Abstract
This study presents a new simple model for predicting activation energy of the thermolysis of various classes of energetic compounds. The new model can help to elucidate the cause of thermal stability and, therefore, shelf life of some energetic compounds. The methodology assumes that activation energy of an energetic compound with general formula C a H b N c O d can be expressed as a function of optimized elemental composition as well as the contribution of specific molecular structural parameters. The new correlation has the root mean square and the average deviations of 9.8 and 7.4 kJ mol−1, respectively, for 86 energetic compounds with different molecular structures. The proposed new method is also tested for 20 energetic compounds, which have complex molecular structures, e.g. 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowurtzitane, 2,4,6-tris(2,4,6-Trinitrophenyl)-1,3,5-triazine and 1-(2,4,6-Trinitrophenyl)-5,7-dinitrobenzotriazole.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energetic materials can be classified into three categories, i.e. propellants, explosives and pyrotechnics [1–4]. Propellants and pyrotechnics release their energy through relatively slow deflagration processes. Meanwhile, explosives can liberate their energy during fast detonation processes in microsecond timescale [5]. Energetic compounds can be divided into different classes such as nitramines, nitroparaffins, polynitro arenes, polynitro heteroarenes and nitrate compounds.
Quantum mechanical and empirical methods can help the chemists to improve systematic and scientific formulation of appropriate futuristic target molecules having enhanced performance as well as good thermal stability, impact and friction sensitivity. Due to the expenditure connected with the development and synthesis of a new energetic material, different theoretical approaches can be used to determine performance, sensitivity and physicochemical properties of energetic compounds before their synthesis [6–10].
High performance, low sensitivity and good shelf life are three important characteristics of an ideal energetic compound. Especially thermal stability of energetic materials is an important feature in their shelf life and safety aspects [11–13]. Low sensitivity and high thermal stability of energetic compounds are necessary to avoid undesirable decomposition or self-initiation during their handling, storing and the application themselves. Prediction of thermal stability on mentioned compounds is an important starting point to evaluate the stability.
For thermolysis of nitramines, it was found that the homolysis of the N–NO2 bond is a primary step of the secondary nitramines whereas the homolysis of primary nitramines is a bimolecular autoprotolytic reaction [14]. However, the longest N–N bonds are responsible for homolytic reactivity of nitramines, which may contribute strongly to the intermolecular potential in the crystal state. Moreover, some linear relationships have been introduced for selected classes of nitramines between their activation energies of decomposition with 15N NMR chemical shifts of nitrogen atoms of nitramino groups [15], heats of detonation or the electronic charges at nitrogen atoms of the nitramines [16, 17]. The electronic charges at nitrogen atoms of nitramines can be computed on the basis of the Muliken population analysis of the electron densities obtained by ab initio DFT B3LYP/6-31G** method [18]. The values of crystal lattice energies of nitramines do not generally differ from those of polynitroaromatics [19]. For evaluation of the results of non-isothermal differential thermal analysis, the activation energy as the slope in the Kissinger relationship [20] can be used.
The study of thermolysis of energetic compounds can be used for estimation of their thermal stability [21, 22]. The experimental data for thermal reactivity can be obtained by various methods of thermal analysis and gasometry or by a variety of methods based on thermal explosion [19, 20, 23–25]. For example, differential thermal analysis (DTA), differential scanning calorimetry (DSC) and thermogravimetric analysis methods are thermoanalytical methods that are used widely to examine the kinetic parameters of thermolysis of energetic materials. Also the Soviet Manometric Method (SMM) is the isothermal manometric method with a glass-compensating manometer of the Bourdon type to examine the kinetics of thermolysis of energetic materials in vacuum. The data obtained by this method are the basic data on the Arrhenius parameters of non-autocatalyzed thermal decomposition of energetic materials. Moreover, if a relationship such as calibration curve exists between the results of DTA and DSC with the results of SMM, the results of DTA and DSC can be converted to SMM data [26–31]. There is no uniform classification of a large majority of results obtained in various laboratories all over the world because there is a discrepancy in principles and physical conditions of kinetics measurements in the thermolysis of energetic materials.
