Abstract
LiFe5O8 solid-phase synthesis at radiation-thermal (RT) annealing of lithium carbonate and iron oxide mechanical mixture was studied using thermal analysis (TG/DSC) and X-ray powder diffraction (XRD) techniques. The RT annealing was proceeded with high-power pulsing beam of 2.4 MeV electrons. It was shown that RT synthesis of the precursors considerably enhances the reactivity of the solid system within temperatures range 600–800 °C. In particular, lithium ferrite can be obtained at lower temperatures than those necessary in the absence of RT annealing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lithium ferrites have attracted continuous interest of researchers because of their technological applications as a perspective cathode materials in lithium batteries and low-cost magnetic material of microwave techniques. The perfection of traditional methods of synthesis and the elaboration of new methods for the lithium ferrites are realize in order to improve the quality of production [1–3]. It was shown [3, 4] that a heating up of reactionary mixture under powerful fast electron flow is a perspective method, advantage of which consists in rapidity and a low iterance of material warming up, lack of contact between heated experimental material and heater, homogeneity of a material heating in the whole volume. Influence of electron beam, unlike microwaves, is insensitive to the dielectric properties of materials. It is necessary to notice, that radiation effects at use of electronic beams find out in the diversified researches, including researches of decontamination of phytotherapeutic materials [5].
The objective of the present work is to investigate the radiation effects in lithium pentaferrite synthesis using TG/DSC and XRD methods. The TG/DSC method is caused by its efficiency at studying of solid-state synthesis of ferrite [1, 6].
The effects of radiation were evaluated by comparing the concentration LiFe5O8 phase in the irradiated samples with those in the samples that received the heat treatments at the same temperatures and for the same time.
Experimental
Powders Li2CO3 and α-Fe2O3 were used as starting materials for lithium pentaferrite synthesis. Reagents were dried at a temperature of 200 °C during 7 h and then weighted in proportion corresponding to the reaction: 5Fe2O3 + Li2CO3 → 2LiFe5O8 + CO2. The mixture was pressed into tablets under pressure of 220 MPa.
Radiation-thermal annealing of samples was carried out by pulse electron accelerator ILU-6 (Institute of Nuclear Physics, Novosibirsk, Russia). Electron energy was 2.4 MeV, beam current in the pulse 400 mA, pulse duration 500 μs, pulse repetition rate 7÷15 Hz. Average radiation dose was ~5 kGy s−1 at heating and ~3 kGy s−1 at isothermal annealing. Pulse radiation dose is 800 kGy s−1. Duration of nonisothermal stages (heating and cooling) did not exceed 3 min.
Thermal annealing was carried out using a silit resistance furnace. Rates of heating and cooling were close to the corresponding rates under radiation-thermal annealing.
The calorimetric measurements of synthesized samples were performed up to 800 °C at a heating rate of 50 °C min−1 in air atmosphere using thermal analyzer STA 449C Jupiter (Netzsch, Germany). We used Netzsch’s “Peak Separation” software for separation of the complicated peaks into components. The permanent magnets (H ~ 5 Oe) were placed near measuring system to control magnetic properties of the sample.
The content of LiFe5O8 phase was derived from endothermic DSC peak at 755 °C (“order–disorder” transition) and mass change at the Curie temperature (T c). These data were compared with results of full-profile XRD analysis using Powder Cell 2.4 software. X-ray measurements were made using diffractometer ARL X’TRA (Switzerland) with Cu Kα radiation.
Results and discussions
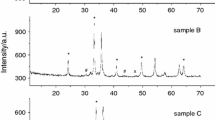
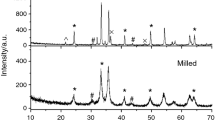
X-ray powder diffraction patterns of samples after synthesis contain reflections from α-Fe2O3, α-LiFeO2 and spinel phase. The spinel phase consists substantially from α-LiFe5O8 and ~3% γ-Fe2O3. Starting mixture does not contain γ-Fe2O3, it is the result of thermal treating.
