Abstract
In the present work, the possibilities of application of the thermomagnetometry TG(M)/DTG(M) method for estimation of the phase homogeneity of end products of synthesis at different stages of ferritizing annealing are considered on the example of solid-state synthesis of Li0.5(1−x)Fe2.5−0.5xZnxO4 and Li0.5(1 + x)Fe2.5−1.5xTixO4 (x = 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6) lithium-substituted ferrospinels. Results of thermomagnetometric analysis are compared with XRD data. It is shown that the resolution of the TG(M)/DTG(M) method on two orders exceeds capabilities of the traditional XRD method. Considerable influence of both intermediate grinding and mixing of samples and concentration of an inducted impurity on the homogeneity of the synthesized powders is confirmed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An important stage of multicomponent ferrite synthesis is the preparation of initial powders with a maximum degree of homogenization in the stage of their synthesis. Among the simplest and most technological approaches used to increase the degree of homogeneity of powders is the inclusion of intermediate grinding and mixing operations into the scheme of synthesizing annealing. Careful control of the phase composition of powders is required in all annealing stages to optimize the temperature and time regimes and multiplicity of grinding for this technological scheme.
The method of X-ray powder diffraction (XRD) is traditional for investigations of the phase composition of materials. However, with application to lithium-substituted ferrospinels, the XRD quantitative data and their reliability call for additional verification since it is difficult to separate different spinel phases that can be formed by this method in Li2CO3–TiO2–Fe2O3 and Li2CO3–ZnO–Fe2O3 systems [1]. For a large set of Li0.5(1−x)Fe2.5−0.5xZnxO4 and Li0.5(1 + x)Fe2.5−1.5xTixO4 intermediate phases with identical types of the crystal lattices and close values of the lattice parameters, reflections from planes of these phases in the registered X-ray diffraction patterns merge into a single reflection. In the absence of splitting discrimination parameters, it is almost impossible to separate these reflections. For this reason, the thermomagnetometry TG(M)/DTG(M) method, which represents the method of thermogravimetric analysis in the magnetic field applied to the sample [2], can be used to analyze phase transformations in the synthesis of lithium-substituted ferrites. This method is used to determine the Curie temperature in iron-containing systems, such as in [3, 4]. A similar approach was used in the study of lithium ferrite LiFe5O8 [5] and definite composition lithium–zinc ferrite [6–8] radiation-thermal solid-state synthesis in an accelerated electron beam.
To prove the possibility of application of the thermomagnetometry method for estimation of ferrite material phase homogeneity in the course of their synthesis, it is necessary to conduct similar research for ferrites with wide compositions. Thus, in the present work, the phase homogeneity of Li0.5(1−x)Fe2.5−0.5xZnxO4 and Li0.5(1 + x)Fe2.5−1.5xTixO4 lithium-substituted solid-state synthesis products for all values x at synthesis for various stages was studied. Results of thermomagnetometric analysis were supplemented with the XRD data.
Experimental
The lithium-substituted ferrospinels were prepared by the solid-state method in accordance with the following reactions:
where x Zn, Ti = 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6.
Commercial Fe2O3, Li2CO3, ZnO, and TiO2 powders preliminary dried and mixed in an agate mortar served as initial reagents for sample synthesis. Then, samples were compacted by single-ended cold pressing under a pressure of 200 MPa in the form of pellets 15 mm in diameter and 2 mm in thickness.
The solid-state synthesis of the samples, including intermediate grinding and mixing operations was performed in air in a resistance furnace at a temperature of 800 °C. The grinding and mixing operations were performed every 120 min during isothermal annealing, after which the samples were subject to the XRD and TG(M)/DTG(M) analyses.
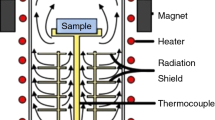
The thermomagnetometric TG(M)/DTG(M) and calorimetric DSC analyses were performed in air atmosphere using thermal analyzer STA 449C Jupiter (Netzsch, Germany). To control the magnetic properties of the samples, an assembly of two permanent magnets creating a field of ~5 Oe was attached on the outer side of the measuring cell. Measurements were performed in linear heating regime at a heating rate of 50 °C min−1.