Zeman [32] have also used the modified Evans–Polanyi–Semenov (E–P–S) equation in the study of the chemical micromechanism governing the initiation of detonation of energetic materials. The original E–P–S equation [33, 34] describes a relationship between the activation energy, E, of the most substitution reactions and corresponding heats of reaction, ∆H. Zeman [32] has substituted ∆H by heat of explosion and E by E a of the low temperature thermal decomposition, which can lead to the modified E–P–S equation for energetic materials.
Some new correlations have been developed to estimate activation energy of the thermolysis of different classes of energetic compounds separately [35–37], but they cannot be used for variety energetic compounds with different molecular structure. The purpose of this work is to improve a new general correlation for prediction of activation energy of thermolysis of organic energetic materials with general molecular formula CaHbNcOd. How elemental composition and several structural parameters can be used to derive a novel correlation will be shown. When decision is to design new energetic materials, it is necessary to consider contribution of non-additive structural parameters in addition to elemental composition.
Materials and methods
More accurate and reliable data source on the activation energies of the non-autocatalyzed thermal decomposition in the condensed state of energetic compounds is on the basis of SMM [26–31]. Since disagreement in principles and physical conditions prevents a uniform classification of a large majority of results obtained in various laboratories, the existing literature also lacks a more complete survey of the published activation energy of the monomolecular non-autocatalyzed thermal decompositions. Table 1 shows experimental data of various classes of energetic compounds contacting energetic groups –NNO2, –ONO2, –CNO2 or –NNO groups, which can act as active sites for thermal decomposition of energetic compounds. Most data of activation energy of energetic compounds in literature are based on SMM because it can minimize the effects of consecutive reactions of intermediates and products of thermal decomposition both with each other and with the starting energetic material.
Due to the presence of various factors affecting the experimental data of activation energies for different classes of energetic compounds [26], at first it seems difficult to obtain a general correlation to predict E a for these compounds. Fortunately, the study of the activation energies for thermal decomposition of various explosives has shown that it is possible to derive a general novel correlation for predicting activation energy of energetic materials.
The results show that the important factors can be divided into additive and non-additive structural parameters. Thus, the following equation can be used as a suitable correlation:
where E a is activation energy in kJ mol−1; the parameters \( n_{\text{C}} \) and \( n_{\text{OH}} \) represent the number of carbon atoms and hydroxyl groups, respectively; the functions \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \) show increasing and decreasing contribution of non-additive structural parameters, respectively. The existence of some molecular moieties in form \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \) can correct the predicted results on the basis of additive part.
Experimental data from different sources given in Table 1 were used to derive Eq. (1) through multiple linear regression method [38]. Deviations of experimental data from the estimated results on the basis of additive part have been used to find non-additive structural parameters. Since the existence of a variety of different factors influences activation energy, large deviations exist between experimental data for some energetic compounds. Fortunately, R 2 value or the coefficient of determination of Eq. (1) is 0.92. Structural moieties affecting \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \)are described in next sections.
Results and discussion
Prediction of \( E_{\text{non-add}}^{ + } \)
The presence of some structural parameters can increase activation energy because they may enhance thermal stability of energetic compound.
-
(a)
Cyclic nitramines This condition can be applied for cyclic nitramines belonging to one of the following classes:
-
(i)
more than six-membered ring and two –NNO2 groups;
-
(ii)
more than four-membered ring with only one −NNO2 group;
-
(iii)
bicyclic ring with only two –NNO2 groups per cycle. The value of \( E_{\text{non-add}}^{ + } \) is 1.0 for these compounds. For cyclic nitramines, this condition is consistent with correlation of activation energy in which their activation energies increase with the size of ring [36].
-
(i)
-
(b)
Nitroalkanes For nitroalkanes with molecular moieties R–CH2NO2 and R–C(NO2)2–R′, the values of \( E_{\text{non-add}}^{ + } \) are 2.0 and 1.0, respectively.
-
(c)
Niroaromatics Two different situations are considered here: (i) For the presence of amino pyridine derivative,
 , triazine ring or four adjacent nitrogens in nitroaromatics, the value of \( E_{\text{non-add}}^{ + } \) is equal to 1.0. (ii) The value of \( E_{\text{non-add}}^{ + } \) equals 2.0 for the existence of molecular fragment
, triazine ring or four adjacent nitrogens in nitroaromatics, the value of \( E_{\text{non-add}}^{ + } \) is equal to 1.0. (ii) The value of \( E_{\text{non-add}}^{ + } \) equals 2.0 for the existence of molecular fragment  , where TNP is 2,46-trinitrophenyl.
