (1–10 mass%) at wavelength 590 nm is given on Fig. 4. The figure shows that the K/S value increases as the quantity of the pigment increased up to 5 mass%. Higher concentration of pigment added into the ceramic glaze than 5 mass% caused the stabilization of the Kubelka–Munk absorption. Thus, the hiding is complete right at the amount of 5 mass% of the pigment in the glaze and this amount was used in all other pigment applications into the ceramic glaze.
In Table 4 the colour parameters of all samples applied into the ceramic glaze are summarised. The glass surfaces of all applications prepared from the powders synthesized by mechanical solid-state reaction are very low quality and the colour isn′t very intensive, especially at the lowest content of cobalt oxide. The colour of these applications varies with the increasing temperature and content of CoO from green–grey to blue hue, but the colour is not uniform. On the surface are visible dark blue to black particles. The lightness L* of applications decreases with an increasing content of CoO and with growth of the temperature; it means that applications become darker. The opposite situation is in the change of the parameters C, where with higher content of CoO the chroma increases. The applications prepared from pigments synthetized by simple solid-state reaction with LiF indicate similar behaviour. But the difference is that simple solid-state reaction with the mineralizer LiF allows to prepare the blue glass surfaces only in the case of samples x = 0.2 and 0.5. Sample x = 0.05 provides rather very light grey than blue colouration. Pigment Y2O3–(CoO)0.5–(Al2O3)0.5 prepared by both ways at the temperature 1,100 °C caused the least glossy surface with a lot of bubbles. The most interesting colour properties provide the pigments prepared by solid-state reaction without mineralizer. The reason is in the phase composition of the samples. They composed of the minor phase of spinel CoAl2O4, which produces intensive blue colouration. All these applications are very glossy, the most interesting, dark blue hue provided by sample Y2O3–(CoO)0.2–(Al2O3)0.8.
As it can be seen from Table 5, the optical properties of the samples applied into the organic binder are completely different in comparison to the ceramic glaze. The powders containing perovskite phase of yttrium aluminium oxide, thus all samples prepared with the mineralizer LiF, have sandy-yellow or grey–brown to grey–green hue in application into organic binder in mass tone. For composition of x = 0.05, their hues are the lightest and the most saturated. Growth of the temperature caused their darkening and decrease of the chroma. The lightness of samples x = 0.5 is not sensitive to the change of temperature, but colour hue of the samples calcinated at 1,200 °C is the most diverse of all. Colour hues of these samples are yellow–orange with very low saturation. The powders prepared by simple solid-state reaction without the mineralizer have intensive, bright blue–turquoise hue. In these samples, values of lightness decrease and values of chroma increase with a growing content of cobalt oxide. The growth of the calcination temperature does not affect the lightness of the pigment applications in an organic binder. The saturation of hues rises with growth of the temperature from 1,200 to 1,400 °C.
Particle size distribution belongs to fundamental measured properties of powders and can affect the rheological and optical properties such as colour or hiding power. For inorganic pigments for the most applications, an average particle size must be between 1 and 15 μm. For the application into ceramic glaze, the optimal particle size is between 5 and 15 μm, but on the other hand, for the applications into plastics, it is less than 2 μm. The composition of powders, the heating temperature and also the way of preparation affected the particle size distribution of the resulting products. Increase of the temperature is connected with the formation of coarser particles in all three ways of synthesis. The biggest growth of the mean of particle size was recorded in the case of powders prepared by simple solid-state reaction with using of the mineralizer LiF. In this case, the calcination at the temperature 1,400 °C brought slightly sintered products with values of d 50 > 20 μm. For technological use, such samples have to be put to the intensive milling process. The optimal particles, which do not need any else technological treatment, were prepared by solid-state reaction without LiF. The smallest particles were prepared by mechanical solid-state reaction with LiF, in which the intensive ball milling of reagents helps readily react to form finer particles 7 μm < d 50 < 13 μm. Increase of CoO content in the samples enhanced the subtly values of d 50. The growth is only between 1 and 2 μm (Table 6).
Conclusions
The aim of the present work was to synthetized the ceramic pigments based on yttrium aluminium oxide doped by CoO as a colourant agent and to evaluate resulted powders with respect to their phase composition, colour properties and particle size distribution. The powders were synthesised by conventional ceramic method, i.e. solid-state reaction with heating at high temperatures (1,100–1,400 °C). The main objective was to observe how increase of reactivity of starting materials by intensive milling process or mineraliser LiF may affect the phase composition and pigment-application properties of the products. The results can be summarised in the following statements.
The result of TG/DTA analysis of the reaction mixtures showed that mechanical solid-state reaction leads to the formation of the yttrium aluminium double oxide easier than simple solid-state reaction and most probably does not provide the formation of spinel compound. The analysis of phase composition of the samples clarified the different optical properties of the samples applied into the organic binder. The turquoise samples prepared by simple solid-state reaction without mineralizer are composed mainly from blue CoAl2O4 spinel and as a minority phase the samples contain also YAP and YAG phases. Simple solid-state reaction with the mineralizer LiF leads to the formation of sandy-yellow to grey–brown colour hue, although the powders also contain small amount of CoAl spinel next to yttrium aluminium double oxides. The powders of sandy yellow to grey–green colour prepared by intensive milling process do not contain any CoAl spinel phase, only yttrium aluminium double oxide and unreacted cobalt oxide.
