Abstract
Egg protein is an important part of our food to get protein in our daily diet, and makes this protein more important to researchers to understand its kinetic behavior to understand the energy involved in the digestion of the egg protein. Hence, the present study explores the denaturing kinetics of the protein obtained from the hen’s egg white (EW) using high resolution calorimetric technique. Fresh EW was scanned for heating and cooling to see the thermodynamics from 10 to 100 °C at different heating ramp rates varying from 1 to 20 °C min−1. An endothermic peak was found on heating scan showing denaturing of protein which was found absent at the cooling indicating the absence of any residue after heating. The denature peak shifted towards higher temperature as ramp rate increases following Arrhenius behavior and shows an activated denaturing kinetics of the egg protein. This peak was also compared with the water to avoid water effects. Behavior of denaturing peak can be explained in terms of Arrhenius theory and further discussed to get the energy involved in digestion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egg white (EW) is of great interest for many culinary and industrial applications. EW is used for coating, gluing, thickening and so on in pasta, desserts, etc. There is thus a great interest from the industrial point of view to better know this raw material, used in very large amounts in the dessert production or food product for example, and to obtain EW fractions with different functional properties [1–3].
Albumen, the egg protein is known as EW. Albumen accounts for most of an egg’s liquid weight, about 67%. It contains more than half the egg’s total protein, niacin, riboflavin, chlorine, magnesium, potassium, sodium, and sulfur. The albumen consists of four alternating layers of thick and thin consistencies. From the yolk outward, they are designated as the inner thick or chalaziferous white, the inner thin white, the outer thick white, and the outer thin white. Since this albumen is rich in protein, it is used in the daily diet of food very widely and frequently.
Egg protein is an important part of our food to get protein in our daily diet, hence it draws our attention to understand the kinetic behavior of egg protein to understand the energy involved in the digestion of the egg protein in daily diet. There are some interesting literatures available on egg protein research talking about concentration effect or heat induced phase transitions in protein mixtures or oil–water emulsions [4–7]. Our interest is to study a raw pure egg protein white to understand its thermal behavior in terms of molecular interaction during its denaturing transition. Therefore, the present study explores the non-isothermal denaturing transition kinetics of the egg protein obtained from the hen’s EW using high resolution calorimetric technique. Calorimetric technique is proven an important tool to study the thermal and kinetic behavior of soft materials in the area of soft matter, material science, and biological sciences [4–14]. Some reports can be seen in the literature on egg protein as an mixture with some additives studying local structure and stability with alanine substitute glycine, globular protein interaction with bovine, EW fractions, etc. [15–18], but almost no study has been found on the activated kinetics of the fresh EW protein which draws the author’s interest to report the present article reporting some important uncovered facts about fresh EW protein using calorimetric technique.
Experimental details
Fresh standard hen EW was studied by differential scanning calorimetry (DSC) using a model of TA instrument calorimeter at various heating scan ramp rates for 1, 5, 10, 15, and 20 °C min−1. The fresh EW was used direct from the refrigerator in the sealed cell and placed in the instrument with the water as reference material at ~1 °C and kept isothermal for 5 min to avoid temperature fluctuation and then heated from 1 to 100 °C with 5 °C min−1 heating scan rate. The respective heat flow of the sample was recorded along with the temperature change during heating scans. An endothermic peak was found on heating scan showing denaturing transition of the protein. This denaturing transition was observed at 60.5 °C for this particular heating rate. The sample was cooled immediate with the same scan rate and then a second heating run was also performed and no reversible phenomena was observed neither in cooling nor in second heating. This peak was also compared with the water to avoid water effects. There was no residue of peak found which clarified that the protein was completely denatured after first heating. Sample weights were about 2 mg. The same experiment was repeated a couple of times keeping the same heating range and rate and all environmental conditions same as above and found the above results reproducible. For the heating rate experiments, the same experiments were performed varying heating scan rates keeping other parameters same to compare heating rate effect on the denaturing transition of protein. All environments were kept identical for all runs to compare parameters (temperature, enthalpy, heat energy) of the sample of EW.
Kinetic theory of egg white (EW)
To understand the kinetic theory and denaturing kinetics of EW, it is important first to find the answers the following questions: (1) How does heating affect an EW? (2) What is the role of rate of heating on EW? (3) Does it really matter if an egg is cooked slowly or quickly? (4) Can we use Arrhenius theory in the kitchen? (5) What role does dissipation play in terms of energy involved in denaturing of EW? (6) Is there any time correlation with denaturing of EW protein?
Arrhenius theory in the kitchen
According to Arrhenius theory [19, 20], the heating rate of EW can be given by
where β is the effective heating rate in K min−1, β0 is a constant in K min−1, ΔE is the activation energy in J/mol, R is universal gas constant in J mol−1 K−1, and T is the absolute temperature in kelvin. This equation can also be given as:
where ΔE is determined from the slope of the graph which is plotted between ln β and 1/T, and ln β0 is the intercept on y-axis.
