Abstract
The thermodynamic properties and phase transitions of Tutton salt (NH4)2Fe(SO4)2·6H2O were investigated using thermogravimetric analysis, differential scanning calorimetry, and nuclear magnetic resonance. The first mass loss occurs around 330 K (T d), which is interpreted as the onset of partial thermal decomposition. Phase transitions were found at 387 K (=T C1) and 500 K (=T C2). The temperature dependences of the spin–lattice relaxation time in the rotating frame, T 1ρ, and that in the laboratory frame, T 1, for the H nuclei change abruptly near T C1. These changes are associated with changes in the geometry of the arrangement of octahedral water molecules and ammonium protons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tutton salts have played a significant role in physics and chemistry; considerable attention is currently focused on the development of materials suitable for strong energy absorption by solar collectors. For domestic heating and hot water supplies, this energy might be stored chemically in reversible reactions or thermally in phase changes or temperature increases of the storage materials [1, 2]. Tutton salts are an isomorphous series of monoclinic crystals with the general formula M I2 MII(SO4)2·6H2O. They contain two octahedral hexahydrate complexes [MII(H2O)6]2+ in the crystal unit cell, where MII is a divalent cation (Co, Zn, Fe, or an ion of the 3d group), and MI is a monovalent cation (K, Rb, Cs, or NH4) [3–10]. The unit cell dimensions and molecular structures of the crystals of this family are very similar. Montgomery and Lingafelter described the structural characteristics of the crystals in this series, including the details of their hydrogen bond networks [11]. One M I2 MII(SO4)2·6H2O compound, (NH4)2Fe(SO4)2·6H2O, has a monoclinic structure with space group P21/a. Six water molecules surround the divalent inversion symmetric sites. The divalent atoms in the unit cell are located at (0, 0, 0) and (½, ½, 0), whereas all the other atoms are in general positions.
Previous study has reported the physical properties; the thermal decomposition of (NH4)2Fe(SO4)2·6H2O has been studied and identified using the Mőssbauer effect, X-ray diffraction, infrared spectroscopy, gravimetric, and thermal differential methods [12]. Recently, Ganesh et al. [13] investigated thermal behavior and dielectric characterization of (NH4)2Fe(SO4)2·6H2O single crystal.
The interesting properties of Tutton salts have been studied by many methods in recent years. However, the thermodynamic properties and phase transition temperatures of (NH4)2Fe(SO4)2·6H2O crystals have not been reported. Further, some questions about Tutton salts remain open, especially those related to the nature of their structural changes and their thermodynamic properties. The hydrogen bond protons in (NH4)2Fe(SO4)2·6H2O crystals are expected to play a dominant role in their physical properties and phase transition mechanisms. The relationship between the loss of water protons and structural phase transitions is a subject of keen interest. To probe the variations with temperature in the physical properties of (NH4)2Fe(SO4)2·6H2O crystals, studying the 1H NMR spectrum and relaxation times is preferable because they are likely to be very sensitive to changes in the symmetry of the crystal.
This paper discusses the thermodynamic properties on the basis of differential scanning calorimetry (DSC) and thermogravimetric analysis (TG) measurements. In addition, the temperature dependences of the spin–lattice relaxation time in the rotating frame, T 1ρ, and that in the laboratory frame, T 1, for the 1H nuclei in (NH4)2Fe(SO4)2·6H2O were investigated using a pulse nuclear magnetic resonance (NMR) spectrometer to obtain detailed information about the physical properties. This is the first investigation of the structural changes in (NH4)2Fe(SO4)2·6H2O crystals, and we use these results to analyze the environments of their 1H nuclei. It is noteworthy that their thermodynamic properties and phase transitions were studied by analyzing these environments.
Crystal structure
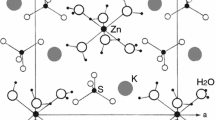
(NH4)2Fe(SO4)2·6H2O single crystals have monoclinic structure with space group P21/a and lattice parameters a = 6.2383 Å, b = 12.6076 Å, c = 9.2652 Å, α = γ = 90°, and β = 106.524° [14]. The unit cell contains two Fe2+ ions, each surrounded by six water molecules forming an octahedron, as shown in Fig. 1. (NH4)2Fe(SO4)2·6H2O is built up from Fe(H2O)6 octahedra, SO4 square planar forms, and NH4 tetrahedra. The Fe(H2O)6 octahedra is highly distorted, as indicated by the Fe–O bond. Each [Fe(H2O)6]2+ complex cation is surrounded by four sulfate anion acceptor groups and four ammonium cation donor groups. The crystal structure is stabilized by the N–H···O and O–H···O hydrogen bonds viewed along the a-axis.
