Abstract
In this study, polyfurfuryl alcohol was absorbed/adsorbed on soy protein isolate films by immersing the SPI films in acid-catalysed furfuryl alcohol solution for 60 h followed by complete curing at 145–150 °C for 2 h. PFA absorbed/adsorbed soy protein films designated as SPI-PFA were then arylated in the presence of 0.5% (w/v) 2,2-diphenyl-2-hydroxyethanoic acid for 4 h to fabricate arylated soy protein films designated as SPI-PFA-Ar. The incorporation of PFA and introduction of aromatic backbone through arylation is revealed from Fourier transform infrared study. Thermal studies of SPI, SPI-PFA and SPI-PFA-Ar were carried out by thermogravimetric analysis, differential scanning calorimetry and dynamic mechanical thermal analysis. In addition, mechanical properties, surface morphology and water uptake of all the three different types of the films were evaluated. Results indicated that PFA absorbed/adsorbed arylated soy protein films showed higher thermal stability than native SPI films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soy protein/wheat gluten-based plastics and composites are being researched for possible replacement of synthetic plastics in some sectors such as biodegradable containers or packaging materials [1–7]. Plasticizers are added to soy protein powder to remove the brittleness of soy protein-based plastics and composites [8–10]. Several works have been carried out to study the denaturation behaviour of enzymatically modified soy protein [11, 12]. However, the thermal stability of soy protein is low and it starts degrading above 170–180 °C [13]. Recently, structural transformations were induced in protein components under influence of gamma irradiation to increase the thermal stability [14]. Mechanism of the degradation of the soy protein polymer has been discussed by Nanda et al. and Swain et al. using several models [15, 16]. We have found earlier that the arylation of soy protein by 2,2-diphenyl-2-hydroxyethanoic acid (DPHEAc) leads to soy protein-based plastics and composites with higher thermal stability [17–20]. Higher thermal stability of the arylated soy protein is attributed to the introduction of aromatic backbone in soy protein moieties (see the mechanism below) [17, 18]. DPHM shown below in reaction mechanism refers to 2,2-diphenyl-hydroxy methane.
Polyfurfuryl alcohol (PFA) is a thermoset biopolymer derived from sugar cane bagasse. PFA has inherently very high thermal stability with high char yield and T max (T max represents the temperature at which mass loss is maximum) [21–24]. We have fabricated biofilms from soy protein isolate and PFA absorbed/adsorbed on it [25]. It has been reported that the thermal stability of soy protein has increased considerably with the absorption/adsorption of PFA on it [25].
There have been no reports of PFA absorbed/adsorbed on arylated soy protein. In this article, we have prepared PFA absorbed/adsorbed on soy protein films followed by arylation. Absorption/adsorption of PFA on soy protein film was structurally characterised by Fourier transform infrared (FTIR) spectroscopy. The resulting native and modified soy protein films were characterized by thermogravimetric analysis (TG), differential scanning calorimetry (DSC) and dynamic mechanical thermal analysis (DMTA). Surface morphology of the native and modified soy protein films was carried by scanning electron microscopy (SEM). The incorporation of PFA and introduction of aromatic backbone through arylation is revealed from FTIR study. In addition, mechanical properties and water uptake of all the three different types of films were evaluated. Results indicated that PFA adsorbed/adsorbed arylated soy protein films showed higher thermal stability than native SPI films.
Experimental
Materials
Soy proten isolate (SPI) with about 90.27% (dry basis) of a protein was purchased from Zhenghou Ruikang Enterprise Co., Ltd. (Zhengzhou, China). Thiodiglycol (TDG) (bp: 164–166 °C, mol wt: 122.19, and density: 1.182 g cm−3), DPHEAc (mp: 149–151 °C, mol wt: 228.25), furfuryl alcohol (FA) and p-toulenesulphonic acid (PTSA) monohydrate were purchased from Sigma-Aldrich and used as received.