The XRD analysis was carried out using an X-ray diffractometer ARL X’TRA with a semiconductor Peltier Si(Li) detector and Cu Kα radiation. XRD diffraction patterns were measured in the range of 2θ = (10–140)° with a scanning rate of 0.02° s−1. Phases were identified by the PDF-4 powder database of the International Center for Diffraction data (ICDD). The XRD patterns were processed by the full-profile analysis by means of Powder Cell 2.5 software.
Results and discussion
To study the character of response of DTG(M) dependences to the homogeneity of the phase composition, two batches of samples of each composition were prepared by annealing at 800 °C. Samples A, after compaction of the initial reactants’ mixture, were annealed for 120 min and samples B were annealed for 360 min with inclusion of intermediate powder grinding and mixing operations every 120 min.
Figures 1 and 2 show a fragments of X-ray diffraction patterns for both zinc- (Fig. 1) and titanium- (Fig. 2) substituted ferrites with x = 0.2, 0.4, and 0.6. All observable reflections belong to the spinel phase. Superstructural (110), (210), and (211) reflections that testify to the presence of the α-LiFe5O8 phases are observed in the small-angle region of X-diffraction patterns (Fig. 2a).
An increase in the duration of ferrospinel synthesis leads to a noticeable decrease in the (311) reflection half width (Figs. 1, 2 for sample B). In this case, the intensities of weak (110), (210), and (211) superstructural reflections also decrease and completely vanish for zinc-substituted ferrites. The foregoing results indicate the high level of homogeneity of lithium–zinc ferrites for the given synthesis conditions. An increase in the duration of synthesis at a temperature of 800 °C and in the grinding and mixing multiplicities does not lead to the formation of a single-phase end product of lithium–titanium ferrite. This conclusion is in agreement with the above-indicated results of thermomagnetometric analysis of the samples.
In this work, it was established that with increasing concentration of the incorporated impurity, the reflection widths increase, and with increasing duration of annealing (and mixing multiplicity), they decrease. These patterns were typical for all examined structures.
Figures 3 and 4 show the results of thermomagnetometry and differential scanning calorimetry for Li0.4Fe2.4Zn0.2O4 (Fig. 3a), Li0.3Fe2.3Zn0.4O4 (Fig. 3b), and Li0.2Fe2.2Zn0.6O4 (Fig. 3c) samples and Li0.6Fe2.2Ti0.2O4 (Fig. 4a), Li0.7Fe1.9Ti0.4O4 (Fig. 4b), and Li0.8Fe1.6Ti0.6O4 (Fig. 4c) samples synthesized in different conditions. The results obtained for samples with other compositions were analogous.
The TG(M)/DTG(M) and DSC analyses of samples examined in this work allowed us to observe changes caused by the formation of magnetic phases in all stages of synthesis. The general behavior of TG(M) curves demonstrates the presence of mass jumps at temperatures of ferrimagnet-paramagnet transitions for every magnetic phase presented in the sample. The DTG(M) dependences, being derivatives of the TG(M) curves, allowed us to determine more exactly the temperatures of these transitions and hence to characterize more correctly the phase composition of the sample. The number of peaks on the DTG(M) curve characterizes the number of magnetic phases dominating in the sample. The DSC curves represent the dependences with a weakly pronounced magnetocaloric effect.
For samples A of lithium–zinc ferrites and samples A, B of lithium–titanium ferrites of all compositions, weak endothermic peaks, the area of which decreases with increasing x Zn or x Ti, are seen on the DSC curve at ~750 °C (Figs. 3, 4). They are caused by the order–disorder α → β transition in the LiFe5O8 phase [9]. The presence of the LiFe5O8 phase in the samples was also confirmed by the mass jumps on TG(M) curves at a temperature of ~630 °C, corresponding to the magnetic phase transition at the Curie point for LiFe5O8 [10].
Only for sample B of both lithium–zinc and lithium–titanium ferrites, a wide DTG(M) peak caused by the formation of magnetic lithium-substituted phases is observed. Positions of maxima of DTG(M) peaks for lithium-substituted phases depend on the zinc or titanium concentration in the initial mixture and are naturally displaced toward low temperatures with increasing x Zn or x Ti.
