Abstract
Graphite/n-docosane composite phase change materials (PCM) were prepared. 4, 10, and 16% graphite were added into n-docosane in order to study the effect of the amount of graphite to the thermal properties of the composite PCM. The structure of the composite PCM was characterized using scanning electron microscopy. The thermal properties of the composite PCM were determined using thermal constant analysis, heat storage/release curve, differential scanning calorimetry, and thermogravimetry analysis. The results revealed that the heat storage/release rate and the thermal conductivity increased with an increase in the amount of graphite, whereas the latent heat of the composite PCM decreased with the increase in the amount of graphite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal energy storage is gaining an increasing interest because it can prompt effective use of solar energy and waste heat. The most common way for thermal energy storage is to utilize the sensible heat change. The advantage of sensible heat storage is the simplicity; however, large volumes are required and the energy is released at varying temperature. Latent heat storage with phase change material (PCM) has higher storage density and constant temperature during phase change compared to the sensible heat change. Therefore, latent heat storage with PCM is an effective way for heat storage [1–5].
Traditional PCM that have been studied and used in applications are alkanes, paraffin wax, fatty acid, and salt hydrates [6–10]. Alkanes have a large heat of fusion per unit weight and are non-corrosive and chemical stable. They are suitable for latent heat storage. However, alkanes undergo a reversible solid to liquid phase change at the transition temperature and usually have to be encapsulated to contain the liquid phase. In addition, alkanes have a low conductivity, which hinders the transfer of the latent heat of PCM [11, 12].
The additives such as fins, carbon fibers, carbon nanotubes, carbon foams, and graphite could increase the thermal conductivity [13–20]. Graphite has advantages include porous, high surface energy, and strong absorbability. Moreover, graphite has excellent thermal conduction performance. It is a good thermal conductivity improvement material.
The heat transfer rate of the paraffin/graphite composite PCM was obviously higher than that of the pure paraffin owing to the combination with the expanded graphite [21]. Ping Zhang [22] found that when the mass fraction of 5% graphite replaced the equiponderant intumescent flame retardant, the thermal conductivities of the composite PCM increased as 82.14%. Xia [23] performed an experiment study on thermal conductivity improvement of PCM. The result showed that an addition of 10 wt% expanded graphite results in a more than 10-fold increase in the thermal conductivity compared to that of the pure paraffin. Ahmet Sari [24] studied the amount of the effect of expanded graphite on the thermal conductivity of paraffin. Thermal conductivity of the pure paraffin and the composite PCMs including 2, 4, 7, and 10 wt% EG was measured as 0.22, 0.40, 0.52, 0.68, and 0.82 W/m K. Lafdi [16] proposed a numerical study to investigate and predict the thermal performance of graphite foams infiltrated with phase change materials. The results showed that the average value of the output power of the new energy storage system has been increased by more than eight times. Some studies investigated the heat storage enhancement of exfoliated graphite nanoplatelets to paraffin [25, 26]. Andrew Mills [27] increased the thermal conductivity of paraffin wax by two orders of magnitude by impregnating porous graphite matrices with the paraffin.
Although investigations have been carried out on some PCM/graphite composites, data still lack in the effect of graphite on the thermal properties of PCM. The microstructure, pore distribution, and size distribution of graphite may influence the performance of the composite PCM. Further studies are necessary on the thermal properties prior to the application of PCM/graphite composite.
This study reports on the thermal properties of graphite/n-docosane composite PCM. The influences of the amount of graphite on the thermal conductivity, latent heat capacity, heat release, and storage rate of n-docosane are investigated. The structure of graphite/n-docosane composite PCM is characterized.
Experimental
Materials
Graphite (EXP-P) and n-docosane was supplied by Kanto Chemical Co., Ltd. The melting point of n-docosane is 42–44 °C, and the density of n-docosane is 0.9 g/ml at 20 °C. The apparent density of graphite is 0.06 g/cm3.