Experimental
Single crystals of (NH4)2Fe(SO4)2·6H2O were grown by slow evaporation from an aqueous solution at 293 K. The resulting single crystals were hexagonal shape and jade green color with a size of ~4 × 7 × 3 mm3.
The 1H magic angle spinning (MAS) NMR spectrum and the spin–lattice relaxation time T 1ρ in the rotating frame were measured in (NH4)2Fe(SO4)2·6H2O by the Varian 200 MHz NMR spectrometer at the Korea Basic Science Institute. The Larmor frequency was set to 200 MHz, and the sample powder was placed in a 4 mm MAS probe. The rotor was spun at 10 kHz to minimize spinning sideband overlap. T 1ρ was measured by varying the duration of a 1H spin-locking pulse applied after direct polarization, i.e., through π/2–τ acquisition. The π/2 pulse time for 1H was 2.7 μs, which was equivalent to a spin-locking field strength of 92.59 kHz. In addition, the 1H NMR spectrum and spin–lattice relaxation time T 1 in the laboratory frame were obtained in (NH4)2Fe(SO4)2·6H2O single crystals. The spin–lattice relaxation time was measured using a saturation recovery pulse sequence, π–τ–π/2 acquisition; the nuclear magnetizations of the 1H nuclei at time τ after the sat pulse were determined following the π/2 excitation pulse. The width of the π pulse was 2.5 μs for 1H. Temperature-dependent NMR measurements were conducted at 200–400 K. The sample temperature was maintained at the required constant value with an accuracy of ±0.5 °C by controlling the helium gas flow and heater current.
Experimental results and discussion
To determine the structure of a single (NH4)2Fe(SO4)2·6H2O crystal, X-ray diffractometry was performed using the Bruker AXS GMBH instrument with a Cu target at the Korea Basic Science Institute. The crystal structure is monoclinic, and the lattice constants are a = 6.245 Å, b = 12.591 Å, c = 9.287 Å, α = γ = 90°, and β = 106.784°. Further, the space group is obtained as P21/c. These results are consistent with the data of Ganesh et al. [13]. In addition, DSC (DuPont 2010 DSC) was used to determine the phase transition temperatures of the crystals. The measurement was performed at a heating rate of 10 °C min−1. Endothermic and exothermic peaks were observed at 387 and 500 K, respectively, as shown in Fig. 2. This result is consistent with the 381.7 K reported by Voight and Goring [15]. TG was then used to determine whether these high-temperature transformations are structural phase transitions or chemical reactions. The curve of (NH4)2Fe(SO4)2·6H2O is shown in Fig. 3. The first mass loss begins around 330 K and reaches 14 % as (NH4)2Fe(SO4)2·3H2O at 366 K. Near 537 K, the thermal decomposition enters a new stage, and the residue of the final products reaches a value of 72.15 %, accompanied by the escape of H2O. Optical polarizing microscopy shows that the crystals are jade green color at room temperature and that their color changes to white with increasing temperature. This color change may be related to the loss of H2O. The bulk mass of (NH4)2Fe(SO4)2·6H2O decreases at 330 K (T d), which is interpreted as the onset of partial thermal decomposition, and reaches complete thermal decomposition into (NH4)2Fe(SO4)2 around 537 K. The DSC, TG, and optical polarizing microscopy results for (NH4)2Fe(SO4)2·6H2O crystals show that the mass loss around 330 K (=T d) is due to the onset of partial thermal decomposition. The transformation anomalies at 387 K (= T C1) and 500 K (= T C2) are related to phase transitions from (NH4)2Fe(SO4)2·6H2O to (NH4)2Fe(SO4)2·2H2O, and from (NH4)2Fe(SO4)2·6H2O to (NH4)2Fe(SO4)2·H2O, respectively.
Structural analysis of the protons in (NH4)2Fe(SO4)2·6H2O was conducted using solid-state NMR. The 1H MAS NMR spectrum of (NH4)2Fe(SO4)2·6H2O at room temperature is shown in the inset in Fig. 4. The NMR spectrum consists of one peak at a chemical shift of δ = 11.1 ppm, and this signal is associated with the ammonium and hydrogen bond protons. The spinning sidebands are marked with asterisks. Here, the signals for the two types of protons cannot be distinguished because they overlap. The temperature dependence of the chemical shift in the 1H NMR signal with respect to a reference signal is presented in Fig. 4. The chemical shift is generally sensitive to the electrical environment of the nucleus. Near T C1, the chemical shift abruptly changes with increasing temperature, which is due to the phase transition. The shift in the resonance lines of (NH4)2Fe(SO4)2·6H2O might be due to the dipole–dipole interactions between the magnetic moments of the H+ nuclei and the magnetic moments of the Fe2+ atoms. The chemical shift near T d is the beginning of a decrease of H2O.
