Abstract
The experimental values of heat capacity and thermal expansion for lutetium boride LuB66 in the temperature range of 2–300 K were analysed in the Debye–Einstein approximation. It was found that the vibration of the boron sub-lattice can be considered within the Debye model with high characteristic temperatures; low-frequency vibration of weakly connected metal atoms is described by the Einstein model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interest in studying the properties of borides, particularly of rare-earth elements with high boron content, is due to the unusual behaviour of the borides’ magnetic, lattice subsystems, and the possibility of practical applications [1–5]. The RB66 borides series (where R denotes a rare-earth metal) was extensively studied in the latter part of the twentieth century, with investigation of their structural, electric and optical properties and thermal conductivity. In recent works [6–9], the magnetic transformations in some RB66 compounds were revealed at temperatures lower than 1 K, a result which was unexpected for substances with such a small concentration of paramagnetic ions.
To successfully study the peculiarities of the magnetic subsystems of substances, it is often necessary to determine effect of the lattice contribution on the overall magnetic characteristics. To achieve this, in the majority of cases, one draws a comparison with the isostructural non-magnetic analogue. For RB66 compounds, this analogue is lutetium boride LuB66, which is a diamagnetic substance of the semi-conductive type.
Lutetium boride LuB66 has a YB66 type cubic crystalline structure, with Fm3c space group. For each unit cell, there are 24 formula units (24 metal atoms and 1,584 boron atoms). The large number of crystal voids in the LuB66 structure is responsible for a number of its properties, such as its glass-like character. Lutetium atoms fill the voids with centres (0.0589, 1/4, 1/4) and (0.05629, 1/4, 1/4); the occupancy rate is around 0.5.
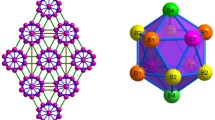
The boron framework is made up of eight super-icosahedrons B12(B12)12, each of which consists of thirteen icosahedrons B12 (Fig. 1). The peculiarities of LuB66 crystalline structure and a large difference in mass between lutetium and boron atoms define the peculiarities of the boride lattice properties.
LuB66 structure scheme [2]. Larger dark circles super-icosahedrons(B12)13, smaller light circles В80 clusters, dumb-bells Lu atoms
In the present work, we experimentally studied the temperature dependency of heat capacity C p(T) and crystalline structure parameter a(T) for lutetium boride LuB66 over a broad range of low temperatures, with the purpose of revealing the peculiarities of the behaviour of the phonon sub-system of boride.
Experiment
A powdered lutetium boride LuB66 sample was obtained via boron-thermal metal reduction from the oxide in a vacuum:
After the homogenising anneal at 1,000 °C, and melting in the arc furnace, the amount of boron in the lutetium boride decreased as its composition got closer to that of the stoichiometric case. The X-ray diffraction pattern of synthesised lutetium boride contained peaks associated with the only LuB66 phase as compared with the ASTM data file. According to the chemical analysis, the sample composition was 19.6 % Lu, 80.2 % B and 0.09 % O2. The pycnometric mass density of the sample, ρ pycn, was 2.430 × 103 kg m−3, which is 93 % of the X-ray density value. The difference between the X-ray and pycnometric density is caused by the porosity of the sample. Spectral analysis showed the presence of a small amount of tungsten admixture in the sample, amounting to around 1 % of the lutetium content.
The heat capacity of the LuB66 samples, at temperatures from 2 to 300 K, was measured in a calorimeter (Termax Ltd.). The calorimeter implements the classical adiabatic method of heat capacity investigation [10, 11]. The experimental error at 1.8–4.2 K is about 3 %, at 4.2–40 K is 2 % and at 40–350 K is 0.5 %.
The lattice parameter a(T) of LuB66 in the range of 5–300 K was determined using a X-ray diffractometer (DRON-7.0) in the Co-Kα radiation mode, with use of a helium cryostat. The calculation of a(T) values was made based on the experimental angular positions of 12 Bragg reflections [12]. The error in calculating the a(T) lattice parameter of LuB66 was ±10−4 nm.