Preparation of the graphite/n-docosane composite PCM
The graphite/n-docosane composite PCM was prepared by melting method. 10 g of n-docosane was put into a beaker, that is, in a water bath at 55 °C. 0.4, 1, and 1.6 g of graphite was added into the beaker after n-docosane is melted. The mixture was stirred for 30 min at 500 rpm and then dispersed by ultrasound for 30 min at 55 °C. The mixture was cooled to the room temperature and ground. Then, the graphite/n-docosane composite PCMs were obtained.
Characterization of the graphite/n-docosane composite PCM
The size distribution of the graphite was measured by Laser Particle Sizer Analyser (Winner 2000, Jinan Winner Instrument Co., Ltd.). The heat storage/release rate of the graphite/n-docosane composite PCM was measured with Multi-channel Temperature Recorder (TP0008U, ShangHai Ruiqin Electronic Co., Ltd.). The temperature change range was from 26 to 54 °C. The scheme of the measurement system is shown in our former work [28]. The thermal conductivity of the graphite/n-docosane composite PCM was tested using Thermal Constant Analysers (1500TPS, Hot Disk Inc. Sweden). The microstructure of graphite and the graphite/n-docosane composite PCM was observed with a SEM (JSM-6700F, JEOL, Japan) at the acceleration voltage of 20 kV under low vacuum. The thermal stability was characterized by TG analysis (209F1 Iris, Netzsch Instrument Inc., Germany) from 0 to 100 °C. The latent heat and phase change temperature were measured with Differential Scanning Calorimetry (DSC200F3, Netzsch Instrument Inc., Germany) at the heating rates of 5 K/min in a nitrogen atmosphere from 0 to 100 °C.
Results and discussion
Morphology of the graphite/n-docosane composite PCM
The size distribution of graphite is shown in Fig. 1. The Granularity parameters of the graphite are presented in Table 1.
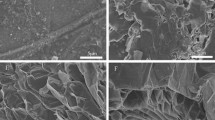
The average particle size of the graphite is 52.13 μm. The morphologies of the graphite with different magnifications are shown in Fig. 2. Figure 2 shows that particles of the graphite are composed by many lamellars. Some micro-cracks are detected in the microstructure of the graphite. After the lamellars are exfoliated by the external force, they can compound with paraffin to form the composite PCM. The morphology of the graphite/n-docosane composite PCM with 10% graphite is presented in Fig. 3. It can be seen that the surface of n-docosane is covered with paraffin. The elements’ content in Fig. 3a are measured by energy-dispersive spectroscopy analysis. The results are shown in Fig 3b. There are two kinds of elements in the graphite. The main element is carbon and its content is 97.09%. The other element is oxygen and the content is 2.91%.
The heat storage/release rate of the graphite/n-docosane composite PCM
The heat storage curve and heat release curve of the graphite/n-docosane composite PCM are shown in Fig. 4. The amounts of graphite in the composite PCM are 4, 10, and 16%.
It is clear from Fig. 4 that the heat storage/release rate increases with an increase in the amount of graphite before the samples arrive the objective temperature. We compared the heat storage/release rate of n-docosane and the composite PCM in Table 2. The heat storage/release rate is calculated by the ratio of the elevated temperature and the desired time. The time range is from 26 to 50 °C.
Table 2 shows that graphite can improve the heat storage/release rate of n-docosane remarkably. When 16% graphite is added into n-docosane, the heat storage rate increase from 0.027 to 0.233 °C/s and the increase percentage is 763.0%. The heat release rate increase from 0.026 to 0.098 °C/s, and the increase percentage is 276.9%. The increase in the amount of graphite will cause the decrease in the mass percentage of n-docosane in the composite PCM. Therefore, the heat storage density of the composite PCM per unit weight will be decreased.
Thermal parameters of the graphite/n-docosane composite PCM
Heat conductivity, thermal diffusivity, and specific heat of the composite PCM are measured to compare the thermal properties of the composite PCM and n-docosane. The results are presented in Table 3.
