Abstract
Biodiesel is a prospective and promising fuel for diesel engines. However, some aspects need improvement, to develop into an ideal fuel, such as flow properties at low temperatures and storage stability at high temperatures with exposure to the air. Thermal analysis is an efficient tool for measuring properties, such as crystallization temperature, and thermal and oxidative stabilities. In this study, the thermal behaviors of biodiesel at low and high temperatures were investigated by using thermogravimetric analyzer, differential scanning calorimetry, pressurized differential scanning calorimetry (PDSC), and sorption analyzer (SA). The soy biodiesel was obtained through a transesterification reaction with a homogeneous catalyst. The constituents of the soy biodiesel as determined by gas chromatography show that methyl esters content was 99 % and of these 84 % were unsaturated fatty acids. TG results illustrate that the total weight loss of the biodiesel was 99 % below 300 °C under nitrogen flow, indicating a high purity biodiesel. The onset decomposition temperature and the peak temperatrue of the soy biodiesel were 193 and 225 °C, respectively, implying the biodiesel has good thermal stability. PDSC results show that the oxidation onset temperature of the soy biodiesel was 152 °C, and the oxidative induction time was 24 min. DSC results demonstrate that the onset crystallization temperature of the soy biodiesel was 1.0 °C. The SA results point out that with increasing temperature and humidity, the soy biodiesel absorbed more water, and in which humidity was the dominant factor. The water absorption and desorption of the soy biodiesel is a non-reversible process. The preferable storage conditions for soy biodiesel occur when humidity is less than 30 % and the temperature is less than 30 °C. In summary, thermal analysis is a faster alternative for thermal behavior studies as compared with conventional standard methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel, synthesized from vegetable oil, is a realistic alternative for diesel fuel since it is not only biodegradable and non-toxic but also produces less particles, smoke, and carbon monoxide as compared with petroleum diesel [1]. However, biodiesel contains more esters from saturated and unsaturated fatty acids, which causes problems such as, flow properties at low temperatures, susceptibility to oxidative processes at high temperatures, and large absorbing capacity for water [2–6]. Thermal analysis has a history of developing scientific applications. It provides efficient tools for measuring thermodynamic properties such as enthalpies, heat capacities, and phase transition temperatures [7]. Moreover, it can measure the thermal characteristics of biodiesel such as decomposition temperature, degree of oxidation, crystallization temperature, polymerization potential, and combustion temperature [5–8]. Teixeira et al. [2] studied the cloud and pour points of biodiesel by using DSC. Castello et al. [4] determined the water content in glycerol by the TG. Conceicao et al. [6] investigated the thermal stability and oxidative degradation of biodiesel using SDT and DSC. TG is a good technique for evaluating the thermal stability and oxidative stability, but it still can also be used for calculating conversion efficiencies. The vegetable oil conversion is very important when synthesizing the biodiesel. The normal method for calculating the conversion of vegetable oil is based on the standard EN 14103, but different individual ester standards are needed to be verified and quantified. Seldom is TG used for calculating oil conversion. Cloud point is correlated with the crystallization temperature of the biodiesel, which is caused by the fatty acid composition in biodiesel. When using ASTM D2500, about 40 mL of sample is needed for testing. However, when using DSC, an easy technique for measuring the crystallization of biodiesel, a 3–5 mg sample is ample. OOT and OIT are crucial parameters for biodiesel because of the content of unsaturated fatty acid in the biodiesel, which make it more susceptive to the oxidation process. PDSC or the Rancimat method is usually used to measure the OIT and OOT. Compared with Rancimat method, PDSC not only saves time but also uses a smaller amount of sample. Moisture content is important for the biodiesel because high moisture content in biodiesel can cause problems such as water accumulation and microbial growth in fuel handling, storage, and transportation equipment [9]. As a result, the moisture absorption characteristic of the biodiesel is very significant. The common method for measuring the water absorption is Karl-Fisher Coulometer or ASTM D2790, but SA is scarcely used [9]. The above investigations partially or singularly focused on one aspect of biodiesel and to date, there is no systematic study on thermal behaviors of biodiesel using thermal analysis techniques, especially the moisture content of the biodiesel. Therefore, the purpose of this study was to investigate the thermal behaviors of the biodiesel, such as thermal stability, crystallization, oxidation, anti-oxidation, and moisture absorption, using TG, DSC, PDSC, and SA, which will not only enlarge the application base of thermal analysis on biodiesel analysis but also provide basic information for biodiesel storage conditions. The thermal profiles of our synthesized biodiesel were compared with reference to commercially available biodiesel.