Thermodynamics of EW
The heat capacity of EW can be given by the following equation:
where C p is the obtained heat capacity of EW from the heat flow, ΔQ is the change in heat flow, and ΔT is the change in absolute temperature. To get the final heat capacity of EW, the excess of heat capacity of EW, can be obtained by subtracting from the specific heat C p a linear background as:
where C p (background) is the base line and C p is the specific heat capacity of the sample. The specific heat capacity (J g−1 K−1) can be calculated by taking ratio of heat flow (W g−1) and heating rate (K s−1).
The answers of the above questions can be seen in the results obtained below.
Results and discussion
Effect of heating on EW
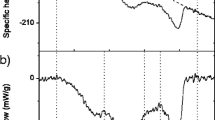
Figure 1 shows the heating scan of EW at 5 °C min−1 heating rate scanning from 1 to 100 °C with 5 °C min−1 heating scan rate. A well-defined endothermic peak was found on heating scan at 60.5 °C, which is the denaturing transition of EW where the protein denatures. The transition on heating scan represents absorption of heat. To see the heat capacity and the enthalpy of the EW’s transition, the excess of specific heat capacity are plotted in Fig. 2 that shows the excess of specific heat capacity of denaturing transition of EW obtained using Eqs. 3 and 4 as mentioned above. The enthalpy of the denaturing of EW is found to be 20.08 kJ g−1. The EW was cooled immediate with the same scan rate and then a second heating run was also performed. There was no reversible transition peak observed neither in cooling or second heating. This peak was also compared with the water to avoid water effects. The absence of any residue of peak on cooling and second heating clarifies that the protein was completely cooked or denatured after first heating.
Effect of heating rates on EW
A heating rate dependent study for EW was also performed at various heating ramp rates. Significant shift in the denaturing transition temperature was observed with different heating rates following Arrhenius behavior. Figure 3 shows the heating rate effect on denaturing transitions of EW for heating scans at different heating ramp rates varying from 1 to 20 °C min−1. As heating ramp rate increases, the denaturing transition shifts towards higher temperature. The rate of shifting shows the kinetics of EW. The rate effect on the transition of EW can be seen clearly in Fig. 4. It is clear that the denaturing transition of EW shows a rate dynamics, and clarifies that the EW cooks slowly (takes longer time in cooking) when heated with slower rate, and cooks quickly (takes lesser time) when heated with the higher rate, seen in Table 1. The slower the egg cooks the larger energy it absorbs whereas the faster the egg cooks the smaller energy it absorbs. The detailed data for the absorbed energy as a factor of heating rate can be seen in Table 1.
A comparative semi-log plot of ramp rate/°C min−1 versus 1/T/°C for denaturing transition of EW following Arrhenius behavior. Dotted lines are linear fits. It represents negative slope due to the absorption of heat. The error bars found in the transition temperature are much smaller than the symbol size. Error in the transition temperature is found ±0.05 °C
Energy activation of the transition of EW
The denaturing transition of EW follows the Arrhenius behavior and indicates the required activation energy for the transitions in the form of the slope of the line as plotted in Fig. 4. The transition peak shifts linearly with the change in heating rate and hence brings a line plot. The slope of the transition is found to be 2.01 kJ mol−1 and it indicates the required activation energy of the transition of EW. The transition shows a negative slope and negative activation due to the absorption of heat because the endothermic peaks found on heating scan shift towards lower temperature with the decrease of ramp rate, and indicates the required absorbed energy for the transition. Hence, the energy dissipated during the transition can be given by the activation energy and the energy absorbed by the transition can be given in the terms of enthalpy of the transition. The energy of dissipation represents the energy required to burn the fat gained by eating an egg whereas the absorbed energy represents the energy taken by egg during heating or cooking the egg. The data details for the denaturing transition of EW can be seen in Table 2.
Time correlation with the transition kinetics of EW
It is found that as heating rate brings transition kinetics in the denaturing transition and this kinetics brings changes in the time required for cooking an EW. It is clear from Fig. 5 that as heating rate increases the time required by the EW decreases and becomes minimum for the highest heating rate. On the other hand, it can be seen too that the denaturing temperature increases as heating rate increases. This effect brings a time correlation of EW cooking with different heating rates.
The denaturing transition of the EW shows a non-isothermal activated kinetics which can be studied using the rate dependent theory of Arrhenius. Using this theory, the activation energy of molecular motion and rearrangement of the EW near the denaturing transition temperature can be calculated by Arrhenius equation. As the heating rate increases, the temperature where the EW denatures moves forward and shows the smaller time taken by the transition which clarifies that the slower heating rates takes longer time to cook the EW where more energy is absorbed by the egg in cooking that makes the egg stiffer or harder whereas the faster heating rates cooks the egg faster by absorbing less energy and keep the egg softer after cooking. Energy details of the transition can be seen in Tables 1 and 2.