Table 3 indicates that the thermal conductivity of the composite PCM is higher than that of n-docosane. The thermal conductivity of the composite PCM is increased with the increase in the graphite. The increase percentages of the composite PCM are 81.5, 148.8, and 264.4% when the amount of graphite is 4, 10, and 16%, respectively. The results show that graphite can improve the thermal conductivity of n-docosane remarkably. The more the mount of graphite is, the greater the thermal conductivity of the composite PCM is. This result is in accordance with the results from the heat storage/release curves. Table 3 also shows that the composite PCM has higher specific heat than n-docosane. The composite PCM with 16% graphite has both higher thermal conductivity and higher thermal diffusivity than n-docosane.
DSC/TG analysis of the graphite/n-docosane composite PCM
The graphite/n-docosane composite PCM was analyzed by using DSC and TG analysis method. The results are shown in Fig. 5.
It can be seen form Fig. 5 that there are two great peaks which are corresponding to the solid–solid phase change and the solid–liquid phase change. The main peak is the solid–liquid phase change phase. Figure 5 indicates that the peak temperatures in the heat storage process are different from those in the heat release process. It means that the freezing point is lower than the melting point of the composite PCM. In addition, the latent heat in the heat storage process and the heat in the heat release process are different slightly. This reflects the difference of the phase change process between melting and freezing. The peak temperature and phase change temperature are marked in Fig. 5. The latent heats of the composite PCMs are calculated based on the DSC curves, and the results are given in Table 4.
Table 4 shows that the peak temperatures and phase change temperature of the composite PCM are lower than that of n-docosane for both solid–solid peak and the solid–liquid peak in the heat storage process. The exception is that the peak temperature of the 4% graphite/n-docosane is slightly higher than that of n-docosane. The reason is that the surface of the composite PCM is covered with n-docosane, and interfaces are formed in the composite PCM. These interfaces affect the phase change process. It can be seen from Table 4 that the latent heat of the composite PCM decreases with the increase in the amount of graphite. It illustrates that the latent heat of the composite PCM is decreased although the addition of graphite is an effective way to improve the thermal conductivity of PCM. TG curves in Fig. 5 show that the thermogravimetry loss is little when the composite PCMs are heated from 0 to 100 °C. The thermogravimetry loss is mainly induced by the water or the impurity in graphite. Moreover, the thermogravimetry loss of the composite PCMs increase with the increase in the amount of graphite. Therefore, the optimum amount of the graphite should be determined by considering synthetically the thermal conductivity, latent heat, and thermal stability.
Conclusions
Different amounts of graphite are used to improve the thermal conductivity of PCM. The structure and the thermal properties are characterized, and the following conclusions are drawn.
-
(1)
The graphite is composed by many lamellars. The microstructure of the prepared composite PCM shows that graphite is covered n-docosane.
-
(2)
The heat storage/release rate increases with an increase in the amount of graphite. When 16% graphite is added into n-docosane, the heat storage and release rate are increased 763.0 and 276.9%, respectively.
-
(3)
The thermal conductivity of the composite PCM is increased with the increase in the amount of graphite. The thermal conductivity of the composite PCM with 16% graphite is increased 264.4%.
-
(4)
The peak temperature, latent heat, and the thermal stability of the composite PCM decreases with the increase in the amount of graphite. The phase change temperature of the composite PCM is lower than that of n-docosane.
References
Frédéric K, Damien D, Kevyn J, Jean JR. A review on phase change materials integrated in building walls. Renew Sustain Energy Rev. 2011;15:379–91.
Beléen Z, Josée MM, Luisa FC, Harald M. Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl Therm Eng. 2003;23:251–83.
Sharma A, Tyagi VV, Chen CR. Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev. 2009;13:318–45.
Tyagi VV, Buddhi D. PCM thermal storage in buildings: a state of art. Renew Sustain Energy Rev. 2007;11(6):1146–66.
Albright G, Farid M, Al-Hallaj S. Development of a model for compensating the influence of temperature gradients within the sample on DSC-results on phase change materials. J Therm Anal Calorim. 2010;101(3):1155–60.
Feldman D, Banu D, Hawes DW. Development and application of organic phase change mixtures in thermal storage gypsum wallboard. Sol Energy Mater Sol Cells. 1995;36(2):147–57.