After short time (<30 min) of synthesis at T = 600 °C, the reflections of lithium carbonate and α-Fe2O3 were detected. This is also reflected in the TG curve where the mass loss due to the decomposition of Li2CO3 takes place at ca. 720 °C. The value of the mass loss decreases with the increase in the time of synthesis. The DSC curve showed a wide endothermic peak in the temperature range 500–760 °C, which consists of the peak with maximum at 620 °C (this stage is caused by diffusion interaction of iron oxide and lithium carbonate, accompanied by allocation carbonic gas), peak near 720 °C (carbonate decomposition) and peak at ca. 755 °C (order–disorder γ–β phase transition in LiFe5O8). We separated of this difficult peak into components using the software.
The X-ray powder diffraction pattern recorded from the mixture heated at 700 and 800 °C showed the disappearance of the Li2CO3 and α-Fe2O3 phases and concomitant growth in those corresponding to α-LiFe5O8. In this case, only a sharp endothermic DSC peak at ca. 755 °C was observed (Fig. 1). The weight changes are not present without magnetic field. Under the condition of exterior magnetic field, the leap Δm appears on TG curve caused by the termination of magnetic interaction between lithium pentaferrite and applied field. The Curie temperature was defined as the maximum on DTG curve (see Fig. 1). For all explored samples, T c = 630 °C and that is in close agreement with literature [7].
Figure 2 shows the yield of LiFe5O8 as a function of time of thermal and radiation-thermal treatment at three temperatures: 600, 700, and 800 °C. Figures 3 and 4 show similar functions for the transition in LiFe5O8 and the leap in mass, respectively. It can be seen that the kinetic curves are characterized by rapid initial and slow second stages of the synthesis process irrespective of the synthesis mode.
Conclusions
The experimental data analysis showed the presence of the strong radiation influence on lithium pentaferrite synthesis, especially at low synthesis temperature. With the increase of synthesis temperature up to 800 °C the effect decreases. Application of powerful electronic irradiation for the heating of reactionary mixture makes it possible to increase the interface between the reactants, leading to the formation of lithium ferrites at lower temperatures. One may assume that the mechanism of solid-phase interaction of lithium carbonate with hematite does not change qualitatively in the conditions of electronic irradiation. The rate of solid-phase reaction changes only.
The analysis of lithium pentaferrite synthesis allows us to conclude that the significant influence of an electron beam is carried out on the initial stage of solid-phase process at small diffusion distances. The contribution of radiation into diffusion factor quickly decreases with increase of radiation treatment.
References
Berbenni V, Marini A, Matteazzi P, Ricceri R, Welham N. Solid-state formation of lithium ferrites from mechanically activated Li2CO3-Fe2O3 mixtures. J Eur Ceram Soc. 2003;23:527–36.
Widatallah HM, Berry FJ. The influence of mechanical milling and subsequent calcination on the formation of lithium ferrites. J Solid State Chem. 2002;164:230–6.
Karagedov GR, Konovalova EA, Boldyrev VV, Lyachov NZ. Influence of reagent biography and reaction conditions on kinetics of lithium ferrite synthesis. J Solid State Ionics. 1990;42:147–51.
Surzhikov AP, Pritulov AM. Radiation-thermal processes in ferrite powder materials. Moscow: EnergyAtomProduce; 2008.
Nemtanu MR, Brasoveanu M, Meltzer V, Pincu E, Oproiu C. Thermal analysis of some phytotherapeutic products irradiated with electron beam. J Therm Anal Calorim. 2009;97:309–13.
Berbenni V, Marini A, Milanese C, Bruni G. Solid state synthesis of CuFe2O4 from Cu(OH)2·CuCO3–4FeC2O4·2H2O mixtures: mechanism of reaction and thermal characterization of CuFe2O4. J Therm Anal Calorim. 2010;99:437–42.
An SY, Shim I-B, Kim CS. Synthesis and magnetic properties of LiFe5O8 powders by a sol-gel process. J Magn Magn Mater. 2005;290:1551–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surzhikov, A.P., Pritulov, A.M., Lysenko, E.N. et al. Calorimetric investigation of radiation-thermal synthesized lithium pentaferrite. J Therm Anal Calorim 101, 11–13 (2010). https://doi.org/10.1007/s10973-010-0788-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0788-7