, where TNP is 2,46-trinitrophenyl.
Estimation of \( E_{\text{non-add}}^{ - } \)
-
(a)
Acyclic nitramines and cyclic nitramines including small ring For those acyclic nitramines with only one –NNO2 group in form Ar(or H)–N(NO2)CH3, the value of \( E_{\text{non-add}}^{ - } \) is equal to 0.75. The value of \( E_{\text{non-add}}^{ - } \) equals 0.4 for cyclic nitramines containing less than five membered ring or five membered ring with more than one N–NO2.
-
(b)
Niroaromatics Several types of molecular fragments can be considered in this category:
-
(i)
For the presence of molecular moieties –O(or S)–R(or Ar), the values of \( E_{\text{non-add}}^{ - } \) equal 1.5;
-
(ii)
For the existence of molecular fragment
 , the value of \( E_{\text{non-add}}^{ - } \) is equal to the number of this molecular moiety;
, the value of \( E_{\text{non-add}}^{ - } \) is equal to the number of this molecular moiety; -
(iii)
The values of \( E_{non-add}^{ - } \) are 1.5, 1.0 and 0.75 for the compound TNP–X where X are –Cl, –NH– and –N<, respectively;
-
(iv)
For energetic compounds with general formula TNP–Y–TNP where Y are –N=N–, –CH2–CH2– and –SO2–, the value of \( E_{\text{non-add}}^{ - } \) equals 2.0.
-
(i)
-
(c)
The presence of nitrate group For the existence of -ONO2 group, the value of \( E_{\text{non-add}}^{ - } \) equals 0.3.
-
(d)
The existence of nitroso group The value of \( E_{\text{non-add}}^{ - } \) is 0.75.
Reliability of the predicted results and the effects of non-additive structural parameters
As seen in Table 1, predicted activation energies for different types of energetic compounds have a root mean square (rms) and the average absolute deviations of 9.8 and 7.4 kJ mol−1, respectively. Moreover, the estimated activation energy by new correlation is more than 20 kJ mol−1 of the reported values only for two energetic compounds. As seen in Table 1, the deviations for experimental values are too high, e.g. compound numbers 24, 50 and 80. However, the best optimized values were derived for \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \) using multiple linear regression method [38].
Table 2 indicates statistical parameters of Eq. (1), which allows comparing the relative weight of the variables in the model. Standard error shows a measure of the precision of the estimation of a coefficient. It can determine precision over repeated measurements. The P value can assess the significance of an observed effect or variation. For P value less than 0.05, it may confirm that the observed effect is not due to random variations and the effect is significant. Thus, suitable statistical parameters and good R 2 value, i.e. 0.92, validate that the predicted results of new method are in good agreement with experimental values.
The predicted activation energies for some complex energetic compounds, e.g. 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowurtzitane (TEX), 2,4,6-tris(2,4,6-Trinitrophenyl)-1,3,5-triazine (TPT) and 1-(2,4,6-rinitrophenyl)-5,7-dinitrobenzotriazole (BTX), are given also in Table 3. As seen in Table 3, the new method gives relatively good results, which confirm the reliability of new method. Although experimental data were obtained from various methods such as DSC, SMM, TGA, DTA, NMR, IR and manometry, the predicted results in both Tables 1 and 3 are consistent with various experimental methods especially SMM method. Introduction of an amino group into a benzene ring containing a nitro group can enhance the thermal stability of explosives. Among various nitro derivatives of benzene, DATB and TATB have qualified as heat resistant explosives [1]. The predicted activation energy for both DATB and TATB is close to corresponding experimental values and higher than PAM. Due to the existence of large deviations for experimental values of some thermally stable explosives such as NONA, the predicted values are satisfactory. Tables 1 and 3 contain some well-known heat resistant polynitro arenes such as N,N-bis(2,4-dinitrophenyl)-2,4,6-trinitroaniline (NTFA), 3,3′-diamino-2,2′,4,4′,6,6′-hexanitrobiphenyl (DIPAM) and 2,2′,2″,4,4′,4″,6,6′,6″-nonanitro-m-terphenyl (NONA), which are highly thermostable and technologically exploited explosives. They have wide applications such as downhole well, achieving stage separation in space rockets, seismic experiments on the moon and booster explosives in space technology [35].