At room temperature, the value of a(T) was 23.3850 Å, which agrees well with values quoted in the literature (a = 23.412 Å, [13]).
Results and discussion
The experimental temperature dependence of heat capacity C p(T) for lutetium boride LuB66 (Fig. 2) does not contain any visible anomalies; however, if the date are plotted as C p/T versus T 2 (inset in Fig. 2) and C p/T 3 versus T 2 coordinates (Fig. 3), one can see that at low temperatures (T < 10 K), the values of the boride heat capacity are anomalously high, and at T = 30 K there is an anomaly similar to the Einstein component of heat capacity.
Temperature dependence of lutetium boride LuB66 heat capacity. Points experimental data. Solid line calculated values [the relation (2)]; dashed line—the same, but without the Einstein contribution. Inset illustrates the presence of the contribution to the heat capacity at the lowest temperatures, which is proportional to 1st power of the temperature
Dependence of C P/T 3 from T 2. Points, the solid line, dashed line—the same as in Fig. 2
The values of the crystalline structure parameter a(T) obtained in the present work are shown in Fig. 4, alongside the calculated temperature dependence of the coefficient of thermal linear expansion α(T) for lutetium boride LuB66, in the interval of 5–300 K. a(T) and α(T) dependencies, similar to the C p(T) dependency, does not exhibit any visible anomalies in the temperature range studied.
The analysis of the temperature dependence of heat capacity and the thermal expansion of LuB66 boride was made on the basis of the combined approach of Debye–Einstein.
For heat capacity:
where D is the Debye function; E is the Einstein function; k i is the fraction of the i-th contribution to the boride total heat capacity; and \( \varTheta_{{D_{\text{i}} }} \) and \( \varTheta_{\text{E}} \) are characteristic temperatures [14]. The latter term describes the glass-like contribution, which linearly depends on the temperature [15–17].
For thermal expansion:
where:
Here, \( \varTheta_{{D_{i} }} \) and \( \varTheta_{E} \) are the characteristic Debye and Einstein temperatures, respectively; the parameters G, F, c, g, f characterise the anharmonic component of the interatomic interaction potential in the crystal; and T 0 = 300 K [18].
In Table 1, the approximate parameters for Eqs. (2) and (3) are provided, which were obtained by the least squares fit to the experimental data.
As can be seen in Figs. 2 and 3, we managed to describe the experimental dependencies of C p(T) and ΔV/V(T) for lutetium boride in the T < 300 K temperature range, following the process of approximating different thermal characteristics and using accepted approximations for similar values of characteristic temperature.
The high values of characteristic temperatures \( \varTheta_{{D_{1} }} \) and \( \varTheta_{{D_{2} }} \) can be ascribed to the boron sub-lattice, where the first value, \( \varTheta_{{D_{1} }} \), characterises the vibration of boron atoms in the sub-lattice, and the second value may characterise the vibration of boron clusters B12, which are the basic structural components of the boron sub-lattice. We should note that the characteristic temperatures of elemental boron, according to different publications, are 1,540, 1,300 and 1,200 K [19]. The vibration of lutetium atoms in a boride can be described by the Einstein model with comparatively low characteristic temperature Θ E = 140 K, which closely matches Θ D = 184 K (equivalent to Θ E = 138.4 K) [20] for metallic lutetium, and is close to Θ E, which is calculated according to the frequency of delocalisation of HO in the soft atomic potentials (SAP model): Θ E = ħω/k = 119 K [21].
The obtained distribution of characteristic temperatures in the LuB66 boride sub-lattice is consistent with the fact that for the calorimetric data, the ratio (k 1 + k 2)/k 3 amounts to =62, which is close to the ratio of boron atoms to lutetium atoms in a LuB66 molecule.
