Abstract
In order to investigate relative reactivity of different oxidants in solid-state reactions of pyrotechnic mixtures, thermal properties of Sn + Sr(NO3)2, Sn + Ba(NO3)2, and Sn + KNO3 pyrotechnic systems have been studied by means of TG, DTA, and DSC methods and the results compared with those of pure oxidants. The apparent activation energy (E), ΔG #, ΔH #, and ΔS # of the combustion processes were obtained from the DSC experiments. The results showed that the nature of oxidant has a significant effect on ignition temperature, and the kinetic of the pyrotechnic mixtures’ reactions, and the relative reactivity of these mixtures was found to obey in the following order: Sn + Sr(NO3)2 > Sn + Ba(NO3)2 > Sn + KNO3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid-state redox reactions occurring in pyrotechnics mixtures are accompanied with generation of heat, light, or color and used in emergency signaling, fireworks, air bag inflators, and special effects devices for the entertainment industry [1]. A pyrotechnic mixture consists of one or more oxidizers together with one or more fuels. Oxidizers used in pyrotechnics, such as potassium nitrate, KNO3, are solids at room temperature and release oxygen when heated to elevated temperatures. The oxygen then combines with the fuel, and heat is generated by the resulting chemical reaction. For such a chemical system, the ignition temperature is defined as onset temperature for a rapid, self-propagating reaction between the oxidizer and the fuel [2].
In order to predict the reaction behavior of a pyrotechnic composition, it is not possible, or only partly possible, to apply the theory of inorganic chemistry which bases on chemical redox reactions in solutions. Pyrotechnic reactions are high-temperature redox reactions; they take place in a temperature range of 1,500–4,000 °C.
Thermal methods of analysis are now firmly established in many fields in which reactions show exothermic or endothermic changes with variation of temperature. Pyrotechnic compositions react at solid state to give out a large amount of heat and, therefore, are suitable subjects for study by these techniques [3, 4].
The rate of combustion process and the ignition temperature for a pyrotechnic system depend on various oxidant properties such as oxidant’s nature, the ability of the oxidizer to release oxygen, its decomposition temperature, and the net heat input required for its decomposition. Therefore, the selection of a suitable oxidant is a critical parameter for the efficiency of pyrotechnic systems [5]. The oxidants used in this study include KNO3, Ba(NO3)2, and Sr(NO3)2, however, the use of KNO3 has been found to be fairly widespread as an oxidizing agent in the pyrotechnic industry [6, 7]. Although, Ba(NO3)2 and Sr(NO3)2 were rarely used as oxidants in pyrotechnic systems, the use of these two nitrate salts in light-producing compositions has been investigated in pyrotechnic studies [8, 9].
In this study, tin powder was used as fuel for pyrotechnic systems. At ordinary temperatures, tin is stable in air. It actually forms a very thin protective oxide film. In powder form, and especially in the presence of moisture, it oxidizes. When heated with oxygen, it forms tin (IV) oxide, SnO2. Several studies could be found on the hazards and safety of tin powder [10–12]. Some articles [13, 14] have established possible application of tin powder as fuel in pyrotechnic systems, but, to the best of our knowledge, there is no report on the thermal properties of pyrotechnic systems containing tin powder as fuel.
The aim of this study is to compare the thermal behavior of three pure nitrate oxidants as individual reactants and also, that of combustion of their tin-binary pyrotechnic systems. Non-isothermal kinetic analysis is used to estimate Arrhenius parameters using isoconversional method. Three new pyrotechnic mixtures, Sn + Sr(NO3)2, Sn + Ba(NO3)2, and Sn + KNO3, have been proposed as pyrotechnic systems containing nitrate salts.
Experimental
Materials
Laboratory reagent grade strontium nitrate, barium nitrate, potassium nitrate, and tin powder were all purchased from Merck (Tehran, Iran). The particle size of powdered tin was about 40 μm, and all other oxidants used were sieved through 30 μm before being mixed with tin powder.
Procedure
The Sn/oxidant binary compositions were initially prepared based on their reaction stoichiometric ratio (as presented in Table 1) through wet mixing in acetone. After evaporation of the solvent, small quantities of the pyrotechnic mixtures were carefully sieved through a slightly coarser sieve than the particles [15].