Experimental
Biodiesel synthesis
The soy biodiesel-1 (named biodiesel-1) was synthesized through a transesterification reaction with soybean oil, methanol, and a potassium hydroxide (KOH) catalyst. Soybean oil (50 g, 0.056 mol), methanol (10.78 g/0.33 mol) corresponding to a 6:1 ratio of alcohol to oil and KOH (1 %, w/w) were refluxed together in a 250 mL glass reactor equipped with a mechanical stirrer. Heating was achieved by means of a water bath. After the mixture reached a temperature of 65 °C, stirring was initiated (600 rpm). In addition, after reacting for 1 h, the mixture was transferred to a separatory funnel and allowed to settle overnight. The upper layer was the coarse biodiesel with catalysts and methanol, while the lower layer was a mixture of glycerol and methanol. The upper layer was vacuum distilled at 80 °C to recover the methanol, washed with hot water (50 °C) until the pH was 7, and then dried with anhydrous sodium sulfate. The soy biodiesel-2 (named biodiesel-2) was obtained from other unspecified methods and was used only for comparison with biodiesel-1. The FAME content was determined by GC using EN14103 by dissolving 125 mg of ester layer in 2.5 mL of n-hexane containing methyl heptadecanoate (5 g L−1 of C17 ester in n-hexane) as the internal standard. The conversion % was calculated using Eq. (1)
where \( \sum A \) is the total peak area of the methyl ester in C14 to that in C24:1, A EI is the peak area corresponding to methyl heptadecanoate; C EI is the concentration, in mg ml−1, of the methyl heptadecanoate solution; V EI is the volume of the methyl heptadecanoate; and m is the mass of the sample [10].
GC analysis
The biodiesel was analyzed on a Varian 3800 gas chromatograph fitted with a polar capillary column (Wax 30 m × 0.25 mm × 0.25 μm, Restek, USA) and a flame ionization detector (GC-FID). The temperature program was as follows: The initial oven temperature was 190 °C, ramping to 200 °C at the rate of 0.5 °C min−1, and then ramping to 240 °C at a rate of 20 °C min−1, isothermal for 2 min. The injector and detector temperatures were 220 and 230 °C, respectively. The split ratio was 1:50.
Thermal stability and oxidative stability
The thermal stability measurement was performed in a TA 2950 TG. About 10 mg of sample was placed in a platinum pan and heated to 600 °C at a heating rate of 10 °C min−1. The experiment took place in an ultra high pure (UHP) nitrogen atmosphere with flow rate of 100 mL min−1. The TG method was also used to measure the conversion of the vegetable oil.
The OOT and OIT were measured using a TA Q20 PDSC. In the OOT experiment, about 3 mg of biodiesel sample was placed in a pinhole aluminum pan and heated to 300 °C at a heating rate of 10 °C min−1. In the OIT experiment, the biodiesel sample was heated to 110 °C at rate of 10 °C min−1 and then isothermal for 100 min. Both experiments took place in an oxygen atmosphere under a pressure of 6.89 bar (100 psi).
Onset crystallization temperature
The crystallization onset temperature was measured using a TA Q2000 DSC. About 3–5 mg of sample was first held isothermal at −70 °C for 5 min, and then heated to 20 °C at a heating rate of 5 °C min−1. The experiment took place in the UHP nitrogen with flow rate of 50 mL min−1.
Moisture absorption
The moisture absorption experiment took place on a TA Q5000 SA. About 5 mg biodiesel sample was placed in a quartz pan and equilibrated at 25, 35, and 45 °C, isothermal for 30 min, at 0 % humidity, and then the humidity ramped to 80 % in steps of 20 %. At each step, the sample was isothermal for 30 min. For the moisture absorption and desorption experiment, about 5 mg sample was equilibrated at 25 °C for 30 min at a humidity level of 0 %, and then stepwise to 80 % with increments of 10 %. After stabilization, the humidity was decreased 80 to 10 % in steps of 10 %. The experiment took place in UHP nitrogen atmosphere with flow of 200 mL min−1.
Results and discussion
Properties of the biodiesel
GC results show that biodiesel-1 was mainly composed of methyl palmitate, methyl stearate, methyl oleate, methyl linoleate and methyl linolenate, in which methyl linoleate accounted for 55.3 % and the methyl oleate accounted for 21.6 % (Table 1). The total amount of unsaturated methyl esters was 84.7 %, which indicates poor oxidative stability. The methyl content was 98.9 %, which meets the requirement of EN14103 (96.5 %). The purity of biodiesel-1 was similar to that of the commercial biodiesel.
