Abstract
A simplified equation relating water droplet size distribution to crystallization temperature, determined from differential scanning calorimetry (DSC) curves of aqueous emulsions of petroleum is reported in this article. A series of water-in-oil (W/O) emulsions was prepared by dispersion of water in different Mexican crude oils; in a classical DSC experiment, these emulsions were submitted to a regular heating and cooling cycle within temperatures including freezing and heating of dispersed water. The Z-average diameters of the water drops (D dz) were estimated this way and correlated with petroleum composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At the beginning of the twenty-first century, the world’s high consumption of energy has provoked a rapid decline of light crude oil reserves. The production of heavy and super-heavy crude oils is ever increasing, and the problem of oil-in-water (O/W) emulsions, with a high concentration of water and salts turning out to be an important issue. It is necessary to measure the different sizes of water drops dispersed in crude oil, and to design and evaluate chemical products for the desalting and dehydration of heavy and super-heavy petroleum; common techniques such as light scattering are not useful, however, because of the extra high opacity of crude oil continuous phase.
On the other hand, thermal analysis techniques are more frequently employed to characterize petroleum and its derivatives [1–3]. The thermal behavior and degradation of a wide variety of whole crude oils and their fractions, obtained by means of chromatographic or distillation methods, have been studied by thermogravimetrical analysis [4, 5]. Similarly, differential scanning calorimetry (DSC) has shown to be a reliable method to elucidate some of the main features of the saturate, aromatic resins and asphaltenes constituting petroleum [6]. Only the characterization of dry samples of crude oil and derivatives are here reported; even if the event thermal methods had not been originally conceived for characterizing dispersed systems, some pioneer researches were performed, with the aim of investigating the solidification of drops dispersed in a continuous phase, by differential thermal analysis (DTA) and DSC. This last technique has turned out to be a very suitable method for studying the behavior of emulsions submitted to a temperature gradient [7]. The heats released or absorbed when solidification or melting takes place within an emulsion can be both detected and quantified by DSC. Nevertheless, if getting emulsion characteristics from DSC measurements were not obvious, this thermal technique constitutes one of the most suitable methods to investigate the stability and characteristics of water in petroleum emulsions. With the aim of solving this problem, Clausse and Dalmazzone have proposed the use of the thermogranulometry, meaning the application of DSC, to determine the drop distribution of the aqueous phase and associated phenomena [8–15]. They have analyzed both the drop ice nucleation, and its kinetic behavior. These researchers have found that the experimental determination of the theoretic parameters is not allowable and, based on this, they have established an experimental correlation between the most likely solidification temperature of a droplet T*, and the water drop size distribution obtained by microscopic analysis [8]. It is important to state that T* is the main freezing temperature, as determined by Bigg [16], where 50% of the droplets of a specific droplet diameter are frozen [17]. This temperature parameter is also obtained by DSC.
With the aim of characterizing a series of emulsions of water dispersed in Mexican crude oils, a simplified equation was inferred in this study, relating the water droplet size distribution to the crystallization temperatures found by using the DSC curve. The statistical average D dz was also calculated. It was, therefore, possible both, to compare the drop diameter distribution of water dispersed in different crude oils and to explain in which type of petroleum the stability of the water-in-oil (W/O) emulsion was higher.
Experimental methods
Materials
Mexican crude oils: samples of light (36° API), heavy (22° API) and super-heavy (17° API) petroleum belong to Mexican Gulf oil field.
Physical testing
Samples of Mexican crude oils were first submitted to a series of characterizations to determine their main physical features. Kinematic viscosity was measured following the ASTM-D 445 method. Salt and water contents in crude oil were determined, respectively, according to the ASTM-D 3230-06 and ASTM-D 4006-07 procedures. In the case of pour points, they were evaluated according to the ASTM-D 97 method. Wax content was evaluated in accordance with the UOP-46 norm. SARA composition was obtained by precipitation of asphaltene with n-heptane, and maltenes were separated through the HPLC method with Water Spherisorb NH2 Column 10 μm (20 × 250 mm) Preparative [18]. Main characteristics and properties of crude oils are reported in Table 1.