A thermobalance (Stanton, model TR-01, sensitivity 0.1 mg) with a differential thermal analysis attachment (STA 1500) was used for TG/DTA studies. In this study, the TG/DTA thermogram of pure compounds and mixtures were studied, separately under similar conditions (the pressure during heating was 1 bar; the sample’s mass was 3.0 mg and heating rate 10 °C min−1). Argon atmosphere was used in this study to investigate the thermal behavior of pure oxidants and mixtures also; air atmosphere was used for tin powder. Before testing, all the samples were stored in a vacuum oven at 65 °C at least for 3 h. The thermochemical behavior of pure tin powder, Sr(NO3)2, Ba(NO3)2, KNO3, and also mixtures of them was characterized according to Table 1.
DSC experiments were run with a DuPont DSC model 910 at the heating rates of 10, 20, 30, 40 °C min−1 with temperature ranging from 50 °C up to the end of the reaction. DSC measurements were conducted by placing 3.0 mg of mixtures in an alumina pan with a perforated cover under argon atmosphere (40 mL min−1).
Results and discussion
Thermal properties of pure compounds
The oxidation of tin powder was studied by simultaneous TG/DTA in air at the heating rate of 10 °C min−1. The results are shown in Fig. 1a. No thermal event was observed prior to the melting point near 232 °C, when the tin undergoes a sharp endothermic phenomenon involving melting. Above this temperature, the tin fuel, in the first step of its oxidation, is oxidized to SnO by air at a maximum of 577 °C peak temperature, and the sample mass increases, as shown in the DTA and TG curves. The oxidation process is continued, and the produced SnO, in the second step of tin oxidation, is oxidized to SnO2 by air at maximum 750 °C peak temperature, and the sample mass again increases. This second peak is observed as a shoulder of the first oxidation peak. Thus, complete oxidation of tin fuel with a mass gain of 26%, occurred in the temperature range of 550–900 °C [16]. After complete oxidation of the sample, SnO2 was produced (1).
The results of TG/DTA studies for oxidation of tin powder in air at the heating rate of 20 °C min−1 are shown in Fig. 1b. As shown in this figure, at higher heating rates, the oxidation of tin powder takes place in two separate steps, and two exothermic peaks corresponded to the oxidation steps are well resolved. The melting point of tin powder, at the heating rate of 20 °C min−1, was shifted to 233.9 °C, and complete oxidation of tin fuel occurred in the temperature range of 555–920 °C with two exothermic peaks appearing at maximum of 585.5 and 800 °C.
These results show that as the heating rate increases, tin smelt oxidizes to SnO and SnO2 in air at higher temperatures. The following exothermic reactions occur between tin fuel and oxygen:
A comparison of Figs. 1a and b shows that heating rate plays a major role on the oxidation mechanism of tin powder. At low heating rate, the oxidation steps of tin are consecutive, and exothermic peaks are overlapped; however, at higher heating rates, these peaks are resolved and oxidation process takes place during two separate stages.
The DTA and TG curves for pure strontium nitrate are shown in Fig. 1c. The strontium nitrate undergoes two overlapping consecutive endothermic phenomena including melting and decomposition at peak temperatures of 571 and 658 °C, respectively. The thermal events are accompanied with a 52% mass loss. Previous studies [17, 18] have shown that strontium nitrate has two different decomposition mechanisms depending on the reaction temperature. At low temperature, strontium nitrate decomposes endothermically near to its melting point according to the following mechanism:
Strontium nitrite, Sr(NO2)2, is formed as an intermediate in this decomposition reaction, and a substantial quantity of the nitrite can be found in the ash of low flame temperature mixtures. At higher reaction temperatures, the decomposition mechanism is as follows:
Produced NO gas by the reaction is accompanied by nitrogen dioxide/nitric oxide equilibrium as follows:
All oxides of nitrogen can eventually be reduced to molecular nitrogen:
These reactions occur as per the following overall reaction scheme:
This is a very strong endothermic reaction, with a heat of reaction of +385 kJ mol−1, and corresponds to an active oxygen content of 37.7%. Little ash is produced by this high-temperature process, which occurs in mixtures containing magnesium or other “hot” fuels [17].