Preparation of SPI films
TDG (30% w/w with respect to SPI) were mixed with SPI powder separately in an electronic mixer for about 15 min [10]. The resulting mixtures were hot pressed in compression moulding machine fabricated in-house at CSIR Port Elizabeth, South Africa, at 140 °C for 20 min under 50 bar pressure to prepare SPI films. Here, TDG has been used as plasticizer for the preparation of SPI films and the advantages of this plasticizer over commonly used glycerol plasticizer has been reported in the earlier literature [10].
Preparation of SPI-PFA films
The solution of PTSA monohydrate (0.3 phr with respect to the resin) prepared in 10 mL distilled water was added drop by drop to FA at room temperature to catalyze the FA. About 15 cm × 15 cm SPI films, as prepared by previously reported method (Ref 10), were immersed in catalyzed FA for 60 h. After the designated time, the SPI films were taken out from catalysed FA solution with the help of tweezers. In the next step, SPI films with absorbed FA were cured in an air-oven for 2 h at 145–150 °C to get PFA absorbed/adsorbed SPI films designated as SPI-PFA. It is to be noted that once PTSA is added to FA, polymerization starts which is slow in nature [23]. Crosslinking phenomena in PFA take place at/above the curing temperature only and that is the reason why FA absorbed SPI films were cured at 145–150 °C [25].
Preparation of the arylated SPI-PFA films
DPHEAc solution was prepared by dissolving 0.5 g of DPHEAc in 100 mL of boiling water. DPHEAc solution was slightly cooled (~50 °C) under stirring to prevent the formation of DPHEAc crystal. The SPI-PFA films were immersed in slightly cooled DPHEAc solution (0.5% w/v) for 4 h to get arylated films, coded as SPI-PFA-Ar [18]. Subsequently, the films were taken out and put between two steel plates fixed by binder clips to prevent dimensional instability of the SPI-PFA-Ar films [18].
Characterizations
FTIR studies of the modified and unmodified soy protein films were obtained on a Spectrum 100 FT-IR (Perkin Elmer, Buckinghamshire, UK) in the range from 4000 to 650 cm−1. Dynamic mechanical thermal analysis (DMTA) was performed on a dynamic mechanical analyzer (DMA8000, Perkin Elmer, Buckinghamshire, UK) with dual cantilever at a frequency of 1 Hz. The films tested were 50 mm × 10 mm (length × width) in dimensions, and the test temperature ranged from 25 to 200 °C, with a heating rate of 2 °C min−1. The α-relaxation temperature, αr, was determined as the peak value of the loss angle tangent (tan δ).
Thermal denaturation of the SPI samples was studied using a DSC (Perkin Elmer, Buckinghamshire, UK). About 5 mg modified and unmodified SPI or ground soy protein powder was weighed into a DSC pan and the pan was sealed. The samples were measured at a temperature range of 0–250 °C and a heating rate of 10 °C min−1. TG of approximately 5 mg dried films was carried out at a heating rate of 10 °C min−1 between room temperature and 700 °C in nitrogen atmosphere on a TG–IR interface (Perkin Elmer, Buckinghamshire, UK).
The tensile strength (TS) and elongation at break (EB) of the SPI films were measured on an Instron 3369 testing machine at a strain rate of 10 mm min−1 according to ASTM D882 (E). The films tested were 110 mm × 15 mm (length × width) in dimensions. The clamping length for each specimen on each jaw was 15 mm. Three-point bending tests were carried out using an Instron Universal Testing Machine, model 3369 to determine the flexural strength. Flexural testing was carried out in accordance with ASTM D-790, at a crosshead speed of 5 mm min−1 and a span length of 60 mm. The sample dimension was 80 mm × 10 mm for flexural testing. An average value from five replicates of each sample was taken for each of the tests mentioned above.
The water uptake of the different soy protein films was evaluated according to ASTM D570-81. The films were preconditioned at 50 °C for 24 h and weighed. After immersing in distilled water for 24 h, the films were dried with paper towels to remove the excess water on the surface and weighed. The total weight gain of the composites was used to calculate the absorbed water. An average value from three measurements was reported.