Liquid and solid phases are the state of thermal decomposition of energetic compounds. The influence of the condensed phase is expected to make itself felt in the reactivity through the changed character of bond hybridization of the molecule because of the presence of intermolecular interactions [32]. For the formation of the activated complex, the hinder influence of the closely arranged molecules within the crystal should be considered in solid phase decompositions [32]. Although the respective primary nitramines thermolysis is considered to have an autoprotolytic bimolecular course, the new method can be easily used for both primary and secondary nitramines.
Homolysis of the N–NO2 bond as the primary fragmentation in thermolysis of the secondary amines in the condensed phase may be important for \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \) under certain mentioned conditions. The presence of –NNO2 in cyclic nitramine containing small ring provides a suitable path for decomposition of energetic compound with respect to –CNO2. For TNAZ and its derivatives, it was found that the N–NO2 fission is the convenient pathway in the kinetics and thermodynamic properties of their decomposition [39], which is consistent with \( E_{\text{non-add}}^{ - } \).
The present method can be programmed for designing a new energetic compound on the basis of \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \) [40, 41]. Quantum mechanical methods have been also used to study thermal decomposition of some energetic compounds [42, 43]. In contrast to quantum mechanical methods, there is no need to use high speed computer and special computer codes. For some classes of energetic compounds, it is possible to correlate activation energy and the other related decomposition properties, e.g. the onset temperatures of nitro aromatic compounds [44].
Conclusions
A novel reliable simple correlation has been introduced to predict activation energy of energetic compounds containing –NNO2, –ONO2, –CNO2 or –NNO groups. It is based on the number of carbon atoms and hydroxyl groups as well as two non-additive functions \( E_{\text{non-add}}^{ + } \) and \( E_{\text{non-add}}^{ - } \). As shown in Tables 1 and 3, the predictions for 106 (corresponding to 131 measured values) molecules provide reliable results with respect to experimental data. Since different factors can influence activation energy of an energetic compound, the main intent in this work was to investigate the likelihood of a generalized method to evaluate it. There is no need to use any experimental data or computed values in the new model. Equation (1) shows that it is possible to predict activation energies of new energetic compounds for which no data exist.
References
Agrawal JP. Recent trends in high energy materials. Prog Energy Combust Sci. 1998;24:1–30.
Agrawal JP, Hodgson R. Organic chemistry of explosives. England: Wiley; 2007.
Agrawal JP. High energy materials: propellants, explosives and pyrotechnics. Weinheim: Wiley; 2010.
Klapötke TM. Chemistry of high-energy materials. Berlin: Walter De Gruyter; 2011.
Akhavan J. The chemistry of explosives. Cambridge: The Royal Society of Chemistry; 1998.
Politzer P, Murray JS, editors. Energetic materials. Part 2: detonation, combustion. Amesterdam: Elsevier; 2003.
Sikder AK, Maddala G, Agrawal JP, Singh H. Important aspects of behaviour of organic energetic compounds: a review. J Hazard Mater A. 2001;84(1):1–26.
Keshavarz MH. A simple theoretical prediction of detonation velocities of nonideal explosives only from elemental composition (Chapter 9). In: Warey PB, editor. New research on hazardous materials. New York: Nova Science Publishers; 2007. p. 293–310.
Keshavarz MH. Important aspects of sensitivity of energetic compounds: a simple novel approach to predict electric spark sensitivity (Chapter 4). In: Janssen TJ, editor. Explosive materials: classification, composition and properties. New York: Nova Science Publishers; 2011. p. 179–201.
Keshavarz MH. Research progress on heats of formation and detonation of energetic compounds (Chapter 10). In: Brar SK, editor. Hazardous materials: types, risks, and control. NewYork: Nova Science Publishers; 2011. p. 339–59.
Krabbendam-LaHaye ELM, de Klerk WPC, Krämer RE. The kinetic behavior and thermal stability of commercially available explosives. J Thermal Anal Calorim. 2005;80:495–501.
Bunyan P, Baker C, Turner N. Application of heat conduction calorimetry to high explosives. Thermochim Acta. 2003;401:9–16.
Keshavarz MH, Moradi S, Ebrahimi Saatluo B, Rahimi H, Madram A. A simple accurate model for prediction of deflagration temperature of energetic compounds. J Thermal Anal Calorim. 2012;. doi:10.1007/s10973-012-2717-4.