Thermal analysis (TG/DTA) of pure barium nitrate indicated that Ba(NO3)2 melts at 588 °C with a strong endothermic peak (Fig. 1d), which subsequently decomposed at 685 °C. This result agrees with the TG curve showing that barium nitrate decomposes at 575–778 °C temperature range with 42% mass loss. Previous studies [19, 20] have shown that barium nitrate decomposes similar to strontium nitrate with two, low and high temperatures mechanisms as follow:
Figure 1e shows the results of DTA and TG curves of the KNO3 sample used in this study. The endothermic peak at 130 °C corresponds to the crystal change from rhombic to trigonal structure, whereas the endothermic peak at 337 °C corresponds to the melting point of KNO3. DTA and TG analyses from the melting point to 500 °C indicate that KNO3 is melting but still neither decomposing nor gasifying because no change on TG curve is observed. Therefore, KNO3 is stable under melting state during this wide range (163 °C) of temperature. The gasification reaction, indicated as a large decrease of TG curve, started at 500 °C and ended at 800 °C. Accordingly, the reaction processes of KNO3 are suggested as follows:
It is clear that the gasification reaction of KNO3 is an endothermic process. On the other hand, previous investigation on TG-DTA characteristics of KNO3 in nitrogen atmosphere showed that potassium nitrate decomposition occurs above 500 °C in two partially overlapping stages (500–770 and 770–1,000 °C). The mass losses were 72.0 ± 1.2% and 14.0 ± 1.0% between 500 and 770 °C and between 770 and 1,000 °C, respectively [21–23].
The proposed mechanism for this reaction could be written as
K2O is not formed until 1,000 °C and decomposition is only complete above 1,200 °C [21, 24]. The mass of residue in N2 (12.1 ± 1.8% of the original) is less than expected for K2O and supports reports of volatilization of K2O (m.p. 380 °C).
Thermal properties of binary pyrotechnic mixtures
For Sn + Sr(NO3)2, as seen in the DTA curve, no change in the curve of the mixture is observed up to 235 °C (Fig. 2a) when the mixture undergoes a sharp endothermic phenomenon containing melting of tin fuel powder. Above this temperature, there are two exothermic peaks with maxima of 437 and 552.2 corresponding to the ignition of tin with released oxygen from oxidant. Total mass loss was about 24%. By considering mass loss during this reaction and low temperature decomposition mechanisms of strontium nitrate, the following main reaction between fuel and oxidant is proposed:
In the TG and DTA curves for Sn + Ba(NO3)2 shown in Fig. 2b, no thermal event was observed prior to 236 °C, when the tin fuel undergoes a sharp endothermic phenomenon involving melting. Above this temperature, there is an exothermic peak with maximum of 462.6 and which is continued with a small shoulder at 527.7 corresponding to the ignition of tin with released oxygen from oxidant. There is an interval (~65 °C) between the first and second step of ignition where the oxidant releases whole of its oxygen content. The mixture gets ignited during this interval, and the sample mass decreases (by ~21%), as shown in the DTA and TG curves. The reaction between fuel and oxidant is a complete reaction:
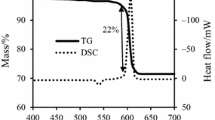
In the DTA and TG curves for Sn + KNO3 mixture (Fig. 2c), no thermal event is observed prior to 131 °C, at which potassium nitrate undergoes an endothermic phenomenon including phase transition. An endothermic peak at 237 °C is observed, corresponding to a melting of tin fuel without any decrease in the sample’s mass. After this, as seen in the DTA curve in Fig. 2c, potassium nitrate in the mixture undergoes fusion at 339 °C. Above this temperature, the mixture is ignited exothermally at 485.5 °C. Thus, this temperature corresponds to an ignition temperature. This result agrees with the TG curve for this sample with 18% mass loss. Such a behavior suggests that this pyrotechnic system gets ignited in single step. The reaction between fuel and oxidant for this sample is that of complete oxidation:
All thermal analysis results are summarized in Table 1.
Effect of heating rate
Figure 3 shows DSC curves for the ignition of Sn + Sr(NO3)2 pyrotechnic mixture at several heating rates. The curves show that as the heating rate was increased, the melting and ignition peaks of mixture were shifted to higher temperatures. As shown in Table 2, this trend was observed for all mixtures.
Kinetics of thermal ignition
Potential hazards associated with the thermal behavior of energetic materials require that stability evaluation and decomposition kinetics be carried out to insure their safe processing, handling, and storage. In this study, kinetic parameters were determined using Kissinger approach [25]:
In this method starting from
where, α is degree of conversion, A the pre-exponential or Arrhenius frequency factor, ϕ the heating rate, E the activation energy (kJ mol−1), R the gas constant, T the temperature of the sample, and n the reaction order.