During glazing process, the most probably spinel phase forms, because the resulting colour of all applications is blue, regardless of the method of synthesis. Evaluation of Kubelka–Munk absorption as a function of the pigment concentration was found that hiding is complete by adding of 5 mass% of pigment to the ceramic glaze. From the colouration point of view, the optimal composition of the powders is Y2O3–(CoO)0.5(Al2O3)0.5. The values of mean size of the particles are between 7 and 26 μm. Mechanical solid-state reaction allows to prepare the smallest particles, whilst simple solid-state reaction with mineralizer LiF causes formation of the biggest, slightly sintered particles.
References
Cruciani G, Ardit M, Dondi M, Matteucci F, Blosi M, Dalconi MCh, Albonetti S. Structural relaxation around Cr3+ in YAlO3–YCrO3 perovskites from electron absorption spectra. J Phys Chem A. 2009;113:13772–8.
Cockayne B. The uses and enigmas of the Al2O3–Y2O3 phase system. J Less-Common Metal. 1985;114:199–206.
Muresan LE, Popovici E-J, Bica E, Cadis AI, Perhaita I, Barbu Tudoran L. Investigation of thermal decomposition of yttrium–aluminium based precursors for YAG phosphors. J Therm Anal Calorim. 2012;110:341–8.
Lach R, Bućko MM, Haberko K, Szumera M, Gajerski R. Dynamic study of ammonium dawsonite doped with yttrium transformation at elevated temperatures. J Therm Anal Calorim. 2013;112:727–30.
Levy RM. Crystal structure and defect property predictions in ceramic materials, Ph.D. Thesis, University of London 2005.
Buxbaum G. Industrial inorganic pigments. 2nd ed. New York: Wiley-VCh; 1997.
Burgyan A., Characterization and Identification of the mixed metal oxides and ceramic pigments manufactured in the U.S. Interceram., NR.1 (1979) 30–32.
Lang AR. Dzes and pigments: new research. New York: Nova Science Publishers Inc; 2009.
Kar JK, Stevens R, Bowen RCh. Processing and characterisation of various mixed oxide and perovskite-based pigments for high temperature ceramic colouring application. Alloy Compd. 2008;461:77–84.
Shirpour M, Faghihi Sani MA, Mirhabibi A. Synthesis and study of red pigments based on perovskite YAlO3 structure. Ceram Int. 2007;33:1427–33.
Ahmadi S, Aghaei A, Eftekhari Yekta B. Synthesis of Y(AlCr)O3 red pigments by co-precipitation method and their interactions with glazes. Ceram Int. 2009;35:3485–8.
Ardit M, Dondi M, Cruciani G, Matteucci F. Ti–Ca–Al-doped YCrO3 pigments: XRD and UV–vis investigation. Matter Res Bull. 2009;44:666–73.
Guo X, Qi J, Sakurai K. Mechanochemical formation of novel catalyst for preaparing carbon nanotubes: nanocrystalline yttrium aluminium iron perovskite. Scripta Mater. 2003;48:1185–8.
Sakurai K, Guo X. Mechanical solid-state formation of Y1−x CeAlO3 and its application as an X-ray scintillator. Mater Sci Eng A. 2001;304–306:403–7.
Blosi M, Albonetti S, Dondi M, Costa AL, Ardit M, Cruciani G. Sol–gel combustion synthesis of chromium doped yttrium aluminium perovskites. J Sol–Gel Sci Technol. 2009;50:449–55.
Blosi M, Dondi M, Albonetti S, Baldi G, Barzanti A, Zanelli C. Microwave-assisted synthesis of Pr-ZrSiO4, V-ZrSiO4 and Cr-YAlO3 ceramic pigments. J Eur Ceram Soc. 2009;29:2951–7.
Shen Z, Ekstrand Å, Nygren M. Oxide/oxide composites in the system Cr2O3–Y2O3–Al2O3. J Eur Ceram Soc. 2000;20:625–30.
Palmero P, Esnouf C, Montanaro L, Fantozzi G. Influence of the co-precipitation temperature on phase evolution in yttrium–aluminium oxide materials. J Eur Ceram Soc. 2005;25:1565–73.
Rane KS, Uskaikar H, Pednekar R, Mhalsikar R. The low temperature synthesis of metal oxides by novel hydrazine method. J Therm Anal Calorim. 2007;90:627–38.
Commission Internationale de l′Eclairage, “Recommendations on uniform colour spaces, colour difference equations, psychometric colopur terms”, Supplement No. 2 of CIE publication No. 15 (E1-1,31) 1971, Paris Bureau Central de la CIE, 1978.
Mercurz JMR, Pena P, Aza AH. On the decomposition of synthetic gibbsite studied by neutron thermodifractometry. J Am Ceram Soc. 2009;89:3728–33.
Liptay G. Atlas of thermoanalytical curves. Budapest: Akadémiai Kiadó; 1974.
Kubelka P, Munk F. Ein beitrag zur optik der farbanstriche. Z Tech Phys. 1931;12:596–601.
Bondioli F. Inorganic pigments to colour ceramic materials: state of the art and future trends, dyes and pigments: new reseach. New York: Nova Science Publishers Inc; 2009.
Li W, Li J, Guo J. Synthesis and characterization of nanocrystalline CoAl2O4 spinel powder by low temperature combustion. J Eur Ceram Soc. 2003;23:2289–95.
Acknowledgements
The Ministry of Education, Youth and Sports of the Czech Republic, Project CZ.1.07/2.3.00/30.0021 “Enhancement of R&D Pools of Excellence at the University of Pardubice”, financially supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dohnalová, Ž., Šulcová, P. & Gorodylova, N. Study of ceramic pigments based on cobalt doped Y2O3–Al2O3 system. J Therm Anal Calorim 116, 647–654 (2014). https://doi.org/10.1007/s10973-014-3649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3649-y