SEM images of the surfaces of the specimens were taken on FEI Quanta 200 (Eindhoven, The Netherlands) electron microscope at an accelerating voltage of 15 kV. Gold sputtering was not required for the preparation of the sample in this instrument.
Results and discussion
Structural characterization by FTIR
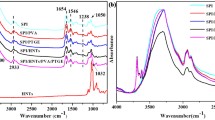
Figure 1 shows the FTIR spectra of SPI, SPI-PFA and SPI-PFA-Ar films. FTIR spectra of all the three sheets exhibit typical amide vibrations including amide A (N–H stretching, 3200–3400 cm−1) (not shown in Figure), amide I (C=O stretching around 1621 cm−1), amide II and amide III (N–H bending and CN stretching, at around 1533 and 1240 cm−1, respectively) [9]. The most relevant peak associated to the presence of furan ring at 1010 cm−1 is observed in the FTIR spectra for SPI-PFA samples. Also the presence of band at 746, 802 and 883 cm−1 confirms the presence of 2,5 disubstituted furan ring. Upon arylation there is appearance of peak at 698 cm−1 which is assigned to the out-of-plane deformation vibrations of the hydrogen atoms on the benzene ring [17].
Thermal properties
Figure 2 shows the DSC curves of soy protein powder as well as SPI, SPI-PFA and SPI-PFA-Ar films. Soy protein powder with moisture content of 6.34% shows an endothermic peak at 117 °C and ∆H of 61.98 J g−1. The presence of endothermic peak is attributed to the heating which converts soybean protein from its native state to a denatured state, accompanied by unfolding and disruption of the intramolecular bonding [26]. The intensity of the endothermic peak at 117 °C, (with ∆H of 21.7 J g−1), decreases significantly for soy protein mixed with plasticizer. In addition, the glass transition temperature is observed at lower side of the temperature denoted by the circle and the arrow. The endothermic peak disappeared for the SPI-PFA and SPI-PFA-Ar films. This is attributed to the subjection of the soy protein at 145–150 °C during curing of PFA. Moreover, the absence of exothermic peak in SPI-PFA at ~150 °C, observed due to curing of PFA, indicates complete curing of PFA.
Figure 3 shows the TG curves for the modified and unmodified films. The thermal degradation of the SPI films in the presence of TDG as plasticizer experiences a two-stage mass loss i.e., T max1 (170 °C) and T max2 (353 °C). Here, T max represents the temperature at which mass loss is maximum. T max1 is attributed to the loss of plasticizer and T max2 is attributed to the degradation of soy protein [10]. The thermal stability of the soy protein films increases with the absorption/adsorption of PFA on SPI films with T max2 being observed at 365 °C [25]. Upon arylation T max2 further increases to 396 °C [17–19]. The char yield at 700 °C for the SPI-PFA and SPI-PFA-Ar is higher than SPI samples. The higher thermal stability is attributed to the incorporation of furanic and aromatic rings in SPI as observed from FTIR study (Fig. 1).
Thermomechanical behaviour
The storage modulus curves of the SPI, SPI-PFA and SPI-PFA-Ar films as a function of temperature are presented in Fig. 4. Storage modulus decreases as temperature increases for all the films. SPI-PFA and SPI-PFA-Ar samples showed higher storage modulus than native SPI films. In addition, the temperature at which the storage modulus shifts to zero for SPI-PFA and SPI-PFA-Ar is also higher than that for native SPI films.
DMTA of the soy protein films were also carried out to provide information on the segmental motion of the protein molecules. Figure 5 shows the temperature dependence of the loss factor of the protein films. SPI shows α-relaxation around 51 °C assigned to protein rich domain [27]. SPI-PFA and SPI-PFA-Ar films showed α-relaxation around 62.5 °C owing to the rigidity in the samples as evident from lower elongation at break (discussed in next section). SPI-PFA and SPI-PFA-Ar films also showed the presence of α-relaxation at higher temperature (183 °C). This may be attributed to the presence of aromatic and furanic rings. Hence, incorporation of PFA or PFA followed by arylation of soy protein lead to materials with less flexibility and higher stiffness and this has also been reported in earlier literature [17–20, 25].