The spin–lattice relaxation time T 1ρ in the rotating frame for the protons as a function of temperature was studied. The nuclear magnetization recovery traces obtained for protons at all temperatures are described by the following single exponential function: M(t) = Moexp(−t/T 1ρ), where M(t) is the magnetization at time t, and Mo is the total nuclear magnetization of 1H at thermal equilibrium [16]. The slopes of the recovery trace are different at several temperatures; one of them is shown in the inset in Fig. 5. The temperature dependence of T 1ρ for the protons is shown in Fig. 5. The T 1ρ value of the protons in (NH4)2Fe(SO4)2·6H2O did not change significantly near T d, but it decreased considerably near the phase transition temperature of T C1, in addition to the change in the chemical shift at T C1.
The NMR spectrum for the 1H nuclei in a single (NH4)2Fe(SO4)2·6H2O crystal was measured at various temperatures. As mentioned above, there are two types of protons in (NH4)2Fe(SO4)2·6H2O: ammonium protons, the relaxation of which is determined mainly by the hindered rotation of the NH4 groups, and hydrogen bond protons, the relaxation of which is determined mainly by the motion of the hydrogens in H2O. In our results, the proton signals due to the ammonium and hydrogen bond protons overlap, as shown in the inset in Fig. 6, and the line width due to the ammonium and hydrogen bond protons is very broad (70 kHz) at 300 K. Therefore, we cannot distinguish the two types. As the temperature is increased, the intensity of the signal decreases, as shown in Fig. 6, and it becomes quite weak above T C1, which indicates that the protons play an important role in this phase transition. We conclude that the decrease in the intensity of the signal with temperature is related to the loss of H2O.
The 1H spin–lattice relaxation time T 1 in the laboratory frame was obtained for a single (NH4)2Fe(SO4)2·6H2O crystal at a frequency of 200 MHz. Here, the magnetic field was applied along the c-axis of the crystal. The saturation recovery traces of the magnetization of 1H at several different temperatures were measured. The measured magnetization recovery was found to be satisfactorily fitted with the single exponential function [M(∞) − M(t)]/M(∞) = exp(−Wt), where M(t) is the nuclear magnetization at time t, and W is the transition probability corresponding to Δm = ±1. The relaxation time is given by T 1 = 1/W [17]. The magnetization recovery at room temperature is shown in the inset in Fig. 7. The slopes of the recovery traces at each temperature are different. The variation in the proton dynamics of the hydrogen bond networks is associated with the phase transition. The 1H relaxation time was obtained in terms of W, and the spin–lattice relaxation time T 1 was found to have a very strong temperature dependence, as shown in Fig. 7. The change in the temperature dependence of T 1 near T C1 (=387 K) is related to the loss of H2O; the forms of the octahedra of water molecules surrounding Fe2+ are probably disrupted by the loss of H2O.
Conclusions
The thermodynamic properties of (NH4)2Fe(SO4)2·6H2O were investigated, and phase transitions were found at 387 and 500 K. This crystal loses H2O with increasing temperature. The first mass loss occurs near 330 K (T d), which is interpreted as the onset of partial thermal decomposition. The transformation anomalies at 387 K (=T C1) and 500 K (=T C2) are related to phase transitions from (NH4)2Fe(SO4)2·6H2O to (NH4)2Fe(SO4)2·2H2O, and from (NH4)2Fe(SO4)2·6H2O to (NH4)2Fe(SO4)2·H2O, respectively. From the 1H T 1ρ determined by MAS NMR and 1H T 1 obtained in a single-crystal NMR experiment, the changes in the temperature dependence of T 1ρ and T 1 near T C1 are associated with the structural phase transitions, which are due to the loss of H2O and indicate that the forms of the octahedra of water molecules surrounding Fe2+ might be disrupted. This transformation is due to proton hopping and the breaking of hydrogen bonds. Near T d, the relaxation time for the 1H nuclei slowly decreases. This temperature is related to the beginning of the loss of H2O, as observed in the TG results.