Zeman S. Analysis and prediction of the arrhenius parameters of low temperature thermolysis of nitramines by means of the 15N NMR spectroscopy. Thermochim Acta. 1999;333:121–9.
Zeman S. New aspects of initiation reactivities of energetic materials demonstrated on nitramines. J Hazard Mater A. 2006;132:155–64.
Zeman S, Friedl Z. Relationship between electronic charges at nitrogen atoms of nitro groups and thermal reactivity of nitramines. J Thermal Anal Calorim. 2004;77(1):217–22.
Sorescu DC, Rice BM, Thompson DL. Molecular packing and molecular dynamics study of the transferability of a generalized nitramine intermolecular potential to non-nitramine crystals. J Phys Chem A. 1999;103(8):989–98.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Lee JS, Hsu CK, Chang CL. A study on the thermal decomposition behaviors of PETN, RDX, HNS and HMX. Thermochim Acta. 2002;392–393:173–6.
Zhao-Xu C, Heming X. Impact sensitivity and activation energy of pyrolysis for tetrazole compounds. Int J Quantum Chem. 2000;79:350–7.
Pourmortazavi SM, Rahimi-Nasrabadi M, Kohsari I, Hajimirsadeghi SS. Non-isothermal kinetic studies on thermal decomposition of energetic materials. J Thermal Anal Calorim. 2012;110(2):857–63.
Cusu JP, Musuc AM, Matache M, Oancea D. Kinetics of exothermal decomposition of some ketone-2,4-dinitrophenylhydrazones. J Thermal Anal Calorim. 2012;110(3):1259–66.
Sinditskii VP, Egorshev VY. Thermal decomposition of NTO: an explanation of the high activation energy. Propellant Explos Pyrot. 2007;32(4):277–87.
Oxley JC, Smith JL, Ye H. Thermal stability studies on a homologous series of nitroarenes. J Phys Chem. 1995;99:9593–602.
Oxley JC, Kooh AB, Szekeres R, Zheng W. Mechanisms of nitramine thermolysis. J Phys Chem. 1994;98:7004–8.
Zeman S, Dimun M, Truchlik Š. The relationship between kinetic data of the low-temperature thermolysis and the heats of explosion of organic polynitro compounds. Thermochim Acta. 1984;78:181–209.
Zeman S. Thermal stabilities of polynitroaromatic compounds and their derivatives. Thermochim Acta. 1979;31:269–83.
Zeman S. Non-isothermal differential thermal analysis in the study of the initial state of the thermal decomposition of polynitroaromatic compounds in the condensed state. Thermochim Acta. 1980;39:117–24.
Zeman S. The thermoanalytical study of some amino derivatives of 1,3,5-trinitrobenzene. Thermochim Acta. 1993;216:157–68.
Zeman S. Relationship between the Arrhenius parameters of the low temperature thermolysis and the 13C and 15N chemical shifts of nitramines. Thermochim Acta. 1992;202:191–200.
Zeman S. Kinetic compensation effect and thermolysis mechanisms of organic polynitroso and polynitro compounds. Thermochim Acta. 1997;290:199–217.
Zeman S. Modified Evans–Polanyi–Semenov relationship in the study of chemical micromechanism governing detonation initiation of individual energetic materials. Thermochim Acta. 2002;384:137–54.
Semenov NN. O nekotorykh problemakh khimicheskoy kinetiki i reaksionnoy sposobnosti (some problems of chemical kinetics and of reaction capability). Moscow: USSR Academy of Sciences; 1958. p. 41–101.
Zavitas AA. Chemtech. Washington: ACS; 1972. p. 434.
Keshavarz MH, Pouretedal HR, Shokrolahi A, Zali A, Semnani A. Predicting activation energy of thermolysis of polynitroarenes through molecular structure. J Hazard Mater. 2008;160(1):142–7.
Keshavarz MH. Simple method for prediction of activation energies of the thermal decomposition of nitramines. J Hazard Mater. 2009;162(2–3):1557–62.
Keshavarz MH. A new method to predict activation energies of nitroparaffins. Indian J Eng Mater S. 2009;16(6):429–32.
Palm WJ III. Introduction to matlab for engineers. New York: McGraw-Hil; 2005. p. 4–328.