DSC sample preparation
It has been shown that thermal methods, such as thermogravimetry (TG) and differential scanning calorimetry (DSC) may be useful to characterize crude oils and their fractions [18, 19]. In the specific case of W/O emulsions, it is important to define the preparation procedure. Emulsions were elaborated in special graduated glass bottles (180 mL) with lids, to prevent any segregation and to obtain a homogeneous mixture. The treated crude, was then placed in a water thermal bath and heated to a temperature that approximates the formation temperature of the geological formation from which the crude oil was obtained (in this case, about 60 °C) during 10 min [7]. A volume of 100 mL of crude oil was measured and introduced into each glass bottle. Afterward, distilled water was added to reach a concentration of 5% (v/v). A continuous vertical movement by hand, of the glass bottle was made for 3 min, to improve macro-stirring of the system—like a bottle test procedure.
It was also important to carry out rigorous sampling, after emulsions’ preparation. The sample had to be stable and representative of the entire mixture; a 1-mL syringe was used for this purpose, one droplet was taken and gently deposited on a perfectly clean Al crucible, and this procedure was repeated with samples with 10 and 20% (v/v) content of distilled water.
DSC characterization
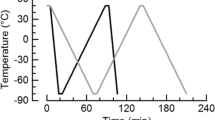
When DSC was performed on emulsions, two parameters were of utmost importance: sample volume and scanning rate [7]; the smallest ones are the best. A compromise had to be made, and this normally needs practice and preliminary experiments. Crystallization temperatures of the W/O emulsions were detected by DSC using a Shimadzu DSC-60A with a TA-60WS interface. A calibration procedure was performed using two calibration materials [20] with melting temperatures—one above and the other below temperature range of interest. Water and Indium were used as reference standards. The apparatus was continuously flushed with nitrogen at 20 mL/min, and an empty crucible was held in the reference cell. Samples weighed between 8 and 22 mg, containing roughly 106 droplets of water [11, 21]. The sample weight was limited by the dimension of the emulsion droplet. As a result of this, global information on the sizes of a very large number of droplets, from 5 × 106 to 200 × 106, depending on droplets size and the volume of the emulsion sample under study, is obtained [8]. All the heating and cooling rates were \( \mathop q\limits^{.} \; = \; 5\;^\circ {\text{C}}/\min \) according to Dalmazzone’s recommendations [9, 22]. First, the sample was heated to 30 °C and held for 3 min; it was then cooled by using liquid nitrogen up to −60 °C, holding it for 3 min and reheating again to 30 °C. This was the first cycle. A similar second cycle was performed to analyze the influence of the first cycle regarding the sample’s stability, and to corroborate the peak’s position. These low-temperature tests are non-destructive; several measurements can be performed by using the same sample, considering that at 30 °C, the thermal stability of the crude oil is significant.
Results
The crude oils were first evaluated to determine their main characteristics and properties, as reported in Table 1. All samples consisted of a little content of water and salt, specially the light crude oil. Similar wax contents were also determined for the three petroleum samples. A perfect correlation between the kinematic viscosity and the pour point was observed for all the series of crude oils; the light petroleum, with a reduced viscosity, begins to flow at a low temperature of −45.0 °C whereas for the very viscous heavy crude oil, a pour point of −20 °C is required to start displacing it. The SARA analysis revealed a saturate content decrease with the API density of the samples. An opposite dependence of the other compound fractions with the API density may be established; specifically apparent in the case of asphaltenes. The highest asphaltene content was detected in super-heavy crude oil. This parameter is very important it is well known that water droplets are stabilized in petroleum mainly by asphaltenes [22–25].
Samples of the three dry crude oils were thoroughly characterized by DSC. The three types of petroleum were submitted to a first cooling cycle, the obtained curves are shown in Fig. 1. Just a reduced water percentage, close to 0.30% in the heavy crude oil was determined from these curves. In the other small samples of light and heavy petroleum, no water was detected.
Afterward, samples of crude oil, dry, and with different contents of emulsified water (5, 10, and 20%) were characterized by DSC. New peaks in the cooling and heating steps were found when water was added—associated to the crystallization and fusion of the water droplets in light, heavy, and super-heavy crude oils—as shown in Fig. 2, for the heavy oil.
The curves showed that the superior and the maximum levels in the fusion peak of the W/O emulsions in light, and heavy crude oils were shifted to higher temperatures, when the water content was increased. These signals represent the overlay of several individual Gaussian-like peaks, obtained for each emulsified water content corresponding to each droplet size. The curve of water emulsified in super-heavy crude oil had the maximum at a water content of 10% (w/w) instead. Five similar signals were assigned to crystallization temperatures of W/O emulsions of the three crude oils, and are shown in Fig. 3. It was supposed in this context, that the water minimum crystallization temperature did not exceed −60 °C. Therefore, as an example, Clausse and Dalmazzone have found crystallization temperatures lower than −45 °C with water solutions with NaCl or urea, up to −82 or −60 °C, respectively [13, 25]. It was considered that crude oil components, such as asphaltenes, resins, and present solids could diminish the crystallization temperature up to −60 °C.