Kissinger [25] had suggested several approaches for apparent kinetic parameter calculation, from DSC data:
where ϕ is the heating rate, R is the gas constant, E is the activation energy, T m is the temperature of max/min peaks of DSC curve, α m is the conversion degree to temperature, T m, and S is the form factor which presents the absolute value of the gradients of DSC curves in the points of min/max. For the reaction order other than 1, parameters are calculated from [26, 27]:
which state that activation energy could be calculated from ln \( \left( {{\phi \mathord{\left/ {\vphantom {\phi {\mathop T\nolimits_{m}^{2} }}} \right. \kern-\nulldelimiterspace} {\mathop T\nolimits_{m}^{2} }}} \right) \) as the relation 1/T m without interfering with the values of n. On the other hand, the Arrhenius frequency factor (A) was found for all mixtures from the following relation [28, 29]:
The calculated activation parameters for all mixtures are given in Table 3.
The entropy of activation (ΔS #), the enthalpy of activation (ΔH #), and the free energy of activation (ΔG #) corresponding to the each mixture were obtained [30]. Table 3 gives the calculated thermodynamic parameters for the studied pyrotechnic systems.
Critical ignition temperature
The critical ignition temperature (T b) an important parameter required to insure safe storage and process operations involving explosives, propellants, and pyrotechnics. It is defined as the lowest temperature to which a specific charge may be heated without undergoing thermal runaway [31]. T b may be calculated from inflammation theory and appropriate thermokinetic parameters, namely, the activation energy, pre-exponential factor, and heat of reaction. In order to obtain the critical temperature of thermal ignition (T b) for the pyrotechnic mixtures, Eqs. 28 and 29 were used [32].
where b and c are the coefficients, R is the gas constant, and E is the value of activation energy obtained by kinetic method.
The value (T e0) of the onset temperature (T e) corresponding to \( \phi \to 0 \) obtained by Eq. 28 is 422.15, 436.15, and 482.85 °C for Sn + Sr(NO3)2, Sn + Ba(NO3)2, and Sn + KNO3 mixture, respectively.
The critical temperatures of thermal explosion (T b) obtained from Eq. 29 is 436.85, 447.53, and 494.53 °C for Sn + Sr(NO3)2, Sn + Ba(NO3)2, and Sn + KNO3 mixture, respectively.
Effect of cation type on oxidant thermal behavior
In this study, the thermal behavior of three nitrate salts containing various cations (strontium, barium, and potassium) commonly used as pyrotechnic oxidants was studied in identical conditions. As shown in Fig. 1 and Table 1, the maximum decomposition temperatures for strontium, barium, and potassium nitrates in argon atmosphere, are 658, 685, and 695 °C, respectively. Many factors are involved in the thermodynamics of decomposition, including ionic size and charge, the hardness or softness of the ions the crystal structure of the solid, and the electronic structure of each of the ions. This stability trend may be related to changes in the degree of interaction between the nitrate and the cations. The interactions can be expressed in terms of hard and soft acids and bases (HSAB), in which the metal cation acts as a Lewis acid, and the nitrate anion as the Lewis base. These cations belong to hard acid group, and also nitrate ion is known as a hard base [33, 34]. Hard acids and bases are small and rigid ions; and hence, ionic interactions between two hard species are stronger than those between one hard and one soft species. Relative stability of ionic bonding in these nitrate salts can be rationalized based on the ionic potential of metal cations (i.e., cation charge to radius ratio), because higher ionic potential in cation causes lowering ionic bonding strength in nitrate salts. Therefore, the most stable salt, KNO3 in which ionic potential of K+ is lower than that of Ba2+ and Sr2+ in Ba(NO3)2 and Sr(NO3)2 respectively, has the greatest stability and the lowest tendency to undergo decomposition.
Comparison of ignition temperatures of mixtures
In this study, the thermal behavior of three pyrotechnic mixtures was studied in identical conditions. Tin powder, as seen in Table 1 and Fig. 1a and b, is a slack fuel and oxidizes at above 500 °C and may react with oxidant at higher temperatures (the common temperature for ignition of pyrotechnic mixture is below 500 °C). Therefore, oxidant nature and heating rate could have significant effects on tin thermal oxidation. At low heating rate, air oxidation of tin powder starts at 550 °C, and tin oxidizes to SnO intermediate and then, in the next step, SnO reacts to produce SnO2 at 750 °C. By increasing the heating rate, the exothermic peaks of oxidation reactions are completely resolved and appearing within a 365 °C temperature range. These results show that by increasing heating rate, oxidation temperature of tin fuel shifts to higher temperatures.