The presence of the LiFe5O8 phase and the observed blurring of the magnetic transition testified not only to the extremely low degree of homogeneity of the material, but also to the incomplete synthesis reaction itself. In this case, the phase homogeneity of ferrite materials decreases with increasing concentration of incorporated impurity for the same conditions of synthesis. An increase in the duration of synthesis and inclusion of intermediate powder grinding and mixing operations decrease the width of the magnetic transition in the lithium-substituted phases and the content of the LiFe5O8 phase down to its vanishing. As follows from the data obtained in this work, the high degree of homogeneity of lithium–titanium ferrite can be obtained only by increasing the temperature of the sample synthesis to 1,000 °C with inclusion of intermediate grinding and mixing operations. Thus, in samples of both ferrites prepared for long synthesis times, there is one sharp DTG(M) peak with a maximum at the Curie temperature that testifies to the high degree of homogeneity of synthesized ferrites with the above-indicated compositions.
Figure 5 shows the DTG(M) curves for Li0.5(1−x)Fe2.5−0.5xZnxO4 (Fig. 5a) and Li0.5(1 + x)Fe2.5−1.5xTixO4 samples (Fig. 5b) prepared in the synthesis conditions corresponding to obtaining the end product with a maximum content of the single phase. The DTG(M) curves comprise individual sharp peaks. With increasing zinc or titanium content, the peak intensities decreased, and the positions of maxima are displaced toward lower temperatures.
As is well known, titanium and zinc ions in lithium-substituted ferrospinels occupy octahedral and tetrahedral positions in the cation sublattice, respectively [11]. Therefore, the decrease of DTG(M) peak intensities is the result of the diamagnetic substitution of iron cations. According to the Néel theory, this causes the magnetization of domains in ferrimagnets to decrease [12]. The displacement of peaks toward lower temperatures is also caused by the diamagnetic substitution that weakens the indirect exchange interaction of magnetic cations.
The position of maxima of every DTG(M) curve corresponds to the Curie point of the composition with x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6. To determine the phase composition from the DTG(M) data, the positions of their temperature maxima must be related to the parameter x characterizing the titanium or zinc content in the corresponding lithium-substituted ferrospinels.
In the insert of Fig. 5a, b, the concentration dependence of the Curie temperature decreasing linearly (which is typical of alloyed ferrospinels [12]) is shown. The given dependence can serve as a calibration curve for identification of the phase composition of ferritized mixtures. The x values of dominating phases can be determined from the positions of the DTG(M) peak maxima, and the relationships of phase concentrations can be judged from those of the peak intensities.
An approximation of the dependences of heights I of DTG(M) peaks and of their halfwidths U on the molar content x yields the following expressions:
For 0 ≤ x ≤ 0.6, the product I·U is constant for lithium–zinc and lithium–titanium ferrospinels and is 6.86 and 5.94, respectively. The constancy of the product demonstrates that broadening of the magnetic transition depends weakly on the structural deficiency of the material and is mainly determined by its magnetization (that is, the DTG(M) peak intensity). Thus, in the examined range of zinc and titanium concentrations, the peak widths are related to their intensities by the expression U = 6.86 I −1 for lithium–zinc and U = 5.94 I −1 for lithium–titanium ferrites.
To elucidate the resolution of the DTG(M) method, a comparison with the XRD data on the ferrospinel crystal lattice parameter was performed. Thus, the lattice parameters, for example, for Li0.5(1 + x)Fe2.5−1.5xTixO4 at x = 0 and 0.6 were 8.330 and 8.348 Å, respectively. For the same x values, the DTG(M) peaks were observed at 630 and 300 °C. Thus, the relative change of the lattice parameter was ~0.2 %, whereas the relative displacement of the DTG(M) temperature peak was 52 %.
Analogous estimates for lithium–zinc spinel at x = 0 and 0.6 were the following. The relative change of the lattice parameter was ~1 % (8.330 and 8.410 Å), whereas the DTG(M) peak was displaced from 630 to 220 °C, which made ~65 %.
Hence, the resolution of the thermomagnetometric method for lithium-substituted ferrospinels is by more than two and a half orders of magnitude higher than that of the XRD method. However, because of the strong decrease in magnetization of phases with increasing x, the sensitivity of the DTG(M) method at x ~ 0.6 decreased approximately by an order of magnitude. This disadvantage can be eliminated by increasing the external magnetic field strength, if necessary. However, since the concentration of the impurity incorporated into microwave ferrites of the lithium group, as a rule, does not exceed x = 0.4 [1], it is not necessary to change the thermomagnetometry conditions in the synthesis of lithium-substituted ferrites.