It is important to indicate that the IV signal in Fig. 3, between −36 and −46 °C, was the most regular one, associated to the normal highest crystallization temperature of pure water [26–28]. Furthermore, the regularity of the signals pointed out that the interfacial tension between the water and the three kinds of petroleum was similar in the range of temperature evaluated, enabling the association of a unique drop diameter to the crystallization temperature determined by DSC.
The second cycle measurements had a high influence of the first cycle scanning on the W/O light crude emulsions, producing a shift to higher temperature in the curve. If this shift to higher temperature is associated to a higher drop diameter; then this shift indicates a coalescence of the droplets. In contrast, the second cycle did not even have an influence in respect of the curves in W/O heavy and super heavy crude emulsions.
Discussion
It was considered that the water droplet did not change its diameter during the cooling process, to evaluate the water droplet diameter in the W/O emulsions. Consequently, the difference of pressure between the water droplet and the surrounding oil \( \Updelta P_{\text{w,o}} \; = \;P_{\text{w}} - P_{\text{o}} \) changed with the water–oil interfacial tension γwo in accordance to Laplace equation:
where D d is the drop diameter. Because this interfacial tension changes slowly [25], it was considered that the difference of pressure was almost constant, as shown in Fig. 4. An assumption was then made that the temperature obtained in DSC was the equilibrium freezing temperature of water \( T_{{f_{2} }} \), given by the Clausius Clapeyron equation [26, 29–31]:
where \( T_{{f_{1} }} \)is the normal freezing temperature of water at 1 atmosphere, say, 273.15 K; \( \Updelta P_{{T_{{f_{2} }} ,T_{{f_{1} }} }} = P_{{T_{{f_{2} }} }} - P_{{T_{{f_{1} }} }} \) is the pressure difference between these equilibrium temperatures; \( \Updelta V_{\text{m,c}} \) is the change of molar volume in the crystallization temperature; and \( \Updelta H_{{{\text{m}},{\text{f}}}} \) is the fusion molar enthalpy. The effect of the droplet curvature [17] was not included in this equation. It was considered that when the temperature declined, the cooling rate was so slow that the crystallization temperature of the drop of water was the equilibrium crystallization temperature \( T_{{f_{2} }} \), and the pressure at this temperature, \( P_{{T_{{f_{2} }} }} \), was equal to the pressure inside this drop P W:
And the drop diameter D d is finally obtained as follows:
Water phase diagram of temperature versus pressure showing that the equilibrium crystallization temperature \( P_{{T_{{f_{2} }} }} \) equals the pressure of the spherical water drop P W [34]
It is considered that \( \Updelta H_{\text{m,f}} \) does not change with the temperature, although the fusion enthalpy decreases with the decrement of temperature [17, 32–34]. Calculations show that the variation of enthalpy is not important in the statistical analysis of the diameter droplet distribution. Besides, the interfacial tension γ wo was calculated using the equation [35, 36].
With
where γ w and γ o are the surface tensions of water and oil, respectively; and V m,w and V m,o are the molar volumes of the water and oil, respectively. We have not considered that the surface tension of water γ w had an asymptotic value close to −45 °C as was assumed by Pruppacher [17], with a total crystallization of water at this temperature.
It is important to make it clear that the calculated diameter values of the droplets were in the order of nanometers at a temperature close to −40 °C, two orders of magnitude less than the values at this temperature found by Dalmazzone [8]. These calculated frozen droplet diameters were approximately of the ice germs, of nanometric size [17, 32, 33], but not of the real frozen droplet diameters. Nevertheless, the values were useful, both for comparing the size distributions between the different W/O emulsions of the crude oil, and finding out in which W/O crude emulsion, the stability changed with the sequential addition of distilled water.
In Fig. 5, histograms of the diameter distribution versus weight fraction showed that higher percentage of crystallization corresponded to signal IV, in accordance with Dalmazzone [9]. This figure shows the droplet diameter histogram of the crudes with 20% distilled water.