One cannot help noticing the considerable difference between the coefficients of the Einstein component k 3 in the approximation of the calorimetric data and the thermal expansion values. In this case, if heat capacity \( \sum\nolimits_{\text{i} = 1}^{3} {k_{\text{i}} } = 0. 9 9 7 \), i.e. close to 1 (one), as it has to be, then for thermal expansion a much greater value k 3 leads to \( \sum\nolimits_{\text{i} = 1}^{3} {k_{\text{i}} } = 1. 2 9 \). We should note that while conducting similar analysis of the thermal properties of rare-earth tetraborides [22] (unpublished work), we revealed the twofold excess of k 3 values obtained from X-ray data, over the calorimetric values of this coefficient. The sum \( \sum\nolimits_{\text{i} = 1}^{3} {k_{\text{i}} } \) for X-ray data also turned out to be more than one, although in this case it was marginal. Such anomalous behaviour of X-ray k 3 values is not clear and needs further study.
From the outlined arguments, looms the following picture: low-temperature data on heat capacity and thermal expansion for rare-earth borides can be described with the help of the Debye–Einstein combined approach; the values of the Debye components, describing the vibration of the boron sub-lattice, turn out to be similar for calorimetric and X-ray data; low-frequency vibrations, which we ascribe to the metal sub-lattice within the Einstein approximation, largely affect borides heat capacity at low temperatures and thermal expansion in the middle part of low-temperature region.
Conclusions
Calorimetric and X-ray research into the thermal properties of icosahedral lutetium boride LuB66 in the broad interval of low temperatures and the analysis of experimental data in the Debye–Einstein approximation enabled us to determine the character of ion oscillations in boron and metallic sub-lattices of a boride and to define the sub-lattices characteristic temperatures. It was found that vibrations in the boron sub-lattice, which are characterised by comparatively small masses of atoms and high values of bond energy, can be satisfactorily described by the Debye model. The vibrations of massive and comparatively weakly bonded lutetium atoms can be described well by the Einstein approximation. The latter conclusion clarifies why in earlier works [12, 22–24], it was difficult to separate the electronic and lattice contributions to the low-temperature heat capacity of rare-earth tetraborides in the simplest approximation, C v = aT + bT 3: the lattice contribution, besides the cubic Debye term, contains the Einstein component.
References
Richards SM, Kasper JS. The crystal structure of YB66. Acta Crystallogr B. 1969;25(2):237–51.
Mori T. Higher Borides. In: Gschneidner KA, Bunzl J-C, Pecharsky V, editors. Handbook on the physics and chemistry of rare-earths. Amsterdam: Elsevier; 2008. p. 105–73.
Wong J, Tanaka T, Rowen M, Schäfers F, Müller BR, Rek ZU. YB66 a new soft X-ray monochromator for synchrotron radiation. II. Characterization. J Synchrotron Radiat. 1999;6(6):1086–95.
Tanaka T, Rek ZU, Wong J, Rowen M. FZ crystal growth of monochromator-grade YB66 single crystals as guided by topographic and double-crystal diffraction characterization. J Cryst Growth. 1998;192(1–2):141–51.
Wong J, Shimkaveg G, Goldstein W, Eckart M, Tanaka T, Rek ZU, Tompkins H. YB66: a new soft-X-ray monochromator for synchrotron radiation. Nucl Instrum Methods A. 1990;291(1–2):243–9.
Flachbart K, Gabani S, Mori T, Siemensmeyer K. Magnetic ordering in RB66 boron-rich borides.In: APS March Meeting, 2010, Portland. K1.034.
Flachbart K, Gabani S, Mori T, Siemensmeyer K. Magnetic ordering in boron-rich borides TbB66 and GdB66. Acta Phys Pol A. 2010;118:875.