For the Sn + Sr(NO3)2 mixture, as seen in Table 1, the fuel melts at 235 °C and, in the next thermal event, ignition happens at 437 °C; then, combustion continues until 610 °C with a fall in the mass of sample. By considering mechanisms for thermal decomposition of pure strontium nitrate and mass loss of the mixture, it could be concluded that strontium nitrate decomposes in this mixture according to the low temperature mechanism, and the combustion occurs within a 200 °C temperature range. This relatively wide temperature range implying that combustion power of the system is not so great as to be suitable for pyrotechnic system. By replacement of Sr(NO3)2 in this mixture with Ba(NO3)2, the tin fuel undergoes melting at 236 °C and ignition steps occur at 462.6 and 527.6 °C. A comparison of the combustion characteristics of these mixtures shows that Sn + Ba(NO3)2 is a more efficient pyrotechnic system than Sn + Sr(NO3)2 since combustion power of the former is higher than that of the latter.
On the other hand, by replacing Ba(NO3)2 with KNO3 powder, the sensitivity of the mixture is decreased. As seen in Table 1 and Fig. 2, the melting point for the fuel in this mixture is 237 °C. Figure 2c shows that the mixture of Sn + KNO3 ignites at 485.5 °C. The results also show that the ignition temperature of Sn + KNO3 mixture is higher than that of other mixtures. However, the combustion of this mixture occurs during single step within a 30 °C temperature range so that this pyrotechnic mixture is a more useful and efficient system than the other two. It may be related to the fact that both fuel and oxidant melt before the ignition process. A comparison of ignition temperature of these mixtures is shown in Table 1.
Conclusions
The thermal studies carried out using non-isothermal TG and DTA in argon atmosphere to characterize thermal ignition of tin-fueled pyrotechnic mixtures with different nitrate-based oxidants. The result shows that Sn + KNO3 pyrotechnic system with the highest ignition temperature is an efficient pyrotechnic mixture. However, inclusion of Ba(NO3)2 in a mixture with tin powder results in lower stability and lower ignition temperature, because Ba(NO3)2 is less stable than KNO3. In addition, mixing tin powder as fuel with Sr(NO3)2 instead of Ba(NO3)2 in a pyrotechnic composition decreases ignition temperature of the mixture. However, this mixture has the lowest efficiency between the pyrotechnic systems investigated.
It was observed that nature of the cation in nitrate oxidant is an important factor in the thermal ignition of pyrotechnic mixtures. Finally, the values E, ΔS #, ΔG #, ΔH #, and T b for the ignition reaction of pyrotechnic mixtures were computed, and based on thermal properties of mixtures; the following order in the reactivity of the mixtures was noticed: Sn + Sr(NO3)2 > Sn + Ba(NO3)2 > Sn + KNO3.
References
Brown ME. Some thermal studies on pyrotechnic compositions. J Therm Anal Calorim. 2001;65:323–34.
Sivapirakasam SP, Surianarayanan M, Chandrasekaran F, Swaminathan G. Thermal hazards of cracker mixture using DSC. J Therm Anal Calorim. 2004;78:799–808.
Roduit B, Borgeat C, Berger B, Folly P, Andres H, Schädeli U, Vogelsanger B. UP-scaling of DSC DATA of high energetic materials, simulation of cook-off experiments. J Therm Anal Calorim. 2006;85:195–202.
Berger B, Brammer AJ, Charsley EL, Rooney JJ, Warrington SB. Thermal analysis studies on the boron–potassium perchlorate–nitrocellulose pyrotechnic system. J Therm Anal. 1997;49:1327–55.
Hosseini SG, Pourmortazavi SM, Hajimirsadeghi SS. Thermal decomposition of pyrotechnic mixtures containing sucrose with either potassium chlorate or potassium perchlorate. Combust Flame. 2005;14:322–6.
Roduit B, Borgeat C, Berger B, Folly P, Alonso B, Aebischer JN, Stoessel F. Advanced kinetic tools for the evaluation of decomposition reactions, determination of thermal stability of energetic materials. J Therm Anal Calorim. 2005;80:229–36.
Charsley EL, Laye PG, Brown ME. Handbook of thermal analysis and calorimetry: pyrotechnics. 1st ed. Amsterdam: Elsevier; 2003. p. 777–815.