Mechanical properties and water uptake
Table 1 shows the mechanical properties and water uptake of the SPI, SPI-PFA and SPI-PFA-Ar films. SPI films showed TS of 8.4 MPa. The TS decreases for SPI-PFA and SPI-PFA-Ar. Incorporation of aromatic or furanic rings in soy protein induce rigidity in SPI films and is well supported by the decrease in percentage EB. It has been observed from Table 1 that EB decrease to ~3% for SPI-PFA and SPI-PFA-Ar. Similarly, there are decrease in flexural stress (FS) and flexural modulus (FM) for SPI-PFA and SPI-PFA-Ar films. It was found in our earlier study that the TS of the arylated SPI films are in the range of 12–15 MPa. But in this study, we have observed that if arylation is done on SPI-PFA films there are reductions in the mechanical properties unlike arylation carried out on native SPI films [17, 18].
Water uptake of the SPI-PFA and SPI-PFA-Ar films was found to be less compared to native SPI films. However, water uptake is slightly higher as reported in the literature for only arylated SPI films [17, 18].
Surface morphology
Figure 6 shows the surface morphology of SPI, SPI-PFA and SPI-PFA-Ar films. SPI films displayed a smooth and homogeneous structure on the surface. Upon curing, PFA as crosslinked moieties appeared on the surface of SPI films which indicates the absorbtion/adsorbtion of PFA on SPI [25]. Surface morphology of the SPI-PFA-Ar shows the presence of 2,2-diphenyl-2-hydroxy methane (DPHM) microparticles on the crosslinked structures of SPI-PFA resulting in the masking of the crosslinked structure of PFA on SPI similar to what have been reported earlier [17, 18].
Conclusions
We have successfully arylated soy protein films with PFA absorbed/adsorbed on it. Thermal stability of the protein films increased with the absorption/adsorption of PFA and it further increased with the arylation process. Storage modulus and tanδ for arylated PFA absorbed/adsorbed soy protein films was found to be highest. Higher thermal stability of SPI-PFA and SPI-PFA-Ar is attributed to the incorporation of furanic and aromatic rings. However, mechanical properties of the soy protein films were found to decrease upon incorporation of PFA or aromatic backbone through arylation when compared to native SPI films.
References
Lodha P, Netravali AN. Characterization of interfacial and mechanical properties of ‘‘green” composites with soy protein isolate and ramie fiber. J Mat Sci. 2002;37:3657–65.
Lodha P, Netravali AN. Characterization of stearic acid modified soy protein isolate resin and ramie fiber reinforced ‘green’ composites. Compos Sci Tech. 2005;65:1211–25.
Liu W, Mohanty AK, Askeland P, Drzal LT, Misra M. Influence of fiber surface treatment on properties of Indian grass fiber reinforced soy protein based biocomposites. Polymer. 2004;45:7589–96.
Chabba S, Netravali AN. ‘Green’ composites Part 1: characterization of flax fabric and glutaraldehyde modified soy protein concentrate composites. J Mat Sci. 2005;40:6263–73.
Huang X, Netravali AN. Characterization of flax fiber reinforced soy protein resin based green composites modified with nano-clay particles. Compos Sci Tech. 2007;67:2005–14.
Kumar R, Liu D, Zhang L. Advances in proteinous biomaterials. J Biobased Mat Bioenergy. 2008;2:1–24.
Mojumdar SC, Moresoli C, Simon LC, Legge RL. Edible wheat gluten (WG) protein films. J Therm Anal Cal. 2011;104:929–36.