In general, light crude oil showed a decline of the smallest droplet weight fraction and, at the same time, an increase in the proportion of the larger droplets with the increment of water content. Thus, the heavy crude oil had only a small coalescence with the increment of water content. The super-heavy W/O emulsion showed two phenomena: an increase of the smallest droplets and an increment of larger droplets (Fig. 6). The Z-average diameter D dz given by Collins [37] was determined to analyze the statistics of the water drop diameter:
It was found that the emulsions prepared in light and heavy crude oils showed the same D dz with 5 and 10% of distilled water. When the percentage of distilled water was 20%, the light crude oil emulsions showed a higher coalescence than those formed in heavy crude oils. Besides, the super-heavy crude showed almost the same average diameter D dz in each W/O emulsion, and with 5 and 10% of distilled water, its diameter values D dz were higher than the other oils.
In order to analyze the stability in the emulsions through kinetic parameters, the nucleation rate J was calculated as per the following equation [38]:
where v g is the volume of one drop; Δt is the time increment between the experimental measurements; A t is the area of the curve between the time of total freezing; and t is the time of the measurement; \( A_{{\left( {t + \Updelta t} \right)}} \) is the area between the time of total freezing, and the next measurement at time t + Δt. The values of J are in the order of 1015–1022 drops/cm3/s. It was found in the W/O emulsions of light crude oil that the nucleation rate J with 10% of distilled water was higher than the emulsions with 5 and 20% of distilled water. On the other hand, the W/O emulsions of the heavy crude oil had a higher instability with 5% of water content compared to the 10 and 20% emulsions, as shown in Fig. 7. A temperature close to −10 °C was also found with a local maximum in J, and a temperature close to 50 °C with a local minimum in J in this 5% heavy crude oil emulsion. Regarding the super-heavy crude oil emulsions, they presented higher maxima of J at lower temperatures than the other crude oil emulsions with the same content of distilled water.
A second cycle of heating and cooling was performed to clarify whether the first cooling up to −60 °C and the first heating of crude oils affected their emulsion stability. It was found that the droplet size distribution did not appreciably change in the heavy and super-heavy crude oil emulsions; while the size distribution was displaced to higher diameters in the light crude oil emulsion. Furthermore, at 20% of distilled water, the highest frequency in solidification was not the signal IV, but a lower diameter contradicting Dalmazzone’s statement. This behavior is shown in Fig. 8.
The nucleation rate J was also calculated for the first and second cooling cycles, for light crude oil emulsified with 20% of distilled water, and two maxima were determined for the second cooling cycle, instead of a single maximum as in the first cycle (see Fig. 9). It could thus be stated, with the last two figures, that the light crude oil emulsion had a strong instability regarding temperature. It is also important to add that the curves shown in the cooling cycles, in specific zones, showed an increase of temperature [9, 14], instead of a corresponding decline. This was associated to an extra high change of nucleation rate J, producing a high heating of the sample, and then a rapid decline in the J value, as shown in Fig. 9.
An analysis of the concepts, in which the deduction of this simplified drop diameter assignation is based, shows that it is necessary to add the effect of the curvature in the nucleation of the frozen droplet. If this effect is mentioned and considered based on Pruppacher [17] methodology, then the diameter of the droplet is:
Here, V m,i is the ice molar volume [39]; γ iw and γ ow are the liquid water–ice and liquid water–oil interfacial tensions, respectively; \( \left( {\overline{{h_{{{\text{i}},1 - 2}} }} - \overline{{h_{{{\text{o}},1 - 2}} }} } \right) \) is the average difference of enthalpy between the ice and the oil from the crystallization temperature of reference,\( T_{{f_{1} }} \), as defined above, to the main freezing temperature, \( T_{{f_{2} }} \), determined by the DSC-measurements. It was supposed that the water drop was surrounded by oil, in the deduction of the last equation, and the ice germ was enclosed by the water drop. It was also considered that in the mean freezing temperature, there was 50% of ice and 50% of water in the droplet, and then the ice diameter was 80% of the total drop diameter. Based on this assumption, it was also found in Eq. 8, that the term \( {{4V_{\text{m,i}} \gamma_{\text{iw}} } \mathord{\left/ {\vphantom {{4V_{\text{m,i}} \gamma_{\text{iw}} } {0.8}}} \right. \kern-\nulldelimiterspace} {0.8}} \) had a smaller value that the term \( 4V_{\text{m,i}} \gamma_{\text{wo}} \). This agrees with the idea that the interfacial tension γ ow influenced the diameter of the droplet, thus validating Eq. 4. However, there are also experiments, not granting importance to the interfacial tension γ ow in the estimation of the droplet diameter; and of importance for determining the crystallization temperature [40]. Equation 9 gave unreasonable droplet diameters for the super-heavy crude, v g at −50 °C; due to the high enthalpy value of this crude oil. Because of this, it was inferred that Eq. 4 had a better applicability. It is worth clarifying that Eq. 4 gave results similar to those originally stated by Thomson [17, 41] to determine the germ diameter, but Thomson’s following equation is related to ice germs surrounded by liquid water, where only one germ is assigned to one droplet:
where \( \overline{{\Updelta H_{\text{m,f}} }} \) is the average enthalpy between \( T_{{f_{1} }} \) and\( T_{{f_{2} }} \); and D germ is the diameter of the ice germ.