Conclusions
The non-isothermal unfolding/denaturing kinetics of the fresh EW protein was studied using a rate dependent Arrhenius theory with calorimetric techniques. Well-defined endothermic peak was found on heating scan for the denaturing transition of EW at 60.5 °C with heating rate of 5 °C min−1. Same experiments were performed with other heating rates and have found rate dependent behavior in the EW denaturing transition. The rate dependent study of the EW showed an increased activated kinetics following Arrhenius behavior. The rate of the shifting of the transition temperature increased as the heating rate increased and showed the rate dynamics. The energy dissipation during the denaturing of EW can be given by the activation energy of the denaturing transition. The energy of dissipation for the denaturing of transition was found to be 2.01 kJ mol−1. This energy indicates the energy required to burn the fat gained by eating the EW. The energy absorbed during the denaturing transition changed with the heating rate and has been found that the slower heating rate takes larger energy to cook the egg compared with the faster heating rates. Due to the large amount of energy absorbed at slower rates, the protein molecules becomes stiffer/harder when they are cooked at slower rate and takes longer time in cooking whereas the protein molecules remain softer when cooked with faster rate and denatures in less time.
References
Masumoto K, Ueda T, Motoshima H, Imoto T. Relationship between local structure and stability in hen egg white lysozyme mutant with alanine substituted for glycine. Protein Eng. 2000;13(10):691–5.
Ferreira M, Hofet C, Raemy A. A calorimetric study of egg white proteins. J Therm Anal. 1997;48:683–90.
Ruan C, Chi Y, Zhang R. Kinetics of hydrolysis of egg white protein by pepsin. Czech J. Food Sci. 2010;28:355–63.
Relkin P, Meylheuc T, Launay B, Raynal K. Heat-induced gelation of globular protein mixtures. A DSC and a SEM study. J Therm Anal. 1998;51:747–55.
Relkin P, Launay B. Concentration effects on the kinetics of b-lactoglobulin heat denaturation: a differential scanning calorimetric study. Food Hydrocolloids. 1990;4:17–34.
Relkin P. Using DSC for monitoring protein conformation stability and effect of fat droplets crystallinity in complex food emulsions. In: Lorincz D, editor. The nature of biological systems as revealed by thermal analysis. Londres: Kluwer Academic Publishers; 2004.
Relkin P. Heat-induced phase transformations of protein solutions and fat droplets in oil-in-water emulsions: thermodynamic and kinetic approaches. In: Kaletunc G, editor. Calorimetry in food processing: analysis and design of food systems. New York: Blackwell Publishing; 2009.
Sharma D. Calorimetric study of activated kinetics of the nematic and smectic phase transitions in an aligned nano-colloidal liquid crystal? Aerosil gel. J Therm Anal Calorim. 2008;93:899–906.
Sharma D. Kinetics of nanocolloids in the aligned domain of octylcyanobiphenyl and aerosil dispersion. Liquid Cryst. 2008;35:1215–24.
Sharma D, Iannacchione GS. Kinetics of induced crystallization of the LC1-xSilx system. J Phys Chem B. 2007;111:1916–22.
Sharma D, MacDonald JC, Iannacchione GS. Thermodynamics of activated phase transitions of 8CB: DSC and MC calorimetry. J Phys Chem B. 2006;110:16679–84.
Sharma D. Effect of alignment on the nematic to isotropic phase transition of bulk octylcyanobiphenyl brings possible solutions to liquid crystal display drawback. Appl Phys Lett. 2009;94:134103.
Sharma D, MacDonald JC, Iannacchione GS. Role of aerosil dispersion on the activated kinetics of the LC1-xSilx system. J Phys Chem B. 2006;110:26160–9.
Sharma D. Non-isothermal kinetics of melting and nematic to isotropic phase transitions of 5CB liquid crystal. J Therm Anal Calorim. 2010;102:627–32.
Reddy RC, Lilie H, Rudolph R, Lange C. l-Arginine increases the solubility of unfolded species of hen egg white lysozyme. Protein Sci. 2005;14:929–35.
Osterballe M, Jensen C. Threshold levels in food challenge and specific IgE in patients with egg allergy: Is there a relationship? J Allergy Clin Immunol. 2003;112:196–201.
Yang X, Foegeding E. Effects of sucrose on egg white protein and whey protein isolate foams: factors determining properties of wet and dry foams (cakes). Food Hydrocolloids. 2010;24:227–38.
Krise K, Milosavljevic B. Mobility of molecules and ions solubilized in protein gels: diffusion in the thick fraction of hen egg white. Biomacromolecules. 2011;12:2351–6.
Vogel H. The law of the relation between the viscosity of liquids and the temperature. Phys Z. 1921;22:645–6.
Fulcher GS. Analysis of recent measurements of the viscosity of glasses. J Am Ceram Soc. 1925;8(6):339–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, D. Non-isothermal unfolding/denaturing kinetics of egg white protein. J Therm Anal Calorim 109, 1139–1143 (2012). https://doi.org/10.1007/s10973-012-2225-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2225-6