Wu Q, Zhang L. Properties and structure of soy protein isolate: ethylene glycol sheets obtained by compression molding. Ind Eng Chem Res. 2001;40:1879–83.
Liu D, Zhang L. Structure and properties of soy protein plastics plasticized with acetamide. Macromol Mat Eng. 2006;291:820–8.
Kumar R, Wang L, Zhang L. Structure and mechanical properties of soy protein materials plasticized by thiodiglycol. J Appl Polym Sci. 2009;111:970–7.
Molina Ortiz SE, Añón MC. Enzymatic hydrolysis of soy protein isolates DSC study. J Therm Anal Cal. 2001;66:489–99.
Zhang H, Takenaka M, Isobe S. DSC and electrophoretic studies on soymilk protein denaturation. J Therm Anal Cal. 2004;75:719–26.
Kumar R, Choudhary V, Mishra S, Varma IK. Enzymatically transformed soy protein Part1-Thermal behaviour. J Therm Anal Cal. 2004;75:727–38.
Cies′la K, Vansant EF. Physico-chemical changes taking place in gamma irradiated bovine globulins studied by thermal analysis. J Therm Anal Cal. 2010;99:315–24.
Nanda PK, Rao KK, Kar RK, Nayak PL. Biodegradable polymers Part VI. Biodegradable plastics of soy protein isolate modified with thiourea. J Therm Anal Cal. 2007;89:935–40.
Swain SN, Rao KK, Nayak PL. Biodegradable polymers Part. II. Thermal degradation of biodegradable plastics cross-linked from formaldehyde-soy protein concentrate. J Therm Anal Cal. 2005;79:33–8.
Kumar R, Zhang L. Effect of water on the hydrophobicity of soy protein materials containing 2, 2-diphenyl 2-hydroxyethanoic acid. Biomacromolecules. 2008;9:2430–7.
Kumar R, Zhang L. Soy protein films with the hydrophobic surface created through non-covalent interactions. Ind Crops Prod. 2009;29:485–94.
Kumar R. Effect of water-mediated arylation time on the properties of soy protein films. Ind Eng Chem Res. 2010;49:3479–84.
Kumar R, Zhang L. Aligned ramie fiber reinforced arylated soy protein composites with improved properties. Compos Sci Tech. 2009;69:555–60.
Pranger L, Tannenbaum R. Biobased nanocomposites prepared by in situ polymerization of furfuryl alcohol with cellulose whiskers or montmorillonite clay. Macromolecules. 2008;41:8682–7.
Choura M, Belgacem NM, Gandini A. Acid-catalyzed polycondensation of furfuryl alcohol: mechanisms of chromophore formation and cross-linking. Macromolecules. 1996;29:3839–50.
Trindade WG, Hoareau W, Razera IAT, Ruggiero R, Frollini E, Castellan A. Phenolic thermoset matrix reinforced with sugar cane baggase fibers; Attempt to develop a new fiber surface chemical modification involving formation of Quinones flowed by reaction with furfuryl alcohol. Macromol Mater Eng. 2004;289:728–36.
Lee H, Rajagopalan R, Robinson J, Pantano CG. Processing and characterization of ultrathin carbon coatings on glass. Appl Mater & Interfac. 2009;1:927–33.
Kumar R, Kumar R, Anandjiwala R. Biofilms from soy protein isolate and polyfurfuryl alcohol. Plastics, rubber and composites: macromolecular engineering. 2011 (Accepted for publication on 7/1/2011).
Mo X, Sun X. Plasticization of soy protein polymer by polyol-based plasticizers. J Am Oil Chem Soc. 2002;79:197–202.
Chen P, Zhang L. New evidences of glass transitions and microstructures of soy protein plasticized with glycerol. Macromol Biosci. 2005;5:237–45.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R. Thermal properties of polyfurfuryl alcohol absorbed/adsorbed on arylated soy protein films. J Therm Anal Calorim 107, 1287–1292 (2012). https://doi.org/10.1007/s10973-011-2023-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2023-6