Akhavan J. The chemistry of explosives. 2nd ed. London: The Royal Society of Chemistry; 2004.
Ianoş R, Lazău I, Păcurariu C. Metal nitrate/fuel mixture reactivity and its influence on the solution combustion synthesis of γ-LiAlO2. J Therm Anal Calorim. 2007;97:209–14.
Patnaik P. Handbook of inorganic chemicals. New York: McGraw-Hill Companies, Inc; 2003.
Cashdollar KL, Cashdollar I, Zlochower A. Explosion temperatures and pressures of metals and other elemental dust clouds. J Loss Prev Proc. 2007;20:337–48.
Cashdollar KL. Flammability of metals and other elemental dust clouds. Process Saf Prog. 1994;13:139–45.
Yoganarasimhan SR, Josyulu OS. Reactivity of the ternary pyrotechnic system red lead–silicon-ferric oxide, Defense. Sci J. 1987;37:73–83.
Knowlton GD, Ludwig CP. Auto-ignition composition, WO1997/045294, 12 Apr 1997.
Pourmortazavi SM, Hajimirsadeghi SS, Hosseini SG. Characterization of the aluminum/potassium chlorate mixtures by simultaneous TG-DTA. J Therm Anal Calorim. 2006;84:557–61.
Stets VP, Karlov VP, Butuzov GN, Ryabukha AA. Heat treatment of tin powder. Powder Metall Met C+. 1989;28:837–9.
de Klerk WPC, Krabbendam-LaHaye ELM, Berger B, Brechbuhl H, Popescu C. Thermal studies to determine the accelerated ageing of flares. J Therm Anal Calorim. 2005;80:529–36.
Kosanke KB, Kubota N, Sturman B, Jennings-White C. Pyrotechnic chemistry. Pyrotechnic reference series no.4. USA: Journal of pyrotechnics, Inc.; 2004.
Ellern H. Military and civilian pyrotechnic. New York: Chemical Publishing Company Inc; 1968.
McLain JH. Pyrotechnics from the viewpoint of solid state chemistry. Philadelphia, Penna: The Franklin Institute Press; 1980.
Rugunanan RA, Brown ME. Reactions of powdered silicon with some pyrotechnic oxidants. J Therm Anal Calorim. 1991;37:1193–211.
Turcotte R, Fouchard RC, Turcotte A-M, Jones DEG. Thermal analysis of black powder. J Therm Anal Calorim. 2003;73:105–18.
Freeman ES. The kinetics of the thermal decomposition of potassium nitrate and of the reaction between potassium nitrite and oxygen. J Am Chem Soc. 1957;79:838–42.
Shimizu T. Fireworks. The art science and technique. USA: Pyrotechnica Publications; 1981.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem.1957;29:1702–6.
Lehmann B, Karger-Kocsis J. Isothermal and non-isothermal crystallisation kinetics of pCBT and PBT. J Therm Anal Calorim. 2009;95:221–6.
Hatakeyama T, Quinn FX. Thermal analysis, fundamentals and applications to polymer science fundamentals and applications to polymer science. New York: Wiley; 1994.
ASTM E698-05. Standard test method for Arrhenius kinetic constants for thermally unstable materials. doi:10.1520/E0698-05.
Criado JM, Perez-Maqueda LA, Sanchez-Jimenez PE. Dependence of the preexponential factor on temperature. J Therm Anal Calorim. 2005;82:671–5.
Eslami A, Hosseini SG, Pourmortazavi SM. Thermoanalytical investigation on some boron-fuelled binary pyrotechnic systems. Fuel. 2008;87:3339–43.
Wang T, Lu YX, Zhu ML, Zhang JS, Ji SJ. DSC research on critical temperature in thermal explosion synthesis reaction Ti+ 3Al→TiAl3. J Therm Anal Calorim. 2002;67:605–11.
Zhang TL, Hu RZ, Xie Y, Li FP. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC. Thermochim Acta. 1994;244:171–6.
Missler GL, Tarr A. Inorganic chemistry. 4th ed. Upper Saddle River: Pearson Prentice Hall; 2010.
Markowitz MM, Boryta DA. The differential thermal analysis of perchlorate. J Phys Chem. 1964;68:2282–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, S.G., Eslami, A. Thermoanalytical investigation of relative reactivity of some nitrate oxidants in tin-fueled pyrotechnic systems. J Therm Anal Calorim 101, 1111–1119 (2010). https://doi.org/10.1007/s10973-010-0813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0813-x