Conclusions
The application of thermodynamics in equilibrium has given the possibility of associating a droplet diameter distribution to the crystallization temperatures detected in DSC curves from different kinds of petroleum containing droplets of emulsified water. With these results, the applicability of analytical formulas, instead of empirical correlations, allows a fast and simple analysis of the main physicochemical parameters of W/O emulsions formed in crude oils. An estimation of the average droplet size and emulsion stability may be performed using DSC measurements and the equations obtained from thermodynamical considerations. In the case of emulsions from three Mexican crude oils, it was found that a super-heavy crude oil exhibited a broad drop size distribution of emulsified water, whereas other petroleums, light and heavy crude oils, presented narrow drop distributions, at different water contents. It was also found that the greater the water content of emulsified water in super-heavy crude oil, the greater the fraction of smaller droplets. It is considered that the high content of asphaltenes and resins in this crude oil leads to the dispersion of a high amount of water in the shape of tiny drops, constituting a very stable emulsion. Furthermore, the stability of W/O dispersions from light and heavy crude oils decreases with the increase of water content. Along with these results, the application of concepts related to the nucleation kinetics led to the performance of a deeper analysis of the stability of W/O emulsions produced in crude oils.
References
Ali MA, Siddiqui AB, Zaidi SMJ. Thermal analysis of crude oils and comparison with SIMDIST and TBP distillation data. J Therm Anal Calorim. 1998;51:307–9.
Zanier A. Application of modulated temperature DSC to distillate fuels and lubricant greases. J Therm Anal Calorim. 1998;54:381–90.
Gonçalves MLA, Teixeira MAG, Pereira RCL, Mercury RPL, Matos JR. Contribution of thermal analysis for characterization of asphaltenes from Brazilian crude oil. J Therm Anal Calorim. 2001;64:697–706.
Gonçalves MLA, Pinto da Mota DA, Teixeira AMRF, Teixeira MAG. Thermogravimetric investigation on prediction of thermal behavior of petroleum distillation. J Thermal Anal Calorim. 2005;80:81.
Kök MV, Karakan Ö, Pamir R. Kinetic analysis of oxidation behavior of crude oil Sara constituents. Energ Fuel. 1998;12:580–8.
Kök M, Lettoffé JM, Claudy P, Martin D, Garcin M, Volle J-L. Comparison of wax appearance temperatures of crude oils by differential scanning calorimetry, thermomicroscopy and viscosimetry. Fuel. 1996;75:787–90.
Clausse DJ. Thermal behavior of emulsions studied by differential scanning calorimetry. J Therm Anal Calorim. 1998;51:191–201.
Clausse D, Gómez E, Dalmazzone C, Noïk C. A method for the characterization of emulsions, thermogranulometry: application to water-in-crude oil emulsion. J Colloid Interface Sci. 2005;287:694–703.
Dalmazzone CSH, Clausse D. Microcalorimetry. In: Sjöblom J, editor. Encyclopedic handbook of emulsion technology. New York: Marcel Dekker Inc; 2001. p. 327–48.
Dalmazzone C, Seris H. Use of the DSC technique to characterize water in crude oil emulsions stability. Rev Inst Franc Petr 53 1998;53:463–71.
Broto F, Clausse D. Study of freezing of supercooled water dispersed within emulsions by differential scanning calorimetry. J Phys C. 1976;9:4251–7.
Clausse D, Dumas JP, Meijer PHE, Broto F. Phase transformations in emulsions. 1. Effects of thermal treatments on nucleation phenomena—experiments and model. J Disper Sci Technol. 1987;8:1–28.
Dalmazzone C, Noïk C, Clausse D. Application of DSC for emulsified system characterization. Oil Gas Sci Technol. 2009;64:543–55.
Avendaño-Gómez JR, Grossiord JL, Clausse D. Study of mass transfer in oil-water-oil multiple emulsions by differential scanning calorimetry. J Colloid Interface Sci. 2005;290:533–45.