The abrupt change in T 1ρ and T 1 near T C1 is the only detectable result of the structural transformation. T 1 and T 1ρ are very short, on the order of milliseconds. The T 1ρ and T 1 values of crystals containing paramagnetic ions are shorter than those of pure crystals; the influence of the paramagnetic ions is predominant. The relaxation time is expected to be inversely proportional to the square of the magnetic moment of the paramagnetic ion. Therefore, the T 1ρ and T 1 values of materials containing Fe2+ ions are shorter than those of materials without paramagnetic ions. These short relaxation times indicate rapid energy transfer from the nuclear spin system to the surrounding environment.
References
Gronvold F, Meisingset KK. Thermodynamic properties and phase transitions of salt hydrates between 270 and 400 K I. NH4Al(SO4)2·12H2O, KAl(SO4)2·12H2O, Al2(SO4)3·17H2O, ZnSO4·7H2O, Na2SO4·10H2O, and Na2S2O3·5H2O. J Chem Therm. 1982;14:1083–98.
Lim AR, Lee JH. 23Na and 87Rb relaxation study of the structural phase transitions in the Tutton salts Na2Zn(SO4)2·6H2O and Rb2Zn(SO4)2·6H2O single crystals. Phys Status Solidi B. 2010;247:1242–6.
Jain VK, Venkateswarlu P. On the 57Fe Mossbauer spectra of FeTe and Fe2Te3. J Phys C. 1979;12:865–73.
Marinova D, Georgiev M, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. II. Infrared spectroscopic study of NH4 + and SO4 2− ions included in copper sulfates and selenates. J Mol Struct. 2009;938:179–84.
Marinova D, Georgiev M, Stoilova D. Vibrational behavior of matrix- isolated ions in Tutton compounds. I. Infrared spectroscopic study of NH4 + and SO4 2− ions included in magnesium sulfates and selenates. J Mol Struct. 2009;929:67–72.
Marinova D, Georgiev M, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. IV. Infrared spectroscopic study of NH4 + and SO4 2− ions included in nickel sulfates and selenates. Cryst Res Technol. 2010;45:637–42.
Georgiev M, Marinova D, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. III. Infrared spectroscopic study of NH4 + and SO4 2− ions included in cobalt sulfates and selenates. Vib Spectrosc. 2010;53:233–8.
Riley MJ, Hitchman MA, Mohammed AW. Interpretation of the temperature dependent g values of the Cu(H2O) 2+6 ion in several host lattices using a dynamic vibronic coupling model. J Chem Phys. 1987;87:3766–78.
Hoffmann SK, Goslar J, Hilczer W, Augustyniak MA, Marciniak M. Vibronic behavior and electron spin relaxation of Jahn–Teller complex Cu(H2O) 2+6 in (NH4)2Mg(SO4)2·6H2O single crystal. J Phys Chem A. 1998;102:1697–707.
Parthiban S, Anandalakshmi H, Senthilkumar S, Karthikeyan V, Mojumdar SC. Influence of Vo(II) doping on the thermal and optical properties of magnesium rubidium sulfate hexahydrate crystals. J Therm Anal Calorim. 2012;108:881–5.
Montgomery H, Lingafelter EC. The crystal structure of Tutton’s salts. III. Copper ammonium sulfate hexahydrate. Acta Crystallogr. 1966;20:659–62.
Heilmann I, Knudsen JM, Olsen NB, Buras B, Olsen JS. Studies of thermal decomposition of (NH4)2Fe(SO4)2·6H2O. Solid State Commun. 1974;15:1481–4.
Ganesh G, Ramadoss A, Kannan PS, SubbiahPandi A. Crystal growth, structural, thermal, and dielectric characterization of Tutton salt (NH4)2Fe(SO4)2·6H2O crystals. J Therm Anal Calorim. 2013;112:547–54.
Brown GM, Chidambaram R. The structure of copper ammonium sulfate hexahydrate from neutron diffraction data. Acta Crystallogr B. 1969;25:676–87.
Voight W, Goring S. Melting of Tutton’s salts studied by DSC. Thermochim Acta. 1994;237:13–26.
Lim AR, Jeong SY. Phase transition in triglycine sulfate crystals by 1H and 13C nuclear magnetic resonance in the rotating frame. J Mole Struct. 2013;1048:471–5.
Lim AR, Shin HK. 1H and 7Li nuclear magnetic resonance study of the superionic crystals K4LiH3(SO4)4 and (NH4)4LiH3(SO4)4. J Appl Phys. 2010;107:63513–8.
Acknowledgements
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012001763).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, W.Y., Lim, A.R. Thermodynamic properties and phase transitions of Tutton salt (NH4)2Fe(SO4)2·6H2O from MAS NMR and single-crystal NMR. J Therm Anal Calorim 116, 779–783 (2014). https://doi.org/10.1007/s10973-013-3566-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3566-5