Luyt AS, Krupa I. Thermal behaviour of low and high molecular weight paraffin waxes used for designing phase change materials. Thermochim Acta. 2008;467:117–20.
Kousksou T, Jamil A. Paraffin wax mixtures as phase change materials. Sol Energy Mater Sol Cells. 2010;94:2158–65.
Suppes GJ, Go MJ, Lopes S. Latent heat characteristics of fatty acid derivatives pursuant phase change material applications. Chem Eng Sci. 2003;58:1751–63.
Evers AC, Medina MA, Fang Y. Evaluation of the thermal performance of frame walls enhanced with paraffin and hydrated salt phase change materials using a dynamic wall simulator. Build Environ. 2010;45:1762–8.
He B, Martin V, Setterwall F. Liquid–solid phase equilibrium study of tetradecane and hexadecane binary mixtures as phase change materials (PCMs) for comfort cooling storage. Fluid Phase Equilib. 2003;212:97–109.
Tae WS, Tae HK, Bong SK, Byung GK. Shape-stabilized phase change materials: frozen gel from polypropylene and n-octadecane for latent heat storage. Macromol Symp. 2007;249–50:450–5.
Elgafy A, Lafdi K. Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon. 2005;43:3067–74.
Shaikh S, Lafdi K, Hallinan K. Carbon nanoadditives to enhance latent energy storage of phase change materials. J Appl Phys. 2008;103:094302.
Marin JM, Zalba B, Cabeza LF, Mehling H. Improvement of a thermal energy storage using plates with paraffin–graphite composite. Int J Heat Mass Transf. 2005;48:2561–70.
Lafdi K, Mesalhy O, Elgafy A. Graphite foams infiltrated with phase change materials as alternative materials for space and terrestrial thermal energy storage applications. Carbon. 2008;46:159–68.
Zoubir A, Jérôme L, Elena PD. KNO3/NaNO3-Graphite materials for thermal energy storage at high temperature: Part I. Elaboration methods and thermal properties. Appl Therm Eng. 2010;30:1580–5.
Wang JF, Xie HQ, Xin Z. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol Energy. 2010;84:339–44.
Agyenim F, Eames P, Smyth M. Experimental study on the melting and solidification behaviour of a medium temperature phase change storage material (Erythritol) system augmented with fins to power a LiBr/H2O absorption cooling system. Renew Energy. 2011;36:108–17.
Mesalhy O, Lafdi K, Elgafy A. Carbon foam matrices saturated with PCM for thermal protection purpose. Carbon. 2006;44(10):2080–8.
Zhang ZG, Fang XM. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Energy Convers Manage. 2006;47:303–10.
Zhang P, Hua Y, Song L, Xiong J. Effect of expanded graphite on properties of phase change material. Sol Energy Mater Sol Cells. 2010;94:360–5.
Xia L, Zhang P, Wang RZ. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon. 2010;48:2538–48.
Sarı A, Karaipekli A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl Therm Eng. 2007;27:1271–7.
Kim S, Drzal LT. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol Energy Mater Sol Cells. 2009;93:136–42.
Xiang JL, Drzal LT. Investigation of exfoliated graphite nanoplatelets (xGnP) in improving thermal conductivity of paraffin wax-based phase change material. Sol Energy Mater Sol Cells. 2011;95:1811–8.
Mills A, Farid M, Selman JR. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl Therm Eng. 2006;26:1652–61.
Li M, Wu ZS, Chen ZQ, Peng CH. Effect of carbon fiber on thermal properties of n-docosane phase change materials. J Southeast Univ. 2010;26:346–50.
Acknowledgements
The authors gratefully acknowledge the financial support for this research from the National Natural Science Foundation of China (51178102), Science and technology project of Ministry of Housing and Urban–Rural development of China (2011-k1-40), and 12th Five Years Key Programs for Science and Technology Development of China (2011BAJ03B11-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Wu, Z. Thermal properties of the graphite/n-docosane composite PCM. J Therm Anal Calorim 111, 77–83 (2013). https://doi.org/10.1007/s10973-012-2218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2218-5