Thermal stability
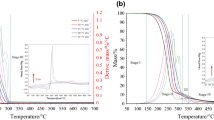
Figure 1 shows that there was one stage of weight loss during heating for biodiesel-1 in nitrogen flow. The weight loss of biodiesel-1 before 300 °C was 99 %, indicating a high purity biodiesel, which is similar to the GC result. The onset decomposition temperature of the biodiesel-1 was 193 °C, a little lower than that of commercial biodiesel (196 °C). Nevertheless, the biodiesel has good thermal stability. Compared with biodiesel samples, the onset temperature of the soybean oil was 377 °C, implying that there was no soybean oil in the biodiesel, confirming that the biodiesel was pure. The peak temperatures of the biodiesel and commercial biodiesel were 225 and 228 °C, respectively.
There were three stages of weight loss during heating for biodiesel-2 in nitrogen flow (Figure 1). The 1st stage was from 30 to 75 °C with the weight loss of 3.8 %, which may be due to the evolution of moisture or impurity (Table 2). The 2nd stage was from 75 to 300 °C with the weight loss of 67.5 %, which was due to the decomposition of biodiesel. The 3rd stage was from 300 to 500 °C, which was due to the decomposition of soybean oil. It was clearly seen that the transesterification reaction was not complete, and the biodiesel content was similar to that of GC result, thus, demonstrating a quick and repeatable way for measuring the conversion of vegetable oil. Only biodiesel-1 was analyzed for DSC, PDSC, and SA.
Oxidative stability
Oxidative stability is very important in quality control for biodiesel because unsaturated fatty methyl esters, such as linoleic and linolenic methyl esters, are more susceptible to oxidation. The OOT and OIT measurements were based on ASTM E2009 and modified ASTM D6186. In ASTM D6186, a pressure of 34.5 bar (500 psi) and oxidative test temperature of 200 °C were specified for measuring the OIT, but if the OIT was under 5 min, the temperature and pressures will be altered. In this study, the pressure of 6.89 bar (100 psi) and the oxidative temperature of 110 °C were used to measure the OIT of the biodiesel. PDSC results show that the OOT and OIT of the biodiesel-1 were 152 °C and 24 min, respectively, while the OOT and OIT of the commercial biodiesel was 141 °C and 15 min, respectively (Figs. 2 and 3). The OOT and OIT of the biodiesel-1 were higher than that of the commercial biodiesel, which may be because the biodiesel was fresh while the commercial has some oxidative degradation. Another important reason was due to the high concentration of linolenic methyl esters in commercial biodiesel (as shown in Table 1), which is more susceptible to oxidation because of triple-double bonds [11]. This observation is similar to other researchers, where the higher unsaturated composition results in lower oxidation stability [3, 6]. From GC data, it can be seen that the unsaturated methyl esters accounted for 84 %, which resulted in low oxidation stability for biodiesel. Therefore, the addition of the antioxidants was necessary for the biodiesel.
Onset crystallization temperature
Cloud and pour points are important parameters for assessing the biodiesel quality. If the temperature is below the cloud point temperature, biodiesel shows the tendency of partial solidification or loss in fluidity, leading to the interruption of fuel flow and clogging of the filtering system, resulting in poor engine starting [2]. Figure 4 shows the onset crystallization temperature of biodiesel-1 and commercial biodiesel was 1.0 and 2.2 °C, respectively, while the onset crystallization temperature of conventional petroleum diesel was −8.0 °C. This is because for petroleum diesel the hydrogen saturated compounds accounted for 80–90 %, which lowers the low temperature properties, such as cloud and pour points. Research shows that high levels of unsaturated fatty acid methyl esters increase the cloud points of the biodiesel, i.e., crystallization temperature of the biodiesel [3]. This explains why biodiesel is suitable for warm weather and why petroleum diesel is necessary for cold weather.