Oftadeh M, Hamadanian Khozani M, Radhoosh M, Keshavarz MH. DFT molecular orbital calculations of initial step in decomposition pathways of TNAZ and some of its derivatives with –F, –CN and –OCH3 groups. Comput Theor Chem. 2011;964(1–3):262–8.
Keshavarz MH, Motamedoshariati H, Moghayadnia R, Ghanbarzadeh M, Nazari HR, Azarniamehraban J. A new computer code to evaluate detonation performance of high explosives and their thermochemical properties, part I. J Hazard Mater. 2009;172(2–3):1218–28.
Keshavarz MH, Motamedoshariati H, Moghayadnia R, Ghanbarzadeh M, Azarniamehraban J. A new computer code for assessment of energetic materials with crystal density, condensed phase enthalpy of formation, and activation energy of thermolysis. Propellant Explos Pyrot. 2012;. doi:10.1002/prep.201100156.
Yan QL, Zeman S. Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods: a brief review. Int J Quantum Chem. 2012;. doi:10.1002/qua.24209.
Yan QL, Zeman S, Šelešovský J, Svoboda R, Elbeih A, Málek J. The effect of crystal structure on the thermal reactivity of CL-20 and its C4-bonded explosives. J Thermal Anal Calorim. 2012;. doi:10.1007/s10973-012-2629-3.
Wang Q, Wang J, Larranaga MD. Simple relationship for predicting onset temperatures of nitro compounds in thermal explosions. J Thermal Anal Calorim. 2012;. doi:10.1007/s10973-012-2377-4.
Shanko VN, Stepanov RS. Fizicheskaya Khimia (physical chemistry). 1974; 1, Krasnoyarsk, 190.
Dubovitskii FI, Korsoonskii BL. Kinetika termicheskogo razlozheniya Nnitrosoedinenii (Kinetics of thermal decomposition of N-nitrocompounds). Usp Khim. 1981;50:1828–34.
Sitonina GV, Korsoonskii BL, Pyatakov NF, Shvayko VG, Abdrakhmanov ISh, Dubovitskii FI Izv. Akad Nauk SSSR, Ser Khim 1979; 311.
Robertson AJB. The thermal decomposition of explosives. Part II: cyclotrimethylenetrinitramine and cyclotetramethylenetetranitramine. Trans Faraday Soc. 1949;45:85–92.
Rogers RN. Differential scanning calorimetric determination of kinetics constants of systems that melt with decomposition. Thermochim Acta. 1972;3:437–47.
Brill TB, Karpowicz RJ. Solid phase transition kinetics: the role of intermolecular forces in the condensed-phase decomposition of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine. J Phys Chem. 1982;86:4260–5.
Rongzu H, Zhengouan Y, Yanjun L. The determination of the most probable mechanism function and three kinetic parameters of exothermic decomposition reaction of energetic materials by a. Thermochim Acta. 1988;123:135–51.
Lbbecke S, Bohn MA, Pfeil A, Krause H. Proceedings of the 29th International Annual Conference, ICT, Karlsruhe, 1998;145/1.
Zeman S, Friedl Z, Roháč M. Molecular structure aspects of initiation of some highly thermostable polynitro arenes. Thermochim Acta. 2006;451:105–14.
Nazin GM, Manelis GB. lzv Akad Nauk SSSR. Ser Khim. 1972;811.
Nazin GM, Manelis GB, Dubovitskii FI. Thermal decomposition of aliphatic nitro-compounds. Russ Chem Rev. 1968;37(8):603–12.
Nazin GM, Manelis GB, Dubovitskii FI. lzv Akad Nauk SSSR. Ser Khim, 1968; 389.
Acknowledgements
We would like to thank the research committee of Iran University of Science and Technology for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keshavarz, M.H., Zohari, N. & Seyedsadjadi, S.A. Validation of improved simple method for prediction of activation energy of the thermal decomposition of energetic compounds. J Therm Anal Calorim 114, 497–510 (2013). https://doi.org/10.1007/s10973-013-3022-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3022-6


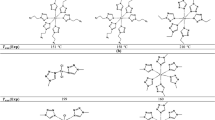
 , triazine ring or four adjacent nitrogens in nitroaromatics, the value of
, triazine ring or four adjacent nitrogens in nitroaromatics, the value of  , where TNP is 2,46-trinitrophenyl.
, where TNP is 2,46-trinitrophenyl. , the value of
, the value of