Conclusions
Based on the results obtained in this work, we can conclude the following:
-
In the example of solid-state synthesis of lithium-substituted ferrospinels, the high resolution of the thermomagnetometry method of estimating the degree of phase homogeneity of products of synthesis in various stages of ferritizing annealing has been demonstrated.
-
The process of synthesis of lithium-substituted ferrites goes through the formation of intermediate Li0.5(1−x)Fe2.5−0.5xZnxO4 and Li0.5(1 + x)Fe2.5−1.5xTixO4 spinel phases, where 0 ≤ x ≤ 0.6. The end product is formed as a result of diffusion interaction of phases.
-
For Li0.5(1−x)Fe2.5−0.5xZnxO4 and Li0.5(1 + x)Fe2.5−1.5xTixO4 (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6) ferrites, the magnetic phase transitions at the Curie point were classified by the DTG(M) analysis. These data provided the basis for estimation of the degree of phase homogeneity of lithium-substituted ferrites.
-
As a whole, the degree of homogeneity of the end product of lithium–titanium ferrospinel synthesis at the same temperatures and annealing durations was lower than of lithium–zinc ferrites. The degree of homogeneity of the synthesized ferrite powders also decreased with an increasing concentration of the alloying element.
-
The suggested method of analysis of the degree of lithium-substituted ferrospinel phase homogeneity by means of TG(M)/DTG(M) is applicable to all ferrospinels that form magnetic solid solutions.
References
Levin BE, Tret’yakov YD, Letyuk LM. Physicochemical principles of preparation, properties, and applications of ferrites. Moscow: Metallurgiya; 1979. p. 471 (in Russian).
Gallagher PK. Thermomagnetometry. J Therm Anal Calorim. 1997;49:33–44.
Suńol JJ, González A, Escoda L, Vilaró A. Curie temperature in Fe(Ni)Nb based mechanically alloyed materials. J Therm Anal Calorim. 2005;80:257–61.
Barbosa GF, Machado FLA, Rodrigues AR, Azevedo WM. On the influence of the milling time in the Curie temperature of Fe56Co7Ni7Zr10B20 investigated by magnetic thermogravimetric analysis. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1791-3.
Surzhikov AP, Pritulov AM, Lysenko EN, Sokolovskiy AN, Vlasov VA, Vasendina EA. Calorimetric investigation of radiation-thermal synthesised lithium pentaferrite. J Therm Anal Calorim. 2010;101:11–3.
Surzhikov AP, Lysenko EN, Vasendina EA, Sokolovskii AN, Vlasov VA, Pritulov AM. Thermogravimetric investigation of the effect of annealing conditions on the soft ferrite phase homogeneity. J Therm Anal Calorim. 2011;104:613–7.
Surzhikov AP, Pritulov AM, Lysenko EN, Sokolovsky AN, Vlasov VA, Vasendina EA. Dependence of lithium–zinc ferrospinel phase composition on the duration of synthesis in an accelerated electron beam. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1947-1.
Surzhikov AP, Pritulov AM, Lysenko EN, Sokolovsky AN, Vlasov VA, Vasendina EA. Influence of solid-phase ferritization method on phase composition of lithium–zinc ferrites with various concentration of zinc. J Therm Anal Calorim. 2012;109(1):63–7.
Ahniyaz A, Fujiwara T, Song S-W, Yoshimura M. Low temperature preparation of β-LiFe5O8 fine particles by hydrothermal ball milling. J Solid State Ionics. 2002;151:419–23.
An SY, Shim I-B, Kim CS. Synthesis and magnetic properties of LiFe5O8 powders by a sol–gel process. J Magn Magn Mater. 2005;290:1551–4.
Smit J, Wijn HPJ. Ferrites. Physical properties of ferrimagnetic oxides in relation to their technical applications. Eindhoven: Philips Technical Library; 1959. p. 299.
Gorter EW. Saturation magnetization and crystal chemistry of ferrimagnetic oxides. J Philips Res Rep. 1954;9:295.
Acknowledgements
This work was supported in part by the Special Federal Program “Research and Development on Priority Directions of Scientific and Technological Complex of Russia for 2007–2013” (State Contract No. 16.513.11.3074).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surzhikov, A.P., Pritulov, A.M., Lysenko, E.N. et al. Analysis of the phase composition and homogeneity of ferrite lithium-substituted powders by the thermomagnetometry method. J Therm Anal Calorim 112, 739–745 (2013). https://doi.org/10.1007/s10973-012-2618-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2618-6