Moisture absorption
The functional group carboxyl, present in biodiesel, have affinity for moisture and therefore, absorb more moisture than the petroleum oil [2]. The higher moisture content in biodiesel augments microbial contamination in the form of bacterial and fungal growth, which may lead to corrosion and filter plugging if not controlled with biocides [9]. Figure 5 shows with increasing humidity or temperature, both the biodiesel-1 and the commercial biodiesel absorbed more water. For the biodiesel-1, at 25 °C, the absorbed moisture amount increased from 0.0263 to 0.079 % as the humidity increased from 20 to 40 %. When the temperature increased from 25 to 35 °C, the absorbed moisture amount increased from 0.0263 to 0.031 % at a humidity level of 20 % and from 0.079 to 0.0776 % at a humidity level of 40 %. This means humidity had a stronger effect on absorbed moisture than temperature. The commercial biodiesel also showed the same trend on humidity and temperature. In addition, it can be seen that the total absorbed moisture for biodiesel-1 was higher than that of commercial biodiesel. This may be due to impurities or the composition of biodiesel-1. This needs further investigation. According to ASTM D 6751, the maximum water content was 0.05 vol.%. Therefore, the best storage temperature and humidity was less than 30 °C and 30 %, respectively. However, it is important to keep the biodiesel in the low humidity condition. Figure 6 shows that the absorption–desorption of biodiesel samples is not a reversible process. Compared with the original sample, the water content of the biodiesel-1 decreased by 0.024 % while for the commercial biodiesel, it decreased by 0.055 %, indicating that more water was lost during the absorption–desorption process.
Conclusions
Biodiesel is an alternative for replacing petroleum diesel. However, it contains more unsaturated compounds, which make it difficult to be an ideal fuel. Thermal techniques provide a faster alternative for evaluating the thermal behaviors of biodiesel. In this study, the thermal behaviors of soy biodiesel were investigated using TG, PDSC, DSC, and SA. GC results show that the methyl ester content was 99 %, of which 84 % were unsaturated. TG results illustrate that the total weight loss was 99 % before 300 °C, indicating a high purity biodiesel. The onset decomposition temperature of the biodiesel was 193 °C. The OOT and OIT for the soy biodiesel were 152 °C and 24 min, respectively. DSC results demonstrate the high crystallization temperature of the biodiesel at 1 °C. The SA results point out that with increasing humidity and temperature, the soy biodiesel adsorbed more water. Humidity was the dominant factor on moisture absorption of soy biodiesel. The preferable storage conditions were found to be with less than 30 % humidity and less than 30 °C. In summary, thermal analysis is a fast, reliable, and effective technique for evaluating thermal characteristics of biodiesel.
References
Wen LB, Wang Y, Lu DL, Hu SY, Han HY. Preparation of KF/CaO nanocatalyst and its application in biodiesel production from Chinese tallow seed oil. Fuel. 2010;89:2267–71.
Teixeira GAA, Maia AS, Santos IMS, Souza AL, Souza AG, Queiroz N. Biodiesel from beef tallow/soybean oil/babassu oil blends: correlation between fluid dynamic properties and TMDSC data. J Therm Anal Calorim. 2011;106:563–7.
Sharma YC, Singh B, Upadhyay SN. Advancements in development and characterization of biodiesel: A review. Fuel. 2008;87:2355–73.
Castello ML, Dweck J, Aranda DAG. Thermal stability and water content determination of glycerol by thermogravimetry. J Therm Anal Calorim. 2009;97:627–30.
Tavares MLA, Queiroz N, Santos IMG, Souza AL, Cavalcanti EHS, Barros AKD, Rosenhaim R, Soledade LEB, Souza AG. Sunflower biodiesel: use of P-DSC in the evaluation of antioxidant efficiency. J Therm Anal Calorim. 2011;106:575–9.
Conceicao MM, Fernandes VJ, Araujo AS, Farias MF, Santos IMG, Souza AG. Thermal and oxidative degradation of castor oil biodiesel. Energy Fuels. 2007;21:1522–7.
Vyazovkin S. Thermal analysis. Anal Chem. 2002;74:2749–62.
Rodriguez RP, Sierens R, Verhelst S. Thermal and kinetic evaluation of biodiesel derived from soybean oil and higuereta oil. J Therm Anal Calorim. 2009;96:897–901.
He BB, Thompson JC, Routt DW, Van Gerpen JH. Moisture absorption in biodiesel and its petro-diesel blends. Appl Eng Agric. 2007;23:71–6.
Rashtizadeh E, Farzaneh F, Ghandi M. A comparative study of KOH loaded on double aluminosilicate layers, microporous and mesoporous materials as catalyst for biodiesel production via transesterification of soybean oil. Fuel. 2010;89:3393–8.
Bouaid A, Martinez M, Aracil J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel. 2007;86:2596–602.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Cao, Y., Orndorff, W. et al. Thermal behaviors of soy biodiesel. J Therm Anal Calorim 109, 1145–1150 (2012). https://doi.org/10.1007/s10973-012-2551-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2551-8