Kim H, Bud’ko SL, Tanatar MA, Avdashchenko DV, Matovnikov AV, Mitroshenkov NV, Novikov VV, Prozorov R. Magnetic properties of RB66 (R = Gd, Tb, Ho, Er, and Lu). J Supercond Nov Magn. 2012;25(7):2371–5.
Novikov VV, Avdashchenko DV, Bud’ko SL, Mitroshenkov NV, Matovnikov AV, Kim H, Tanatar MA, Prozorov R. Spin glass and glass-like lattice behaviour in HoB66 at low temperatures. Philos Mag. 2013;93(9):1110–23.
Sirota NN, Novikov VV, Antjukhov AM. Thermodynamic properties of solid solutions of gallium and indium arsenide in the temperature range of 5–300 K. J Phys Chem. 1983;57(3):542.
Sirota NN, Novikov VV. Heat capacity, mean square ion displacements and lattice parameter of DyB6 at 5–300 K. J Mater Process Manu. 1998;7(1):111–4.
Novikov VV, Mitroshenkov NV, Morozov AV, Matovnikov AV, Avdashchenko DV. Thermal properties of TbB4. J Therm Anal Calorim. 2013;113(2):779–85.
Properties, preparation and application of high-melting compounds: reference book. In: Kosolapova TYa, editor. Moscow: Metallurgy; 1986. p. 927.
Ramirez AP, Kowach GR. Large low temperature specific heat in the negative thermal expansion compound ZrW2O8. Phys Rev Lett. 1998;80:4903.
Slack GA, Oliver DW, Horn FH. Thermal conductivity of boron and some boron compounds. Phys Rev B. 1971;4(6):1714–20.
Cahill DG, Fischer HE, Watson SK, Pohl RO, Slack GA. Thermal properties of boron and borides. Phys Rev B. 1989;40(5):3254–60.
Mori T, Martin J, Nolas G. Thermal conductivity of YbB44Si2. J Appl Phys. 2007;102:073510.
Mukherjee GD, Bansal C, Chatterjee A. Thermal expansion study of ordered and disordered Fe3Al: an effective approach for the determination of vibrational entropy. Phys Rev Lett. 1996;76:1876–9.
Madelung O, Rössler U, Schulz M, editors. Non-Tetrahedrally Bonded Elements and Binary Compounds I, vol. 41C., Landolt-Börnstein—group III condensed matter. Berlin: Springer; 1998. p. 1–4.
Tonnies JJ, Gschneidner KA, Spedding FH. Elastic moduli and thermal expansion of lutetium single crystals from 4.2 to 300 K. J Appl Phys. 1971;42:3275.
Parshin DA. Interactions of soft atomic potentials and universality of low-temperature properties of glasses. Phys Rev B. 1994;49(14):9400–18.
Novikov VV, Mitroshenkov NV, Morozov AV, Matovnikov AV, Avdashchenko DV. Low-frequency modes and peculiarities of lattice thermal properties of RE-tetraborides. Phys Status Solidi B. 2014. In press.
Novikov VV, Morozov AV, Matovnikov AV, Polesskaya YN, Sakhoshko NV, Avdashchenko DV, Kornev BI, Solemennik VD, Novikova VV. Low-temperature heat capacity of rare-earth tetraborides. Solid State Phys. 2011;53(9):1743–7.
Novikov VV, Mitroshenkov NV, Morozov AV, Matovnikov AV, Avdashchenko DV. Heat capacity and thermal expansion of gadolinium tetraboride at low temperatures. J Appl Phys. 2012;111:063907.
Acknowledgements
The work was supported by the Ministry of Education and Science of the Russian Federation (Project 14.B37.21.0886) and the President Grant (Project 14.124.13.7302-MK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novikov, V.V., Avdashchenko, D.V., Matovnikov, A.V. et al. Heat capacity and thermal expansion of icosahedral lutetium boride LuB66 . J Therm Anal Calorim 116, 765–769 (2014). https://doi.org/10.1007/s10973-013-3593-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3593-2