Clausse D, Gomez F, Pezron I, Komunjer L, Dalmazzone C. Morphology characterization of emulsions by differential scanning calorimetry. Adv Colloid Interface. 2005;117:59–74.
Bigg EK. The supercooling water. Proc Phys Soc Lond B. 1953;66:688–94.
Pruppacher HR, Klett JD. Microphysics of clouds and precipitation. 2nd ed. Dordrecht: Kluwer; 1997.
Castro LV, Vazquez F. Fractionation and characterization of Mexican crude oils. Energ Fuel. 2009;23:1603–9.
Goual L, Schabron JF, Turner TF, Towler BF. On column separation of wax and asphaltenes in petroleum fluids. Energ Fuel. 2008;22:4019–22.
ASTM E 967-08. Standard test method for temperature calibration of differential scanning calorimeters and differential thermal analyzers.
Charoenrein S, Reid DS. The use of DSC to study the kinetics of heterogeneous and homogeneous nucleation of ice in aqueous systems. Thermochim Acta. 1989;156:373–81.
Aboul-Gheit AK, Abd-el-Moghny T, Al-Eseimi MM. Characterization of oils by differential scanning calorimetry. Thermochim Acta. 1997;306:127–30.
Rondon M, Bouriat P, Lachaise J, Salager JL. Breaking of water-in-crude oil emulsions. 1. Physicochemical phenomenology of demulsifier action. Energy Fuel. 2006;20:1600.
Rondon M, Pereira JC, Bouriat P, Gracia A, Lachaise J, Salager JL. Breaking of water-in-crude-oil emulsions. 2. Influence of asphaltene concentration and diluent nature on demulsifier action. Energ Fuel. 2008;22:702–7.
Clausse D, Pezron I, Gauthier A. Water transfer in mixed water-in-oil emulsions studied by differential scanning calorimetry. Fluid Phase Equilibr. 1995;110:137–50.
Mossop SC. The freezing of supercooled water. Proc Phys Soc Lond B. 1955;68:193–208.
Langham EJ, Mason BJ. The heterogeneous and homogeneous nucleation of supercooled water. Proc R Soc Lond A. 1958;247:493.
Bogdan A. Phase transitions in finely divided aqueous systems. In: Hubbard AT, editor. Encyclopedia of surface and colloid science. New York: Marcel Dekker; 2002. p. 4002–305.
Levine IN. Physical chemistry. 2nd ed. New York: McGraw-Hill; 1983.
Atkins PW. Physical chemistry. 6th ed. New York: WH Freeman Co; 1997.
Castellan GW, Bedolla CA. Físico-química. 2nd ed. Mexico: Addison-Wesley Iberoamericana; 1987.
Dufour L, Defay R. Thermodynamic of clouds. New York: Academic Press; 1963.
Defay R, Prigonine I, Bellemnas A. Surface tension and adsorption. London: Longmans; 1966.
Bogdan A, Kulmala M, Avramenko N. Reduction of enthalpy of fusion and anomalies during phase transitions in finely divided water. Phys Rev Lett. 1998;81:1042–5.
Becher P. Emulsions: theory and practice. 2nd ed. New York: Reinhold Publishing Corporation; 1965.
Girifalco LA, Good RJ. A theory for the estimation of surface and interfacial energies. 1. Derivation and application to the interfacial tension. J Phys Chem. 1957;61:904–9.
Collins EA. Measurement of particle size and article distribution. In: Lovell PA, El-Aasser MS, editors. Emulsion polymerization and emulsion polymers. New York: John Wiley and Sons; 1997. p. 386–436.
Franks F, Mathias SF, Parsonage P, Tang TB. Differential scanning calorimetric study on ice nucleation in water and in aqueous-solutions of hydroxyethylstarch. Thermochim Acta. 1983;61:195–202.
Angell CA. Supercooled water. In: Franks F, editor. Water a comprehensive treatise, vol. 7. New York: Plenum Press; 1982. p. 1–56.
Montenegro R, Antonietti M, Mastai Y, Landfester K. The crystallization in miniemulsion droplets. J Phys Chem B. 2003;107:5088–94.
Thomson JJ. Application of dynamics to physics and chemistry. 1st ed. London: Cambridge University Press; 1888.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz-Ponce, J.A., Flores, E.A., Lopez-Ortega, A. et al. Differential scanning calorimetry characterization of water-in-oil emulsions from Mexican crude oils. J Therm Anal Calorim 102, 899–906 (2010). https://doi.org/10.1007/s10973-010-0904-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0904-8